Abstract
This study investigated the removal of selenite from wastewater using the fungus Asergillus niger KP isolated from a laboratory scale inverse fluidized bed bioreactor. The effect of different carbon sources and initial selenite concentration on fungal growth, pellet formation and selenite removal was first examined in a batch system. The fungal strain showed a maximum selenite removal efficiency of 86% in the batch system. Analysis of the fungal pellets by field-emission scanning electron microscopy, field-emission transmission electron microscopy and energy-dispersive X-ray spectroscopy revealed the formation of spherical-shaped elemental selenium nanoparticles of size 65–100 nm. An increase in the initial selenite concentration in the media resulted in compact pellets with smooth hyphae structure, whereas the fungal pellets contained hair like hyphae structure when grown in the absence of selenite. Besides, a high initial selenite concentration reduced biomass growth and selenite removal from solution. Using an airlift reactor with fungal pellets, operated under continuous mode, a maximum selenite removal of 94.3% was achieved at 10 mg L−1 of influent selenite concentration and 72 h HRT (hydraulic retention time). Overall, this study demonstrated very good potential of the fungal-pelleted airlift bioreactor system for removal of selenite from wastewater.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is a naturally occurring chalcogen element with both metallic and non-metallic properties. It is an essential element for living beings at low concentrations. The minimum amount of Se in diet is 55 μg/day for adults (Ibrahim et al. 2019), but at high concentration it is toxic to living organisms (Hunter and Manter 2009) as Se generates oxidative stress leading to DNA damage (Wang et al. 2016). In nature, selenium usually occurs in one of the four oxyanion forms, namely selenate (Se (+ 6)), selenite (Se (+ 4)), elemental selenium (Se (0)) and selenide (Se (− 2)) and among the four different selenium species, selenite, i.e., Se (+ 4) or SeO32− is considered the most toxic, whereas elemental selenium (Se (0)) is considered the least toxic.
Hence, water pollution due to discharge of untreated Se (+ 4) wastewater from agricultural and industrial activities, including pesticides, lime, fertilizer, coal mining, oil refinery, coal combustion, glass, rubber, and electronic industry is a major concern (Lenz and Lens 2008; Perkins 2011; Tan et al. 2016). It is therefore necessary to treat such Se containing wastewater before it could be released into the environment.
Different physico-chemical methods such as adsorption (Howarth et al. 2015), membrane separation (Mavrov et al. 2006), precipitation (Jung et al. 2016), electrocoagulation (Hansen et al. 2019), ion exchange (Nabi et al. 2011) and zero valent iron treatment (Li et al. 2018a, 2018b) have been studied for selenite removal from wastewater. However, due to certain drawbacks, including high installation and operation costs, large volume of toxic sludge generation and use of costly chemicals have diverted the research focus towards biological treatment methods which is considered to be sustainable and environmentally friendly. The use of biological agents (bacteria, fungi) is an environmentally friendly and inexpensive alternative to traditional physicochemical treatment methods (Zhang et al. 2019; Sinharoy and Pakshirajan 2019) to remove Se oxyanions from wastewater. Various classes of microorganisms have been reported (Eswayah et al. 2016) to be capable of transforming toxic Se oxyanions, i.e. selenate (SeO42−) and selenite (SeO32−), to less toxic and stable form (Se0) in the nano size range (Staicu et al. 2015). Selenium nanoparticles possess a high surface to volume ratio, which display many unique properties such as high photoconductivity, piezoelectric, thermoelectric and non-linear electric responses (Liang and Qian 2009).
