Abstract
In this study, corn stalk was modified by manganese (Mn) before (MBC1) and after (MBC2) pyrolysis at different temperatures (400~600 °C) under anaerobic conditions for Cd sorption in both water and soil. Batch experiments in aqueous solution were conducted to evaluate the optimum sorption capability by biochar with and without manganese-modified. Both types of manganese modification can improve the sorption capacity of Cd(II) on biochar, which is superior to the corresponding pristine biochar without modification, especially, pyrolyzed at 500 °C with 5:1 modification ratio. Under the optimal preparation conditions, the sorption percentage on MBC2 was 11.01% higher than that of MBC1. The maximum sorption capacity of MBC2 was 191.94 mg g−1 calculated by isotherm model. The performance of MBC2 was also verified in soil stabilization experiments in Cd-contaminated soil. We can conclude from the results of BCR extraction that all the application rates of MBC2 (1%, 2%, and 3%) can reduce the mild acid-soluble fraction Cd. The reducible, oxidizable, and residual fraction Cd showed an upward trend, thus controlling the migration, transformation, and enrichment of Cd in soil. The characteristic analysis showed biochar has more irregular fold and more particle-aggregated surface after modification. The main components of these aggregated particles are manganese oxides (MnOx) with high sorption capacity, such as the MnOx crystal structure loaded on MBC2 is a mixed structure of δ-MnO2 and MnO. However, these particles may block the biochar pores, or some of the pores may collapse at high temperatures during the modification process. The specific surface area was reduced, even if the sorption effect of MBC was strongly enhanced. Meanwhile, under the action of the secondary pyrolysis of MBC2 modification process, the MBC2 has a higher degree of aromatization with more potential active sorption sites for Cd. The study concluded that the MBC2 could be a promising amendment for Cd in both water and soil real field applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pollution of heavy metal has turned into one of the most grievous environmental problems with widely contaminated area and serious hazard in recent years, and there is an urgent need for environmentally friendly and cost-effective remediation technologies. Natural activities, i.e., volcanic eruptions, forest fires, windblown dust, and anthropogenic activities, such as mining, wastewater irrigation, pesticides, fertilizers, have resulted in heavy metal contamination in the environment, of which Cadmium (Cd) contamination is most serious. When heavy metal elements such as Cd are leached from contaminated water or soil, they will further pollute the surrounding environment. At the same time, they will be in an easily absorbed state and will accumulate in the animal and human organs through the food chain, causing a large amount of irreversible damage (Pehlivan et al. 2008). Studies have shown that brief exposure to lower concentrations of Cd may cause nausea, runny nose, muscle cramps, and anemia, and long-term exposure can cause damage to organs such as the lungs, liver, kidneys, bones, , or causes toxic effect of the immune system and cardiovascular system, and may even cause cancer in severe cases (Vuković et al. 2010).
In order to achieve sustainable development, it is necessary to remediate water and soil polluted by heavy metal (Hou and Al-Tabbaa 2014). Currently, many techniques have been introduced to remediate heavy metal contamination, including electro kinetic remediation, sorption, chemical precipitation, and phytoextraction. Among these methods, convention physical and chemical remediation techniques require a large amount of energy and resources investment, which also may further lead to loss of land function (Song et al. 2019), while sorption technology has received widespread attention due to its lower cost, as well as higher efficiency and soil amelioration ability (Mondal 2009; Sullivan et al. 2010).
There have been many previous researchers tested a series of adsorbents, including lime (Cao et al. 2018), phosphate, biochar (Meng et al. 2014), nanomaterials (Wang et al. 2019b), and zeolite (Li et al. 2018b). Among them, biochar (BC) is particularly regarded as an effective reagent, because of its porous structure and multifunctional functional groups (Tan et al. 2015). It can be used to adsorb various contaminants. In addition, BC can adjust the soil structure; it can improve soil physicochemical properties and promote the uptake of soil nutrients during plant growth (Yuan et al. 2019). However, relatively lower sorption of pristine BC undoubtedly limits its in-depth application in the remediation field of high-concentration contamination (Fan et al. 2018). Several methods have been widely used, such as acid and base modification (Goswami et al. 2016), oxidation (Hadjittofi et al. 2014; Shen et al. 2019), nano-zero valent metals (Wang et al. 2019a), and nano metal oxide/hydroxide(Cao et al. 2019) to improve sorption capacity.
