Abstract
Swine production chain generates residues with potential application in environmental processes. This study aimed at the use of swine hair as a potential biofilter for hexavalent chromium (Cr(VI)) removal from wastewater of tannery industry. The hair was pretreated using H2O2 in alkaline medium, and statistical analysis was carried out to evaluate the hair degradation, as well the Cr(VI) removal by the potential pretreated biofilter. The results showed 99% of Cr(VI) removal in 105 min of treatment in large pH range (1–10). Treated and untreated effluents were submitted to cytotoxicity study using vegetable and animal cells, demonstrating a significant reduction on toxicity to both cells. Therefore, swine hair demonstrated to be a promising residue for heavy metal removal on the perspective of an environmentally friendly technique.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Demand for swine meat consumption is growing fast worldwide (USDA 2015; Calayag et al. 2017). In Brazil, exports of swine meat are increasing, and Brazil occupies the fourth position among the largest producers and global exports (USDA 2015; Ali et al. 2017).
The production process of swine meat generates several residues. These residues, mainly liquid effluents, are considered to have a high polluting potential, including swine manure from the creation and concentration of these animals in the same area (Rizzoni et al. 2012; Cherubini et al. 2015). The environmentally adequate destination of most of these wastes is the sanitary or industrial landfills. However, these residues have the potential to be used as products in other processes, since they present high added value, which consequently ends up positively valuing these agroindustrial wastes (Segat et al. 2015).
A by-product from swine production is swine hair, which does not have its potential being exploited, possibly due to its high degradation time due to the presence of high keratin content (Mabrouk 2008). The keratin present in swine hair is hardly degraded, as it presents disulfide-type bonds, hydrogen bonds, and hydrophobic interactions in its structure (Feghelman and Haly 1960; Thankaswamy et al. 2018). Because of this complex structure, hair is a by-product that requires treatment to break the disulfide bonds, before exploring its potential applications (Cai and Zheng 2009).
Complex material treatment has the purpose to facilitate the subsequent processes of use of the material under study, several types of treatment can be applied, such as reactions in the presence of H2O2, use of acid, or high temperatures and pressures (Croce et al. 2016; Paudel et al. 2017; Venturin et al. 2018). The treatment is considered adequate when the final material presents a simple and bioavailable structure (Zhao et al. 2018; Venturin et al. 2018).
A relevant alternative for the application of the pretreated swine hair would be in the treatment of wastewaters containing heavy metals. Some studies have reported the ability of these metals to form complexes between the effluent and the organic compound (Wan et al. 2010; Jiang et al. 2018).
In this context, the objective of this study was to pretreat the swine hair of the food agroindustry aiming at its application in Cr(VI) removal from wastewater, developing a possible alternative to solve an environmental problem that affects many industries, and to make this by-product a promising technology in the treatment of effluents with a heavy metal load.
Material and methods
Swine hair and tannery effluents
Swine hairs were obtained from a food agroindustry, located in the state of Rio Grande do Sul, Brazil. The tannery wastewater was collected from a local industry at the discharge point of the leather processing tanks. The wastewater has an initial concentration of Cr(VI) of 7.22 ± 1.78 mg L−1 and pH 3.49.
Swine hair pretreatment using hydrogen peroxide in alkaline medium
Swine hair was washed with water for removal of the main residual impurities (skin and blood). Thereafter, the pretreatment by alkaline hydrogen peroxide (H2O2) was studied in an orbital shaker. The experiments were designed with a central composite design 23 (CCD) with 8 tries and more than 3 repetitions at the central point (Table 1). The factors investigated were exposure time (1.3–9.7 h), swine hair mass (0.8–9.2 g), and H2O2 concentration (2.0–12.0%). The H2O2 concentration was adjusted according to Rabelo et al. (2011), Sun et al. (2015), and Venturin et al. (2018). The pH was adjusted to 11.5 ± 0.2 (Sun et al. 2015).
The test runs were incubated in an orbital shaker at 28 °C and 150 rpm and maintained for the period determined in the CCD. The samples were maintained at 60 °C during 24 h (Reddy et al. 2017) and weighed in analytical balance, for determining the dry mass after pretreatment.
Results were evaluated based on mass loss of the swine hairs (Reddy et al. 2017). Swine hair degradation was calculated from Eq. 1, where Mo is the dry mass of biomaterial initially added and Mf is the dry mass after the pretreatment process (Cheong et al. 2018).