Compared with bacteria, fungi are known to produce many extracellular enzymes for organics degradation and other environmental applications (Karigar and Rao 2011; Kues 2015), but very little attention has been focused towards the use of fungi for bio-reduction of selenite (Eswayah et al. 2016). In this regard, the use of bioreactor with fungal pellets is scant. Fungal pellets are compact and densely packed form of the hairy region of fungal hyphae (Espinosa-ortiz et al. 2016). Traditionally the formation of fungal pellets are ascribed as either coagulative or non-coagulative type. In the coagulative type, spores forms a cluster quickly and afterwards germinate by hyphal tip growth. Finally, numerous spores of the coagulative type form pellets (Zhang and Zhang 2016). On the opposite, spores of the non-coagulative type arise before pellet formation and, therefore, one pellet can be theoretically formed by one single spore (Pazouki and Panda 2000). Non-coagulative pellet formation is interlinked with agitation and aeration. Hydrophobicity and electrostatic interaction between fungal spore wall are the main factors that trigger the pellet formation (Veiter et al. 2018).
The reduction of selenite to Se (0) by fungi has generally been considered as a detoxification mechanism which results in red coloration of fungal pellet or media (Li et al. 2018b). This study mainly focused on developing a continuous process for treating Se containing wastewater using fungal-pelleted airlift bioreactor, which is not reported thus far in the literature. Effect of carbon source and initial selenite concentration on selenite removal was first studied using batch shake flasks. The effect of hydraulic retention time and influent selenite concentration on the bioreactor performance was then investigated under continuous operation mode. Characterization of biologically synthesized selenium nanoparticles using different instrumental techniques was carried out. Furthermore, for better understanding of removal and recovery of Se in the study, mechanism based on the literature and from the results obtained has been proposed.
Materials and methods
Fungal culture and medium composition
Five different fungal strains, viz. Aspergillus fumigatus, Mucor hiemalis, Cunninghamella elegans and two other isolated strains (F1 and F2) were initially screened for selenite removal. The strains A. fumigatus, M. hiemalis and C. elegans were procured from CSIR-Institute of Microbial Technology (IMTECH), Chandigarh, India. Whereas, the strain F1 was isolated from waste tea leaf and the strain F2 was isolated from effluent of an anaerobic inverse fluidized bed bioreactor treating selenite rich wastewater (Sinharoy et al. 2019). All the fungal strains were initially grown in petri plates containing potato dextrose agar (PDA) media and incubated at 28 °C for 4 days. The fungal spore solutions were prepared by harvesting the spores using Triton X-100 from the 4 days old petri plate and transferring into distilled water. The spore solutions were stored at − 20 °C until future use in the experiments.
Screening of fungal strains for selenite removal
In order to screen the best fungal strain for selenite bio-reduction, batch experiments were carried out using 250-mL Erlenmeyer flasks with a 100-mL synthetic wastewater medium containing (g L−1): glucose (10), KH2PO4 (2), MgSO4·7H2O (0.5), NH4Cl, (0.1), CaCl2·2H2O (0.1), thiamine (0.001) (Espinosa-ortiz et al. 2017) and 5 mL of trace elements solution (Tien and Kirk 1988). The pH of the medium was adjusted to 3.5 and autoclaved at 121 °C and 15 psi pressure for 30 min and then the flasks were inoculated with 1% (v/v) of fungal spore solution, followed by incubation at 30 °C and 150 rpm in an orbital shaking incubator. The low pH condition used in the study is based on optimum pH required for fungal growth. The 3 days old fungal cultures were used as the inoculum (2% v/v) in selenium removal experiments (Espinosa-ortiz et al. 2017). In order to test the selenium reduction capability of the individual fungi, 10 mg L−1of selenite was added to the media. The screening experiment was carried out for 7 days and samples were withdrawn every day for the analysis of selenite and biomass concentrations. All experiments were conducted in triplicates. In order to identify the isolated fungal strain (F2) which showed the best selenite removal capability among the five different fungal strains, the fungal culture was sent to genOmbio Technologies Pvt. Ltd. for identification of the fugal strain; internal transcribed spacer (ITS) region was sequenced and examined by using basic local alignment search tool (BLAST).