It has been proved that manganese oxides (MnOx) have superior immobilization potential (Liu et al. 2016) since heavy metal can enter the amphoteric surface functional group of the MnOx by oxidation/reduction, adsorption, complexation, or co-precipitation, and thereby achieving efficient removal of heavy metal (Komárek et al. 2013). It is worth mentioning that, despite its good immobilization potential, MnOx have not been subjected to extensive chemical stability studies compared with iron oxides. Therefore, we can believe that the effective combination of MnOx and biochar can improve the sorption performance of Cd and have the potential for practical water and soil treatment. Moreover, this combination can increase the stability of the composites, generate significantly lower amounts of leached Mn to reduce the possibility of secondary pollution to the environment (Ourednicek et al. 2019). Liang’s team found that the sorption capacity of Cd with amorphous MnO2 modified biochar was 3.18 times of unmodified biochar (Liang et al. 2017). However, the above research on manganese-modified biochar is mainly focused on its sorption behavior. The influence of the modification methods on the MnOx crystal structure and thus the effect of the resulted different crystal structures on the sorption capacity of biochar have not been well understood. Further efforts are necessary to comprehensively evaluate the modification method and determine the sorption mechanism.
Therefore, this study attempts to combine biochar and MnOx into manganese-modified biochar to verify whether it is a sorption material with good effect on Cd. The effect of different modified materials obtained with different modification methods and different conditions on sorption capacity was compared, aiming to find out the reasons for the difference and the relevant reaction mechanism. This study hopes to offer a better preparation method of manganese-modified biochar and explore its sorption effect toward Cd in the environment.
Materials and methods
Preparation of materials
Two kinds of modification methods were used to prepare manganese-modified biochar(MBC). One was to modify corn stalk with potassium permanganate (KMnO4), before pyrolysis (MBC1), and the other was to modify biochar of corn stalk with manganese, followed by second-time pyrolysis (MBC2).
MBC1: The corn stalk was pulverized and dried, then passed through the 100-mesh sieve, and finally kept in a sealed bag for use. The weight ratio of produced corn stalk biochar (the theoretically yield of biochar = 25%) to KMnO4 in the solution is 5:1, 10:1, 20:1, which is called modification ratio in this research. The corn stalk was firstly immersed in the KMnO4 solution for 24 h. The obtained sample was separated by suction filtration, dried in an oven at 80 °C for 12 h, then pyrolyzed at 400, 500, and 600 °C for 2 h. The material was removed, passed through the 100-mesh sieve, thoroughly washed again with deionized water, and then the dried MBC1 was kept for later use.
MBC2: Using corn stalk as raw material, biochar was produced by slowly pyrolyzing for 2 h under the nitrogen gas (N2) atmosphere by adjusting different pyrolysis temperatures, respectively, at 400, 500, and 600 °C. 5.0 g biochar, having been passed through a 100-mesh sieve, was weighted and then soaked in 40 mL of KMnO4 for 24 h; the weight ratio of biochar to KMnO4 was 5:1, 10:1, 20:1. The material prepared in the previous step was further pyrolyzed at 600 °C for 30 min to obtain the manganese-modified biochar (MBC2).
Modified materials analysis methods
Surface morphology analysis of biochar was performed using a scanning electron microscope (SEM, S-4700, Hitachi, JPN). Surface area and porosity analyzer (ASAP 2460, Micromeritics, USA) was used to measure the BET surface area with BET nitrogen sorption-desorption isotherms. A Fourier transform infrared spectroscope (FTIR, TENSOR II, BRUKER, GER) was used to evaluate the distribution of functional groups on the surface between different materials. The patterns of X-ray diffraction (XRD, Ultima IV, Rigaku, JPN) were collected to determine the mineral composition of BC and MBC.
Batch screening experiments in aqueous solution for Cd(II) sorption
The sorption experiment was repeated three times using a one-time equilibrium method. Under background electrolyte (0.01 mol·L−1 NaNO3), an appropriate amount of Cd(NO3)2·4H2O was dissolved to prepare a stock solution, and then diluted to the desired concentration for the later sorption experiment.
The effect of the modification methods on the sorption behavior of MBC toward cadmium (Cd(II)) was studied. The 100 mg biochar and modified biochar were added respectively in 50-mL polyvinyl tubes containing 20 mL of 100 mg L−1 Cd(II) containing solution. Studies have demonstrated that the optimum pH value of Cd(II) sorption experiments in water is 5–6 (Luo et al. 2019). Therefore, the pH of each solution was adjusted to 5.0 by 0.1 mol L−1 HNO3 to promote the reaction to proceed better. Qiwen Zhou and others proved that the similar modified biochar can achieve sorption equilibrium after 200 min (Liu et al. 2019; Zhou et al. 2018), so the suspension will be shaken at 25 °C for 6 h in this experiment. After shaken and equilibrated, the supernatants were filtered (0.45 μm pore sizes membrane) and stored at 5 °C for measurement by inductively coupled plasma-optical emission spectrometry (ICP-OES)(Optima 5300 DV).