Application of pretreated swine hair for Cr(VI) removal in a synthetic effluent
Firstly, the potential of the pretreated biomaterial for removal of Cr(VI) was tested using a central composite design (CCD 23). Therefore, synthetic effluents were prepared with 20 mg Cr(VI) L−1, added in the form of potassium dichromate solution (K2Cr2O7). The samples were prepared with 50 mL of Cr(VI) solution, 1.0% (m v−1) of the pretreated swine hair, and pH 2.0 (Khelaifia et al. 2016), and incubated in an orbital shaker at 40 °C, 200 rpm for 3 h (Khelaifia et al. 2016).
Then, a central composite rotational design with two factors (CCRD 22) was carried out for evaluation of the effects of concentrations of Cr(VI) (2.0–20.0 mg Cr6+ L−1) and pretreated swine hair (1.0–10% m v−1) on Cr(VI) removal. The pH was maintained at 2.0 (Table 2) and the incubation conditions were the same as in the previous assay.
After the incubation period, the samples were filtered and the Cr(VI) concentration was quantified by colorimetric method described in Standard Methods (APHA 1998), using 1.5 diphenylcarbazide (C13H14N4O). Removal of Cr(VI) of the medium was evaluated by the difference between the concentration at time zero and the concentration after incubation.
The evaluation of contact time was carried out to determine the shortest possible time for Cr(VI) removal by the pretreated biomaterial, with equal efficiency as the 3 h used in the CCRD 22. Thus, destructive samples were prepared and Cr(VI) concentration was evaluated at 0, 15, 30, 45, 60, 90, 105, and 120 min. The best relation of concentration of Cr(VI) and pretreated swine hair was obtained by optimization, as described in the “Application of pretreated swine hair for Cr(VI) removal in a synthetic effluent” section. The samples had the pH set to 2.0 and were maintained in an orbital shaker at 200 rpm and 40 °C.
Subsequently, the effect of sample pH was evaluated at 1.0, 2.0, 3.0, 4.0, 7.0, and 10.0. The Cr (VI) concentration and mass of pretreated swine hair used were the same optimized in the “Application of pretreated swine hair for Cr(VI) removal in a synthetic effluent” section, and the contact time determined in previous assays. The samples were incubated an orbital shaker at 200 rpm and 40 °C.
The concentration of Cr(VI) was determined by the method of 1.5 diphenylcarbazide (C13H14N4O). The Cr(VI) removal was evaluated by the difference between the final and initial concentrations of the medium.
Evaluation of the biomaterial for Cr(VI) removal in real tannery wastewater
The assays in tannery effluent were carried out with 8.7% (m v−1) of pretreated swine hair in 50 mL of wastewater. The samples were placed in orbital shaker at 200 rpm, 40 °C for 105 min. The pH of the effluent was not altered.
Hair morphology and qualitative composition by electronic scanning microscopy and X-ray dispersive energy spectroscopy
Electronic scanning microscopy (SEM) and X-ray dispersive energy spectroscopy (EDX) analyses were performed in a scanning electron microscope (SEM/EDX, TM-3030, Hitachi) in four samples: crude swine hair (control), assays 3 and 6 of the CCD 23, and assay 3 of CCRD 22. Samples were fixed with carbon tape on aluminum holders and then coated by gold sputtering (Ernest Fullam) for 30 s. All the SEM analyses were carried out at 15 kV.
Cytotoxicity assay in a real tannery wastewater
The evaluation of the real tannery wastewater cytotoxicity was carried in vitro, using vegetal meristem of Allium cepa, and the assays were performed as described by Dusman et al. (2014).
The meristems of A. cepa were exposed to real effluent before and after pretreatment with swine hairs, at a dilution of 1:8. The cytotoxicity analysis in the cells war performed by mitotic index (MI) analysis, for which the relation between the total number of cells in the division process (NCM) and the total number of cells observed (NTC) was used.
Statistical analysis of data
The data obtained from the tests were analyzed by analysis of variance (ANOVA) using the software Statistica 8.0 (StatSoft, Tulsa, USA) and online software Protimiza Experimental Design (http://experimental-design.protimiza.com.br/).
Results and discussion
Pretreatment of swine hairs by means of CCD 23 using hydrogen peroxide
The results for the degradation of swine hairs as a function of hair amount, concentration of peroxide, and time of degradation are shown in Table 1. Assay 6 yielded the greatest mass loss (79.58%), with maximum contact time (8 h) and hydrogen peroxide (10 mL), and lower mass (2.5 g of swine hair). The lowest degradation (31.46%) was observed in assay 1, with lower conditions (3 h; 2.5 g of swine hair and 4 mL of hydrogen peroxide).