Effect of carbon source and selenite concentration
To study the effect of carbon source on selenite reduction by Aspergillus niger KP (F2 strain), four different carbon substrates viz. glucose, sucrose, fructose and sodium lactate at a concentration of 10 mgL−1 were taken in the media. The initial selenite concentration and pH of the media were fixed at 10 mgL−1 and 3.5, respectively. The flasks were incubated for 7 days at 30 °C, and samples were withdrawn every day in order to assess the selenite removal and residual chemical oxygen demand (COD). Furthermore, the effect of initial selenite concentration on its removal by the fungus was studied in the range 10-40 mg L−1 and with sucrose as the carbon source.
Continuous selenite removal using airlift reactor with fungal pellets
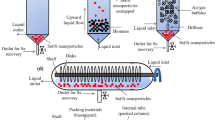
Selenite removal by A. niger KP pellets under continuous mode of operation was performed using a bench scale airlift reactor made of acrylic material (Fig. 1). The reactor had a working volume of 3 L with 10 cm diameter and 90 cm height. An inner draft tube of 5 cm diameter and 45 cm height was positioned concentrically inside the reactor. The experimental setup comprised of a feed tank, an effluent tank, peristaltic pumps for feeding and withdrawing liquid to and from the reactor, an air pump, an air filter of 0.45 μ pore in size, a flow meter to measure and regulate the air supply and airflow velocity in the reactor. The reactor was provided with a fixed airflow at a velocity of 1 VVM throughout the continuous experiments. For initial startup of the reactor, synthetic wastewater as mentioned earlier and A. niger KP pellets (0.25 g dry biomass L−1) were fed into the bioreactor. The bioreactor was then continuously operated at different conditions of hydraulic retention time (HRT) of 24–72 h and influent selenite concentration of 10–60 mg L−1 (Table 1). The bioreactor was initially operated at 72 h HRT, and later the HRT was lowered to 48 h and subsequently to 24 h for examining the effect of HRT. The initial selenite concentration was varied from 10 to 60 mg L−1 for all the three HRT values. The influent and effluent selenite concentrations were measured every day and only after achieving a steady state value of selenite removal for three consecutive reading next set of experiments were performed.
Characterization of selenium nanoparticles
For characterization of selenium nanoparticles formed due to selenite bio-reduction, field-emission transmission electron microscope (FETEM) and energy-dispersive X-ray (EDX) spectroscopy techniques were used. For FETEM and EDX analyses, samples were centrifuged at 12,600×g for 20 min and washed with deionized water (Sinharoy and Pakshirajan 2020). The samples were then fixed onto a carbon-coated copper grid and viewed using FETEM-EDX (JEOL, Model: 2100F, Japan).
For FESEM analysis, liquid sample containing suspended fungal pellets with selenium nanoparticles were centrifuged at 12,600×g for 20 min. The pellets were fixed overnight with 2.5% glutaraldehyde, following which the cells were dehydrated in series of graded ethanol (30–100%). The dehydrated pellet was kept at 37 °C in a hot air oven for overnight, and after fixing it on a metallic grid using adhesive tape, the prepared sample was analysed using FESEM (Zeisss, Model: Sigma, Germany).
Analytical methods
For analysis of selenite concentration, a modified spectrophotometric method was followed (Mal et al. 2016). The liquid samples collected at different time intervals were centrifuged at 12,600×g for 20 min to remove the suspended fungal pellets and Se (0) particles, 1 mL of supernatant was mixed with 0.5 mL of 4 M HCl and 1 mL of 1 M ascorbic acid. After 10 min of incubation at room temperature, absorbance of the mixture was determined at 500 nm using a UV–Vis spectrophotometer (Thermo Scientific, Evolution 201, USA). Selenium removal efficiency (R) was estimated using the following Eq. (1) (Espinosa-ortiz et al. 2016).
where, C0 = Initial concentration (mg L−1) and Ct = Concentration at time t (mg L−1).