The effect of the pyrolysis temperature and modification rate of MBC on the sorption behavior was studied. Add 25 mg, 50 mg, and 100 mg biochar, as well as modified biochar in 50-mL polyvinyl tubes containing 20 mL of 100 mg L−1 Cd(II) containing solution. After the pH was adjusted to 5.0, the suspension was shaken, centrifuged, filtered, and stored until determination.
To study the sorption equilibrium isotherm of sorbent on Cd(II), 50 mg of modified biochar was added to the 20 mL solution with different Cd(II) concentrations of 1–200 mg L−1 and shaken at 25 °C for 6 h.
Stabilization experiments in soil
Through the batch screening experiment in aqueous solution, we can select the better methods and conditions of modification. In order to clarify the application scope of the manganese-modified biochar as an amendment and verify its application effect in the environment, the stabilization experiment in soil was carried out.
Collect contaminated soil samples at multiple points with a sampling depth of 0~20 cm in a polluted farmland in Jiaozuo, Hunan Province, China. After removal of impurities, the soil was mixed evenly, air-dried, sieved, and stored. The pH value was 5.30; the soil organic matter (SOM) was 35.70 g·kg−1; standard soil sample value (GBW07401) and the measurement standard soil sample value are 31.03 g kg−1 and 30.79 g kg−1 with good accuracy (relative standard deviation (RSD) is less than 5.0%); the cation exchange capacity (CEC) was 8.13 cmol kg−1; total concentrations of Cd is 2.78 mg kg−1 in soil.
Select the material with the best sorption effect in the above experiment. Weigh 30 g contaminated soil into a 100-mL centrifuge tube, add the material with a ratio of 1%, 2%, 3%, and stir evenly with a glass rod to simulate the water content of the paddy field to add water to the soil and form a water layer. During the period, the water was added by the weighing method, and three groups of parallel samples were set. On the 35th day, the soil was grounded and sieved through the 40-mesh sieve to be measured.
Sample analyses
The Walkley-Black method was used to determine the soil organic carbon content (Arthur et al. 2016).
CEC in soil was analyzed by the substitution method with BaCl2 described in Huang et al. (2014).
Sequential extraction in soil was performed using the modified four-stage procedure BCR method (Rauret et al. 1999). 0.5 g soil sample was subjected to this extraction process, as following Table 1, in a 100-ml polyethylene centrifuge tube (Nemati et al. 2011).
Results and discussion
Preparation and screening of manganese-modified biochar
The effect of the modification method on the sorption behavior of MBC toward Cd(II) was determined with the results shown in Table 2. It was shown that the MBC sorption of Cd(II) was obviously improved compared with BC and the sorption was increased by 32.44%. However, in the same modification ratio as MBC1, MBC2 has a higher sorption effect toward Cd(II), of which sorption removal efficiency is 98% or higher, regardless of all pyrolysis temperature. Under the same condition that at the pyrolysis temperature of 500 °C, the sorption removal efficiency on MBC2 is higher than that of MBC1 by 11.01%, probably due to the different MnOx crystal structure and the amount of load supported on the biochar surface by two modification methods, or result from the secondary pyrolysis of MBC2 preparation process, since the MBC2 has been observed more aromatization structure.
As can be seen from the above section, MBC2 pyrolyzed at 500 °C (MBC2-500) and pyrolyzed at 600 °C (MBC2-600) have better effects. Therefore, using these two materials, the following sorption experiment was carried out by changing the modification ratio and adjusting the different additional amounts, with the results shown in Fig. 1, to comprehensively improve the sorption effect of the MBC2.
Figure 1 shows that the sorption effect of Cd(II) on MBC2 is positively correlated with its additional amount and its modification ratio. When the amount of addition is 100 mg, the optimal sorption effect of the modified material is obtained. The effect of MBC2-500 is slightly better than MBC2-600. And considering energy conservation and cost-effectiveness, it is determined that 500 °C is the optimal pyrolysis temperature. The sorption effect of the MBC2-500 with 5:1 modification ratio is the best, when the addition amount is 100 mg, the sorption removal efficiency can reach 99.60% with 20 mL of solutions contained 100 mg L−1 Cd(II), which is obviously better than the BC with 72.12% in the same case.