The hair is composed basically of keratin, which is a biopolymer formed by polypeptide bonds of aminoacids, which give their hard and strong appearance (Pienpinijtham et al. 2018). Keratin fibers are incorporated into a matrix by means of many chemical bonds, such as disulfide bonds. The H2O2 has a strong interaction capacity with the keratin chain groups, which can cause chemical changes in the keratin bonds (Zhang et al. 2015). Structural alterations occur by disrupting the disulfide bonds of the keratin structure and oxidation to cysteic acid, causing disintegration of the structure (Baias et al. 2009). The reduction of the disulfide bonds in the keratinolytic material causes the loss of mechanical resistance and can be observed by increasing the alkalization of the medium (Millington 2006).
The mechanism of degradation using H2O2 is strongly dependent on the pH of the reaction, which is reported as pH 11.5–11.6. Under these conditions, the dissociation of H2O2 and the formation of hydroperoxide anions, which will react with other H2O2 molecules, occur, and this reaction will result in the formation of hydroxyl radicals and superoxide radicals (Gould 1985; Venturin et al. 2018).
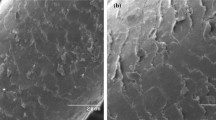
Considering these factors, the results obtained could be explained due to the rapid decomposition of H2O2. So, in the presence of lower mass, longer reaction time, and higher concentration of the oxidizing agent (H2O2), the greater deformation of keratin occurred. Additionally, the increase of the hair mass in lower concentrations of H2O2 yielded a smaller degradation, considering the smaller amount of hydroxyl radicals to break the chemical bonds of the swine hair. The scanning electron microscopy (SEM) micrographies are presented in Fig. 5a, in which it is possible to observe the difference in structures of swine hair in the control sample compared with the sample of greater degradation (run 6 of the CCD 23), with 79.58% degradation and run 3 of the CCD 23, with 36.08% degradation. The Pareto chart presented in Fig. 1 was generated to identify significant effects.
All analyzed parameters were statistically significant (p < 0.05), being positive for H2O2 concentration, time, and interaction of the two respective variables, and negative for swine hairs weight and interaction between swine hair weight and tempo, and swine hair weight and concentration of H2O2.
Removal of hexavalent chromium in synthetic effluent using pretreated swine hair
Based on the degradation results obtained in the CCD 23, the biomaterials were tested in Cr(VI) removal assays of a synthetic medium with 20 mg Cr(VI) L−1, aiming at the evaluation of the behavior of the biomaterial pretreated for detoxification of the solution containing the heavy metal. The results obtained are shown in Fig. 2.
In the assays where the biomaterial presented degradation higher than 60%, the lowest percentages of Cr(VI) removal were observed (tests 6, 9, 10, and 11). Also, a sample was treated using crude swine hairs (without H2O2 treatment), confirming that the non-treated hair was not able to remove the metal.
The affinity between the Cr(VI) ions and the surface layer of the pretreated biomaterial showed satisfactory results after 3 h of contact of the heavy metal solution with the hair. The oxidative process with hydrogen peroxide possibly acted upon disruption of the disulfide bonds. This oxidative process released the amino acid (cysteine and cystine) residues, which have affinity with heavy metals (Sanri and Pant 2006; Baias et al. 2009). Therefore, the variation in Cr(VI) removal capacity between the tests may be closely related to the chemical groups of the hair surface, responsible for the interaction with the metallic ions, such as the metal-cysteine interaction.
Animal hair is rich in cysteine, an amino acid that can form inter- and intra-chain disulfide bonds, resulting in cystine, which plays an important role in the protein structure. The protein keratin is the major component in human and animal hair (Baker and Czarnecki-Maulden 1987; Sato et al. 2019). Cysteine has the ability to compose free radicals, acting as a binder, mainly with metals (Baker and Czarnecki-Maulden 1987). Because of this characteristic, this amino acid is used for the removal of heavy metals from the environment and in drugs and supplementation for cases of intoxication, such as copper, arsenic, and selenium, among others (Albert 1961; Robbins and Baker 1980). This process involves the sequestration of metal ions in the presence of phytochelatins (Panda and Chouldhury 2005).
In higher percentages of degradation, there were smaller removals of the metal. This factor may be associated with the oxidation process by H2O2, which causes loss of hair resistance. This happens since the structure of the hair is formed by three fibrous layers: medulla (internal); cortex (medium); cuticle (external) (Pienpinijtham et al. 2018). The high degradation that resulted in the smaller Cr(VI) removals may have occurred due to the excessive removal of the fibrous layers of keratin.
The removal of Cr(VI) was similar for swine hair degradations of less than 50% of the material, so the hypothesis that the oxidative process results in a similar disruption of disulfide bridges between samples. That is, there are a number of disulfide bonds possible to be ruptured before irreversible degradations of the chemical bonds of keratin occur, oxidizing the molecule to compounds with no affinity for the metal. Furthermore, degradation of swine hairs evaluated by loss of mass results in the quantification of degradation of other compounds, including residues from the skin of the animals that are present in the medium. Thus, this explains why pig hair with degradations between 31.46 and 48.62% removes similar concentrations of Cr(VI).