For chemical oxygen demand (COD) analysis, samples were centrifuged at 12,600×g for 20 min and the supernatant was used for the COD determination by following closed reflux colorimetric method, a standard procedure of American Public Health Association (APHA 5220D) (APHA 1997). For biomass estimation gravimetric method was followed (Espinosa-ortiz et al. 2017), which however did not exclude selenium nanoparticles contained within the biomass.
Results and discussion
Screening and identification of fungal strains for selenite bioreduction in batch culture
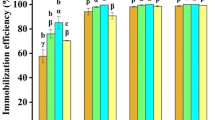
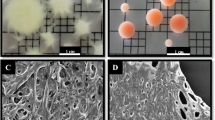
Five fungal strains namely, C. elagans, A. fumigatus, M. hiemalis and two other isolated strains (F1 and F2) were screened for selenite removal from simulated wastewater. The selenite removal profiles using these five strains are shown in Fig. 2, which revealed that a maximum selenite removal efficiency of 86% was obtained with the F2 strain. The strain F2 was later identified as Aspergillus niger KP strain and used for further experiments. Fungal pellets were observed with the different strains, regardless of the presence or absence of selenite, which, depends on various factors such as pH, carbon source, electrostatic interaction and hydrophobicity (Veiter et al. 2018). The morphology of fungal pellets grown in absence of selenite were observed to be hairy and white in colour (Fig. 3a), whereas the fungal pellets exposed to selenite were found to be more tightly packed and smooth, with a characteristic red orange colour, due to reduction of SeO32− to Se0 (Fig. 3b) (Wang et al. 2016). However, FESEM images (Fig. 3c and d) revealed that selenium affected only a change in the surface morphology of the pellets, but showed no effect on the internal hyphae structure of the pellets. Similar findings were reported previously in case of Phanerochaete chrysosporium (Espinosa-ortiz et al. 2017). Li et al. (2018a, 2018b) also observed a change in Aspergillus sp. morphology due to presence of selenite in growth media. Figure 4 shows the biomass growth curve of A. niger KP in the presence and absence of selenite in the media, which revealed that the biomass growth is better in case of control (without selenite) than in selenite containing media, probably due to the selenite-induced oxidative stress on intracellular thiols (Wang et al. 2016) of A. niger KP strain.
Effect of carbon source and selenite concentration
The effect of four different carbon sources, viz. glucose, sucrose, fructose and sodium lactate on selenite bioreduction by A. niger KP strain was investigated at 10 mg L−1 of selenite. A maximum selenite reduction of 88.5% was obtained using either sucrose or glucose as the carbon source (Fig. 5a). A negligible amount of selenite reduction was observed with fructose or sodium lactate as the carbon source. Figure 5b shows COD utilization profile by A. niger KP strain for all the different carbon substrates used in this experiment which further confirms that sucrose and glucose were the most preferred carbon source for the fungus. Lactate and fructose were not utilized by A. niger KP strain, indicating their non-suitability as carbon substrate for selenite bioreduction.
The effect of selenite concentration in the range 10–40 mgL−1 on selenite removal by A. niger KP strain using sucrose as the carbon source was investigated (Fig. 6). An increase in initial selenite concentration reduced the selenite removal efficiency with a maximum selenite reduction of 88% at 10 mgL−1 of initial selenite concentration. The selenite reduction efficiency dropped to 77, 70.3, 67.25% for 20, 30, 40 mgL−1 of initial selenite concentrations, respectively.