Sorption isotherm
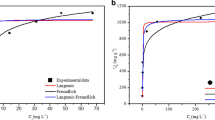
The sorption performance of MBC2 was determined by varying the Cd(II) concentration from 1 to 200 mg L−1. Sorption isotherms of the sorption of Cd(II) on MBC2 pyrolyzed at 500 °C with 5:1 modification ratio (MBC2-500-5:1) at pH = 5.0 are given in Fig. 2. The Langmuir and Freundlich models can be represented by the nonlinear forms (1) and (2) (Xiong et al. 2010). They are widely used in the equilibrium model of many sorption systems. The applicability of the isothermal model of sorption studies is mainly determined by comparing the correlation coefficient R2 (Zhang et al. 2018).
Both Langmuir (1) and Freundlich (2) models equations were tested to fit the data, and the obtained model parameters are shown in Table 3.
By comparing the R2 values in the above table, it can be concluded that both models can well represent the sorption process of Cd(II) by MBC2-500-5:1, though the Freundlich model exhibits a higher R2 (0.9988) among the isotherm models. Then the maximum sorption capacity is 191.94 mg g−1, which is far higher than the sorbent has been reported (Vuković et al. 2010).
Characterization of materials
BC-500, MBC1-500-5:1, and MBC2-500-5:1 appeared macroscopically as black powdery solids, scanning electron microscope (SEM) images of them are shown in Fig. 3. As far as the surface morphology and structure are concerned, the surface of BC is relatively smooth and flat with no obvious pore structure and surface loading particles. This may also be the reason why the specific surface area of both biochar and modified biochar is very small. But the MBC, especially MBC2, were synthesized irregular fold and particle-aggregated surface. It is obvious that there are small hexagonal and cubic particles on the surface of MBC2-500-5:1, which is presumed to be the δ-MnO2 and MgO particles detected below (Bdewi et al. 2015). It is suggesting that modified biochar can generate more potential active sorption sites (Tao et al. 2019a).
The specific surface area of BC, MBC1, and MBC2 is shown in Table 4, which differs significantly depending on the modification methods and preparation temperature. As the pyrolysis temperature increases, the specific surface area increased firstly and then decreased. This might result from the fact that the material contains oxygen, when the raw material is pyrolyzed and carbonized, the carbon element is etched due to the oxidation reaction, and the pore structure is formed. However, when the preparation temperature is too high, the pore structure of the biochar may collapse and the pore characteristics may be deteriorated(Jin et al. 2016).
The specific surface area of the MBC is smaller than BC, the reason may be the loaded MnOx particles were filled into the pore diameter of biochar and potassium permanganate was highly oxidized and which has serious erosion and perforation. Comparing MBC1-500 and MBC2-500, both obtained by BC-500, it is obvious that MBC2-500 has a larger specific surface area. Therefore, it was speculated that the MBC2-500 is loaded with MnOx particles and the effect of sorption is better, which consistent with the above experimental results.
FT-IR spectra of BC-500, MBC1-500-5:1, and MBC2-500-5:1 are shown in Fig. 4, which is usually used to analyze the changes of surface functional groups (Hassan et al. 2014). It is obvious that the surfaces of the three materials are rich in the same functional groups: The bands at 3500~3900 cm−1 are assigned to the stretching vibration of hydroxyl(–OH) groups (Jung et al. 2015). There are several weak absorption peaks between 3100 and 2700 cm−1, which is the C–H stretching vibration region; after the biochar was modified, the peaks in the above two intervals are obviously strengthened; the characteristic peak at 1553 cm−1 and 1405 cm−1 is generated by the stretching vibration of C=O (Luo et al. 2012) and the stretching vibration of methyl-CH3, where MBC2 has the strongest peak, followed by MBC1, and then BC; the in-plane bending vibration zone of -CH is between 1000 and 670 cm−1. In this interval, all three materials show multiple characteristic peaks. The characteristic peak of MBC2 at 690 cm−1 is obviously enhanced, which is likely to be caused by a disubstituted benzene ring. It is worth mentioning that blue/red shift occurs in some bands of MBC compared with BC, indicating that the manganese modification process has successfully changed the surface properties of biochar (Fan et al. 2018).
Meanwhile, MBC2 has characteristic peaks at 1630 cm−1 and 1251 cm−1, which are caused by C=O or C=C double bond stretching vibration of polycyclic aromatic hydrocarbons and aromatic ring C–C stretching vibration (Chen et al. 2015; Tao et al. 2019b), indicating that MBC2 has a high degree of aromatization. Moreover, the new peak at 520 cm−1 of MBC1 and MBC2 can be assigned to the stretching vibration of MnOx (Tan et al. 2019), which shows that manganese modification is successful.
The XRD patterns of BC-500, MBC1-500-5:1, and MBC2-500-5:1 are shown in Fig. 5. That of MBC1 composites presented simply the diffraction peaks corresponding to Alpha manganese dioxide (α-MnO2, PDF card: No. 81-1947), though delta manganese dioxide (δ-MnO2, PDF card: No. 86-0666) and manganese oxide (MnO, PDF card: No. 75-0625) were detected in the MBC2. The three materials all had the characteristic peaks of carbon (C) (Hao et al. 2013). Compared with the peak at the same position in the XRD pattern, the peak strength of the MBC is significantly weakened, which may be due to the fact that the Mn loaded weakens the diffraction peak intensity of other crystals (Escande et al. 2015), which is the same as the study by M. Richter et al. (1999).
Stabilization experiment results in soil
Total concentrations of Cd in the soil with different treatments (control check (CK); 1% MBC2-500-5:1; 2% MBC2-500-5:1; 3% MBC2-500-5:1) were 2.78, 2.39, 2.43, 2.36 mg kg−1, respectively. There was no significant decrease in the data results, mainly because Cd in the contaminated soil did not leave the soil system after treatment, although it would be more stable.
Cd in soil was sequentially fractionated into the mild acid-soluble, reducible, oxidizable, residual fractions. The dynamic changes of different fractions of Cd in the soil are shown in Fig. 6. As shown by the experimental results, Cd was distributed mainly in the mild acid-soluble and reducible fractions. Compared with the CK, the value of w (mild acid-soluble fraction) in the soil is inversely proportional to the amount of MBC2-500-5:1 applied, contrary to other fractions. w (mild acid-soluble fraction) of MBC2-500-5:1 treatment (CK, 1%, 2%, 3%) of soil was decreased from 66.27 to 56.13%, 50.88%, 48.63%; w (reducible fraction) was increased from 19.56 to 23.19%, 22.32%, 25.08%; w (oxidizable fraction) was increased from 8.22 to 11.40%, 14.74%, 15.76%; w (residual fraction) was increased from 5.95 to 9.28%, 12.06%, 10.53%.
When applied to the soil, biochar can affect the pH, CEC, and other physical and chemical properties, thus affecting the occurrence of Cd in the soil. For all existing heavy metal fractions, the mild acid-soluble fraction can be seen as the bioavailable state, because it is easily absorbed by plants; the reducible and oxidizable fractions are potentially bioavailable; the residual fraction is difficult for plants to absorb and use, so it is the most stable (Wu et al. 2019). The results showed that the percentage of the more stable Cd fraction in the soil after application of MBC2-500-5:1 was increased, compared with CK. Adding 3% worked best, which is the same result as Cui et al. (2016), also in line with our expectations.
Sorption mechanisms of Cd
Above all, the MBC was successfully synthesized with high sorption capacity, its physicochemical properties and morphology were examined, and the Cd(II) sorption properties of this material were determined using some experiments and sorption isotherm. The surface areas of MBC are much smaller than that of BC and are less porous, due to the MnOx loaded on the MBC’s surface. The surface of BC and MBC is rich in many efficient functional groups, in which MBC2 is more aromatic. Functional groups such as C–C, C–O, C=O, and –OH rich in the surface can react with Cd(II) for complexation and ion exchange, thereby improving the sorption effect (Zhu et al. 2019). MnOx-related functional groups were added to the surface of biochar. According to the XRD results, the surface load of MBC1 is α-MnO2, while the surface of MBC2 is loaded with δ-MnO2 and MnO. The δ-MnO2 is a layered, hexagonal, poorly crystalline Mn oxide (Sun et al. 2018), which is the most abundant MnOx rich in Mn (IV) in soil with higher sorption performance (Li et al. 2018a), which further improves the sorption effect of MBC2 on Cd(II) (Zhou et al. 2018). Thus, the Cd(II) sorption on MBC2 was mainly due to the formation of surface complexes between Cd(II) and δ-MnO2, as well as between Cd(II) and O-containing groups (such as C–O, C=O, and –OH).