Bioreactor performance under continuous operation mode
In order to study continuous selenite removal from wastewater by fungal pellets in airlift reactor, effect of different influent selenite concentration (10–60 mg L−1) and different HRT in the range 24–72 h was examined. Figure 7a shows the time profile of influent and effluent selenite concentration along with selenite removal efficiency obtained during continuous bioreactor operation. During phase I, the bioreactor was operated at 72 h HRT and the influent selenite concentration was varied from 10 to 60 mg L−1. The selenite removal was initially low but gradually increased to 94.3% after 15 days of bioreactor operation. However, further increase in the inlet selenite concentration to 30 and 60 mg L−1 resulted in reduced selenite removal values of 75.5% and 71.3%, respectively. In Phase II, the HRT was changed to 48 h and the selenite concentration was initially kept at 10 mg L−1; the selenite removal efficiency fluctuated due to the sudden change in HRT, which, however, reached a stable value of 78.2% on 54th day of bioreactor operation. The selenite removal values for 30 and 60 mg L−1 inlet selenite concentrations were similarly low at 48 h HRT compared with that at 72 h HRT. The selenite removal efficiency significantly reduced at a very low HRT of 24 h. The values were 72.5, 66.2 and 58% for 10, 30 and 60 mg L−1, respectively. Figure 7b shows selenite removal rates at different selenite loading rate during the continuous bioreactor operation. Straight line passing through the origin in this figure represents stable reactor performance with maximum selenite removal rate and the points denote experimental values of selenite removal rates obtained. From the figure it is clear that up to a selenite loading rate of 0.83 mg L−1 h−1 stable performance of the bioreactor is achieved, and above which its performance deteriorated.
Use of fungal pellets in bioreactors are more desirable due to the rich source of extracellular enzymes produced by fungi as well as due to their ability to combat harsh conditions, particularly varying pollutant loads, low pH and tolerance to low nutrient concentrations (Cruz-morató et al. 2014). Several fungal strains possess the potential to reduce metalloids, but only a very few studies have been successfully carried out until date for selenite removal using fungi (Oremland et al. 2004; Stolz et al. 2006). Espinosa-Ortiz et al. (2015) investigated selenite removal using fungal pelleted bioreactor, and reported nearly 70% selenite removal in the bioreactor which is comparable with the selenite removal values obtained in this study. Large amounts of enzymes and reductive proteins secreted by fungi are responsible for selenite reduction to selenium. These non-selective intracellular and extracellular reductive enzymes make fungi an ideal candidate for treating not only selenite but also a wide range of organic and inorganic pollutants containing wastewater (Sen et al. 2016). Compared with the nutrient used for fungal growth or any other microbial growth, selenite is highly toxic even at a very low concentrations. Therefore, its removal is essential. Moreover, the nutrient media used for fungal growth in this study is important to maintain activity of the fungus during the removal process. However, low-cost substrates, e.g. lignocellulosic wastes, could be explored to keep the process cost low.
Characterization of selenium nanoparticle
FETEM images shown in Fig. 8 reveal the formation of selenium nanoparticles within the fungal cell, suggesting intracellular formation of Se0 nanoparticle from selenite. By FETEM analysis, numerous nanoparticles were measured and all of these were in the size range 65–100 nm. The EDX analysis further confirmed the nanoparticle formation due to selenium (Fig. 8d). Mukherjee et al. (2001) suggested that intracellular production of nanoparticles is driven by electrostatic interaction between metal ions in solution and enzymes present in the fungal cell wall, and binding of metals on fungal cell surface, which leads to reduction of metals and synthesis of metal nanoparticles. A number of fungal species especially Verticillium sp. (Mukherjee et al. 2001; Sastry et al. 2003) and Aspergillus flavus (Vigneshwaran et al. 2007; Rajakumar et al. 2012) have been reported to synthesize nanoparticles intracellularly. The exact mechanism of Se nanoparticle formation from selenite by fungi is not well studied, but it is attributed to intracellular or extracellular enzymes produced by such fungi (Molnár et al. 2018). Espinosa-ortiz et al. (2017) proposed a mechanism for intracellular selenium nanoparticle formation by Phanerochaete chrysoporium, which involved a series of enzyme mediated steps. Based on these literature reports, Fig. 9 depicts the proposed mechanism for Se nanoparticle formation by A. niger KP strain used in this study. Most fungi are highly tolerant towards metals or metalloids and are capable of binding and intracellular uptake of metals (Alghuthaymi et al. 2015). Once inside the cell many reductase enzymes present act on the metal ions for nanoparticle synthesis (Khandel and Kumar 2018). The size, shape and surface molecules present on Se nanoparticles depend upon environmental conditions such as pH, temperature, specific enzyme activity and nature of the metal/metalloid (Alghuthaymi et al. 2015).
Conclusions
Fungal pellets of Aspergillus niger KP strain isolated from effluent stream of a lab scale bioreactor treating selenite rich wastewater was found highly efficient in reducing selenite to selenium nanoparticle. The spherical shaped selenium nanoparticles were synthesized intercellularly due to enzymatic reduction and the fungal pellets loaded with selenium nanoparticles were compact, smooth and with a red orange colour. Bioreactor experiments under continuous operation mode using airlift bioreactor with fungal pellets revealed that low influent selenite concentration and long HRT are ideal for achieving a stable performance of the system. This study demonstrated the potential of fungal pelleted systems for selenite removal from wastewater.
References
Alghuthaymi MA, Almoammar H, Rai M et al (2015) Myconanoparticles: synthesis and their role in phytopathogens management. Biotechnol Biotechnol Equip 29:221–236. https://doi.org/10.1080/13102818.2015.1008194
APHA (1997) Standard methods for water and wastewater examination. Am Public Heal Assoc Washington, DC, USA 19 ed.:14–19
Cruz-morató C, Lucas D, Llorca M et al (2014) Hospital wastewater treatment by fungal bioreactor: removal efficiency for pharmaceuticals and endocrine disruptor compounds. Sci Total Environ 493:365–376. https://doi.org/10.1016/j.scitotenv.2014.05.117
Espinosa-Ortiz EJ, Rene ER, van Hullebusch ED, Lens PNL (2015) Removal of selenite from wastewater in a Phanerochaete chrysosporium pellet based fungal bioreactor. International Biodeterioration & Biodegradation 102:361–369
Espinosa-ortiz EJ, Rene ER, Pakshirajan K et al (2016) Fungal pelleted reactors in wastewater treatment: applications and perspectives. Chem Eng J 283:553–571. https://doi.org/10.1016/j.cej.2015.07.068
Espinosa-Ortiz EJ, Rene ER, Guyot F et al (2017) Biomineralization of tellurium and selenium-tellurium nanoparticles by the white-rot fungus Phanerochaete chrysosporium. Int Biodeterior Biodegradation 124:258–266. https://doi.org/10.1016/j.ibiod.2017.05.009
Eswayah AS, Smith TJ, Gardiner PHE (2016) Microbial transformations of selenium species of relevance to bioremediation. Appl Environ Microbiol 82:4848–4859. https://doi.org/10.1128/AEM.00877-16.Editor
Hansen HK, Peña SF, Gutiérrez C et al (2019) Selenium removal from petroleum refinery wastewater using an electrocoagulation technique. J Hazard Mater 364:78–81. https://doi.org/10.1016/j.jhazmat.2018.09.090
Howarth AJ, Katz MJ, Wang TC et al (2015) High efficiency adsorption and removal of Selenate and selenite from water using metal − organic frameworks. J Am Chem Soc 137:7488–7494. https://doi.org/10.1021/jacs.5b03904
Hunter WJ, Manter DK (2009) Reduction of selenite to elemental red selenium by pseudomonas. Curr Microbiol 58:493–498. https://doi.org/10.1007/s00284-009-9358-2
Ibrahim SAZ, Kerkadi A, Agouni A (2019) Selenium and health : an update on the situation in the Middle East and North Africa. Nutrients 11:1–13. https://doi.org/10.3390/nu11071457
Jung B, Safan A, Bill B, Abdel-wahab A (2016) Spectroscopic study of Se (IV) removal from water by reductive precipitation using sulfide. Chemosphere 163:351–358. https://doi.org/10.1016/j.chemosphere.2016.08.024
Karigar CS, Rao SS (2011) Role of microbial enzymes in the bioremediation of pollutants: a review. Enzyme Res 2011:1–11. https://doi.org/10.4061/2011/805187