Conclusion
Biochar and manganese-modified biochar were synthesized to investigate their effect on the sorption of Cd. In order to verify the effect, batch screening experiments in aqueous solution and soil stabilization verification experiment were carried out. Batch experiments showed that MBC2 has a much higher sorption capacity as large as 191.94 mg g−1 for Cd(II) than BC and MBC1, although all materials can enhance the sorption of Cd(II). The sorption effect of the MBC2 pyrolyzed at 500 °C with 5:1 modification ratio is the best, when the addition amount is 100 mg, the sorption removal efficiency can reach 99.60% with 20 mL of solutions contained 100 mg L−1 Cd(II). In soil stabilization experiment, 1%, 2%, and 3% addition of MBC2-500-5:1 can all convert the mild acid-soluble fraction Cd to the reducible, oxidizable, residual fraction Cd, thereby controlling the migration, transformation, and enrichment of Cd in the soil. After the biochar was modified by manganese, the MBC2 has a higher degree of aromatization with more potential active sorption sites to Cd, and the MnOx crystal structure supported by MBC2 is a mixed structure of δ-MnO2 and MnO with high sorption capacity. Therefore, the MBC2 could be used as a promising remediation sorbent to reduce the risks of Cd-contaminated water and soil.
References
Arthur E, Tuller M, Moldrup P, De Jonge LW (2016) Evaluation of theoretical and empirical water vapor sorption isotherm models for soils. Water Resour Res 52:190–205
Bdewi S, Mutar A, Aziz B (2015) Catalytic photodegradation of methyl orange using MgO nanoparticles prepared by molten salt method. Asian Trans Eng 05:1–5
Cao XY, Hu PJ, Tan CY, Wu LH, Peng B, Christie P, Luo YM (2018) Effects of a natural sepiolite bearing material and lime on the immobilization and persistence of cadmium in a contaminated acid agricultural soil. Environ Sci Pollut Res 25:22075–22084
Cao Z-f, Wen X, Wang J, Yang F, Zhong H, Wang S, Wu Z-k (2019) In situ nano-Fe3O4/triisopropanolamine functionalized graphene oxide composites to enhance Pb2+ ions removal. Colloids Surf A Physicochem Eng Asp 561:209–217
Chen Z, Xiao X, Chen B, Zhu L (2015) Quantification of chemical states, dissociation constants and contents of oxygen-containing groups on the surface of biochars produced at different temperatures. Environ Sci Technol 49:309–317
Cui L, Pan G, Li L, Bian R, Liu X, Yan J, Quan G, Ding C, Chen T, Liu Y, Liu Y, Yin C, Wei C, Yang Y, Hussain Q (2016) Continuous immobilization of cadmium and lead in biochar amended contaminated paddy soil: a five-year field experiment. Ecol Eng 93:1–8
Escande V, Petit E, Garoux L, Boulanger C, Grison C (2015) Switchable alkene epoxidation/oxidative cleavage with H2O2/NaHCO3: efficient heterogeneous catalysis derived from biosourced eco-Mn. ACS Sustain Chem Eng 3:2704–2715
Fan Z, Zhang Q, Li M, Niu D, Sang W, Verpoort F (2018) Investigating the sorption behavior of cadmium from aqueous solution by potassium permanganate-modified biochar: quantify mechanism and evaluate the modification method. Environ Sci Pollut Res Int 25:8330–8339
Goswami R, Shim J, Deka S, Kumari D, Kataki R, Kumar M (2016) Characterization of cadmium removal from aqueous solution by biochar produced from Ipomoea fistulosa at different pyrolytic temperatures. Ecol Eng 97:444–451
Hadjittofi L, Prodromou M, Pashalidis I (2014) Activated biochar derived from cactus fibres–preparation, characterization and application on Cu (II) removal from aqueous solutions. Bioresour Technol 159:460–464
Hao F, Zhao X, Ouyang W, Lin C, Chen S, Shan Y, Lai X (2013) Molecular structure of corncob-derived biochars and the mechanism of atrazine sorption. Agron J 105:773
Hassan AF, Abdel-Mohsen AM, Fouda MMG (2014) Comparative study of calcium alginate, activated carbon, and their composite beads on methylene blue adsorption. Carbohydr Polym 102:192–198