Khandel P, Kumar S (2018) Mycogenic nanoparticles and their bio - prospective applications : current status and future challenges. J Nanostructure Chem 8:369–391. https://doi.org/10.1007/s40097-018-0285-2
Kues U (2015) Fungal enzymes for environmental management. Curr Opin Biotechnol 33:268–278. https://doi.org/10.1016/j.copbio.2015.03.006
Lenz M, Lens PNL (2008) The essential toxin: the changing perception of selenium in environmental sciences. Sci Total Environ 407:3620–3633. https://doi.org/10.1016/j.scitotenv.2008.07.056
Li Y, Guo X, Dong H et al (2018a) Selenite removal from groundwater by zero-valent iron (ZVI) in combination with oxidants. Chem Eng J 345:432–440. https://doi.org/10.1016/j.cej.2018.03.187
Li Z, Li H, Hu H (2018b) Selenite removal and reduction by growing Aspergillus sp. BioMetals 31:45–50. https://doi.org/10.1007/s10534-017-0063-5
Liang F, Qian H (2009) Synthesis of tellurium nanowires and their transport property. Mater Chem Phys 113:523–526. https://doi.org/10.1016/j.matchemphys.2008.07.101
Mal J, Nancharaiah YV, Van Hullebusch ED, Lens PNL (2016) Effect of heavy metal co-contaminants on selenite bioreduction by anaerobic granular sludge. Bioresour Technol 206:1–8. https://doi.org/10.1016/j.biortech.2016.01.064
Mavrov V, Stamenov S, Todorova E et al (2006) New hybrid electrocoagulation membrane process for removing selenium from industrial wastewater. Desalination 201:290–296. https://doi.org/10.1016/j.desal.2006.06.005
Molnár Z, Bódai V, Szakacs G et al (2018) Green synthesis of gold nanoparticles by thermophilic filamentous fungi. Sci Rep 8:3943. https://doi.org/10.1038/s41598-018-22112-3
Mukherjee P, Ahmad A, Mandal D et al (2001) Bioreduction of AuCl4- ions by the fungus, Verticillium sp. and surface trapping of the gold nanoparticles formed **. Angew Chemie Int Ed 40:3585–3588. https://doi.org/10.1002/1521-3773(20011001)40:19<3585::AID-ANIE3585>3.0.CO;2-K
Nabi SA, Bushra R, Al-Othman ZA, Naushad M (2011) Synthesis, characterization, and analytical applications of a new composite cation exchange material acetonitrile stannic (IV) selenite: adsorption behavior of toxic metal ions in nonionic surfactant medium. Sep Sci Technol 6395:847–857. https://doi.org/10.1080/01496395.2010.534759
Oremland RS, Herbel MJ, Blum JS et al (2004) Structural and spectral features of selenium nanospheres produced by Se-respiring bacteria. Appl Environ Microbiol 70:52–60. https://doi.org/10.1128/AEM.70.1.52
Pazouki M, Panda T (2000) Understanding the morphology of fungi. Bioprocess Eng 22:127–143. https://doi.org/10.1007/s004490050022
Perkins WT (2011) Science of the total environment extreme selenium and tellurium contamination in soils — an eighty year-old industrial legacy surrounding a Ni refinery in the Swansea Valley. Sci Total Environ 412–413:162–169. https://doi.org/10.1016/j.scitotenv.2011.09.056
Rajakumar G, Rahuman AA, Roopan SM et al (2012) Fungus-mediated biosynthesis and characterization of TiO 2 nanoparticles and their activity against pathogenic bacteria. Spectrochim Acta Part A Mol Biomol Spectrosc 91:23–29. https://doi.org/10.1016/j.saa.2012.01.011
Sastry M, Ahmad A, Khan MI, Kumar R (2003) Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr Sci 85:162–170
Sen SK, Raut S, Bandyopadhyay P, Raut S (2016) Fungal decolouration and degradation of azo dyes: a review. Fungal Biol Rev 30:112–133. https://doi.org/10.1016/j.fbr.2016.06.003