Hou D, Al-Tabbaa A (2014) Sustainability: a new imperative in contaminated land remediation. Environ Sci Pol 39:25–34
Huang B, Li Z, Huang J, Guo L, Nie X, Wang Y, Zhang Y, Zeng G (2014) Adsorption characteristics of Cu and Zn onto various size fractions of aggregates from red paddy soil. J Hazard Mater 264:176–183
Jin J, Li Y, Zhang J, Wu S, Cao Y, Liang P, Zhang J, Wong MH, Wang M, Shan S, Christie P (2016) Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge. J Hazard Mater 320:417–426
Jung K-W, Jeong T-U, Hwang M-J, Kim K, Ahn K-H (2015) Phosphate adsorption ability of biochar/Mg–Al assembled nanocomposites prepared by aluminum-electrode based electro-assisted modification method with MgCl2 as electrolyte. Bioresour Technol 198:603–610
Komárek M, Vaněk A, Ettler V (2013) Chemical stabilization of metals and arsenic in contaminated soils using oxides – a review. Environ Pollut 172:9–22
Li Q, Huang X, Su G, Zheng M, Huang C, Wang M, Ma C, Wei D (2018a) The regular/persistent free radicals and associated reaction mechanism for the degradation of 1,2,4-Trichlorobenzene over different MnO2 polymorphs. Environ Sci Technol 52:13351–13360
Li ZT, Wang L, Meng J, Liu XM, Xu JM, Wang F, Brookes P (2018b) Zeolite-supported nanoscale zero-valent iron: new findings on simultaneous adsorption of Cd(II), Pb (II), and As (III) in aqueous solution and soil. J Hazard Mater 344:1–11
Liang J, Li X, Yu Z, Zeng G, Luo Y, Jiang L, Yang Z, Qian Y, Wu H (2017) Amorphous MnO2 modified biochar derived from aerobically composted swine manure for adsorption of Pb (II) and Cd(II). ACS Sustain Chem Eng 5:5049–5058
Liu J, Ge X, Ye X, Wang G, Zhang H, Zhou H, Zhang Y, Zhao H (2016) 3D graphene/δ-MnO2 aerogels for highly efficient and reversible removal of heavy metal ions. J Mater Chem A 4:1970–1979
Liu X, Gao M, Qiu W, Khan ZH, Liu N, Lin L, Song Z (2019) Fe-Mn-Ce oxide-modified biochar composites as efficient adsorbents for removing As (III) from water: adsorption performance and mechanisms. Environ Sci Pollut Res Int 26:17373–17382
Luo M, Lin H, He Y, Li B, Dong Y, Wang L (2019) Efficient simultaneous removal of cadmium and arsenic in aqueous solution by titanium-modified ultrasonic biochar. Bioresour Technol 284:333–339
Luo X, Wang C, Luo S, Dong R, Tu X, Zeng G (2012) Adsorption of As (III) and As (V) from water using magnetite Fe3O4-reduced graphite oxide–MnO2 nanocomposites. Chem Eng J 187:45–52
Meng J, Feng XL, Dai ZM, Liu XM, Wu JJ, Xu JM (2014) Adsorption characteristics of Cu (II) from aqueous solution onto biochar derived from swine manure. Environ Sci Pollut Res 21:7035–7046
Mondal MK (2009) Removal of Pb (II) ions from aqueous solution using activated tea waste: adsorption on a fixed-bed column. J Environ Manag 90:3266–3271
Nemati K, Bakar NKA, Abas MR, Sobhanzadeh E (2011) Speciation of heavy metals by modified BCR sequential extraction procedure in different depths of sediments from Sungai Buloh, Selangor, Malaysia. J Hazard Mater 192:402–410
Ourednicek P, Hudcova B, Trakal L, Pohorely M, Komarek M (2019) Synthesis of modified amorphous manganese oxide using low-cost sugars and biochars: material characterization and metal (loid) sorption properties. Sci Total Environ 670:1159–1169
Pehlivan E, Yanik BH, Ahmetli G, Pehlivan M (2008) Equilibrium isotherm studies for the uptake of cadmium and lead ions onto sugar beet pulp. Bioresour Technol 99:3520–3527
Rauret G, López-Sánchez J, Sahuquillo A, Rubio R, Davidson C, Ure A, Quevauviller P (1999) Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J Environ Monit 1:57–61
Richter M, Berndt H, Eckelt R, Schneider M, Fricke R (1999) Zeolite-mediated removal of NOx by NH3 from exhaust streams at low temperatures. Catal Today 54:531–545
Shen Z, Zhang J, Hou D, Tsang DCW, Ok YS, Alessi DS (2019) Synthesis of MgO-coated corncob biochar and its application in lead stabilization in a soil washing residue. Environ Int 122:357–362
Song Y, Kirkwood N, Maksimovic C, Zheng X, O'Connor D, Jin Y, Hou D (2019) Nature based solutions for contaminated land remediation and brownfield redevelopment in cities: a review. Sci Total Environ 663:568–579
Sullivan C, Tyrer M, Cheeseman CR, Graham NJD (2010) Disposal of water treatment wastes containing arsenic — a review. Sci Total Environ 408:1770–1778
Sun Q, Liu C, Alves ME, Ata-Ul-Karim ST, Zhou D-M, He J-Z, Cui P-X, Wang Y-J (2018) The oxidation and sorption mechanism of Sb on δ-MnO 2. Chem Eng J 342:429–437
Tan G, Liu Y, Xiao D (2019) Preparation of manganese oxides coated porous carbon and its application for lead ion removal. Carbohydr Polym 219:306–315
Tan X, Liu Y, Zeng G, Wang X, Hu X, Gu Y, Yang Z (2015) Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 125:70–85
Tao Q, Chen Y, Zhao J, Li B, Li Y, Tao S, Li M, Li Q, Xu Q, Li Y, Li H, Li B, Chen Y, Wang C (2019a) Enhanced Cd removal from aqueous solution by biologically modified biochar derived from digestion residue of corn straw silage. Sci Total Environ 674:213–222
Tao Y, Hu S, Han S, Shi H, Yang Y, Li H, Jiao Y, Zhang Q, Akindolie MS, Ji M, Chen Z, Zhang Y (2019b) Efficient removal of atrazine by iron-modified biochar loaded Acinetobacter lwoffii DNS32. Sci Total Environ 682:59–69
Vuković GD, Marinković AD, Čolić M, Ristić MĐ, Aleksić R, Perić-Grujić AA, Uskoković PS (2010) Removal of cadmium from aqueous solutions by oxidized and ethylenediamine-functionalized multi-walled carbon nanotubes. Chem Eng J 157:238–248
Wang Y, Li Q, Zhang P, O’Connor D, Varma RS, Yu M, Hou D (2019a) One-pot green synthesis of bimetallic hollow palladium-platinum nanotubes for enhanced catalytic reduction of p-nitrophenol. J Colloid Interface Sci 539:161–167
Wang Y, O’Connor D, Shen Z, Lo IMC, Tsang DCW, Pehkonen S, Pu S, Hou D (2019b) Green synthesis of nanoparticles for the remediation of contaminated waters and soils: constituents, synthesizing methods, and influencing factors. J Clean Prod 226:540–549
Wu C, Shi L, Xue S, Li W, Jiang X, Rajendran M, Qian Z (2019) Effect of sulfur-iron modified biochar on the available cadmium and bacterial community structure in contaminated soils. Sci Total Environ 647:1158–1168
Xiong L, Yang Y, Mai J, Sun W, Zhang C, Wei D, Chen Q, Ni J (2010) Adsorption behavior of methylene blue onto titanate nanotubes. Chem Eng J 156:313–320
Yuan P, Wang J, Pan Y, Shen B, Wu C (2019) Review of biochar for the management of contaminated soil: preparation, application and prospect. Sci Total Environ 659:473–490
Zhang X, Lin Q, Luo S, Ruan K, Peng K (2018) Preparation of novel oxidized mesoporous carbon with excellent adsorption performance for removal of malachite green and lead ion. Appl Surf Sci 442:322–331
Zhou Q, Liao B, Lin L, Qiu W, Song Z (2018) Adsorption of Cu (II) and Cd(II) from aqueous solutions by ferromanganese binary oxide-biochar composites. Sci Total Environ 615:115–122
Zhu L, Tong L, Zhao N, Li J, Lv Y (2019) Coupling interaction between porous biochar and nano zero valent iron/nano alpha-hydroxyl iron oxide improves the remediation efficiency of cadmium in aqueous solution. Chemosphere 219:493–503
Funding
This work was supported by the National Key Research and Development Program of China (2017YFD0801503), the National Natural Science Foundation of China (41877133, 41701367), and the Fundamental Research Funds for the Central Universities (PT1906).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible editor: Zhihong Xu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tan, X., Wei, W., Xu, C. et al. Manganese-modified biochar for highly efficient sorption of cadmium. Environ Sci Pollut Res 27, 9126–9134 (2020). https://doi.org/10.1007/s11356-019-07059-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07059-w