Sinharoy A, Pakshirajan K (2019) Heavy metal sequestration by sulfate reduction using carbon monoxide as the sole carbon and energy source. Process Biochem 82:135–143. https://doi.org/10.1016/j.procbio.2019.04.002
Sinharoy A, Pakshirajan K (2020) A novel application of biologically synthesized nanoparticles for enhanced biohydrogen production and carbon monoxide bioconversion. Renew Energ 147:864–873. https://doi.org/10.1016/j.renene.2019.09.027
Sinharoy A, Saikia S, Pakshirajan K (2019) Biological removal of selenite from wastewater and recovery as selenium nanoparticles using inverse fluidized bed bioreactor. J Water Process Eng 32:100988. https://doi.org/10.1016/j.jwpe.2019.100988
Staicu LC, Van Hullebusch ED, Lens PNL (2015) Production, recovery and reuse of biogenic elemental selenium. Environ Chem Lett 13:89–96. https://doi.org/10.1007/s10311-015-0492-8
Stolz JF, Basu P, Santini JM, Oremland RS (2006) Arsenic and selenium in microbial metabolism. Annu Rev Microbiol 60:107–130. https://doi.org/10.1146/annurev.micro.60.080805.142053
Tan LC, Nancharaiah YV, Van Hullebusch ED, Lens PNL (2016) Selenium: environmental signi fi cance, pollution, and biological treatment technologies. Biotechnol Adv 34:886–907. https://doi.org/10.1016/j.biotechadv.2016.05.005
Tien M, Kirk TK (1988) Lignin peroxidase of Phanerochaete chrysosporium. Methods Enzymol 161:238–249. https://doi.org/10.1016/0076-6879(88)61025-1
Veiter L, Rajamanickam V, Herwig C (2018) The filamentous fungal pellet — relationship between morphology and productivity. Appl Microbiol Biotechnol 102:2997–3006
Vigneshwaran N, Ashtaputre NM, Varadarajan PV et al (2007) Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater Lett 61:1413–1418. https://doi.org/10.1016/j.matlet.2006.07.042
Wang J, Wang B, Zhang D, Wu Y (2016) Selenium uptake , tolerance and reduction in Flammulina velutipes supplied with selenite. Peer J:1–17. https://doi.org/10.7717/peerj.1993
Zhang J, Zhang J (2016) The filamentous fungal pellet and forces driving its formation. Crit Rev Biotechnol 36:1066–1077. https://doi.org/10.3109/07388551.2015.1084262
Zhang Y, Kuroda M, Arai S et al (2019) Biological removal of selenate in saline wastewater by activated sludge under alternating anoxic/oxic conditions. Front Environ Sci Eng 13:1–11. https://doi.org/10.1007/s11783-019-1154-z
Acknowledgements
The authors thank the Department of Biosciences and Bioengineering and Central Instrumentation Facility, IIT Guwahati, for providing the necessary support for this research work. Authors are grateful to Council of Scientific and Industrial Research, Government of India, for funding this research work (CSIR/22(0740)/17/EMR-II).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Bingcai Pan
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
Very high selenite removal using fungal pellets of Aspergillus niger KP is reported.
Se exposure affected morphology of the fungal pellets.
Spherical-shaped selenium nanoparticles were accumulated inside the cell.
Airlift reactor (ALR) is highly suited for continuous Se removal and recovery as nanoparticle.
Low inlet selenite concentration and 72 h HRT favoured the best selenite removal using ALR
Rights and permissions
About this article
Cite this article
Negi, B.B., Sinharoy, A. & Pakshirajan, K. Selenite removal from wastewater using fungal pelleted airlift bioreactor. Environ Sci Pollut Res 27, 992–1003 (2020). https://doi.org/10.1007/s11356-019-06946-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06946-6