In the sequence, three factors were considered for the selection of experimental conditions: the lower concentration of the chemical, because the high consumption of H2O2 increases the economic cost of the process (Alvarez-Vasco and Zhang 2017); the largest swine hair weight; and the shortest processing time. Therefore, the experimental conditions of assay 3 (3 h, 7.5 g of swine hair and 4% (w/v) of H2O2) were used in the subsequent experiments.
Central composite rotational design 22 for evaluation of Cr(VI) removal
Using the contact time of 3 h, a CCRD 22 was performed to analyze the efficiency of the pretreated pig hair in the removal of hexavalent chromium from a synthetic effluent. The tests and the respective percent removal of the metal concentration are shown in Table 2. The higher metal removal was obtained in assay 3 with the conditions 4.6 mg Cr6+ L−1 and 8.7 % (m v−1) of hair. The highest efficiencies were obtained in the tests with larger swine hair masses and lower concentrations of Cr(VI).
The results were statistically analyzed and an optimized mathematical model was obtained (Eq. 2) for the Cr(VI) removal as a function of the Cr(VI) concentration (mg Cr6+ L−1) [Cr] and swine hair pretreated (% m v−1) [W].
Model validation was performed by variance analysis (ANOVA), and the correlation coefficient (0.94) and the value of F (calculated F greater than the tabulated F) validated the model (p < 0.05) and allowed the construction of response surface and contour curve (Fig. 3).
The response surface shows that the highest Cr(VI) removals are obtained at the lower concentrations of Cr(VI) and higher mass quantities, since in this case there is a higher availability of chemical bonds with the metal in the presence of Cr(VI) ions in the solution, that is, more binding sites than metal ions in the medium (Fig. 4). Thus, the result that showed the highest removal of the metal was assay 3 (Fig. 5b), where the biomaterial mass was added to the medium in a ratio of 8.7% (m v−1) and the metal concentration was 4.6 mg Cr6+ L−1, resulting in a removal of 99.41% Cr(VI). This result is promising considering the detoxification of Cr(VI) wastewaters by pig hair.
Evaluation of contact time and pH for Cr(VI) removal by biomaterial
The contact time for chromium removal, previously fixed at 3 h, was further investigated at lower adsorption times. The conditions previously established in assay 3 of CCRD 22 were used and the results are shown in Fig. 4.
In 1 h 45 min (105 min), the removal of chromium was equivalent to that observed in 3 h. Thus, it was possible to reduce the process time by 75 min.
From the pH variation assay and Cr(VI) removal, swine hairs pretreated showed affinity with all forms of Cr(VI) ions in the pH range of 1.0 to 10.0, with removals above 99%. This may result from the affinity of metal ions for functional groups on the surface of the biomaterial (Ullah et al. 2013), causing an attraction and consequent removal of the toxic metal from the medium.
The hexavalent chromium can take the form of chromate (CrO42−), dichromate (Cr2O72−), and hydrogen chromate (HCrO4−), and these oxidation states are strongly dependent on the pH of the solution and the total concentration of Cr(VI). Considering the solubility equilibrium of Cr(VI), the dominant species at pH lower than 1 is H2CrO4, the dominant species at pH between 1 and 6.5 is HCrO4−, and at pH greater than 6.5, the dominant form is CrO42 (Sanri and Pant 2006; Moussavi and Barikbin 2010; Ullah et al. 2013; Yang et al. 2014; Kan et al. 2017).
Several studies have monitored the influence of pH on metal removal. Sanri and Pant (2006) reported that increasing the pH from 1.5 to 9.0 reduced Cr(VI) removal capacity by eucalyptus peel. In that study, the authors pointed out that with increasing pH, there was a gradual decrease in the percentage of Cr(VI) removal, and from 1.5 to 5.0, the removal efficiency of the metal fell from 99 to 93%, and the maximum removal occurred in pH 2.0 with a percentage near 99%. The same was observed by Al-Homaidan et al. (2018) who evaluated the Cr(VI) removal using three algae. The removal efficiency of the metal was observed at pH 2.0, with a decline when increasing pH from 2.0 to 6.0. Jiang et al. (2018) used bimetallic Fe/Cu nanoparticles stabilized by chitosan to remove Cr(VI) in wastewater. The same pH relationship was observed, with maximum efficiency obtained at pH 3 of 80.3%, declining to 50.2% at pH 9.
In this context, the results obtained in this study are relevant considering the efficiency of Cr(VI) removal by swine hair pretreated in a wide range of pH, and can be applied for several purposes without the need for pH control to increase efficiency.
Application of pretreated swine hair in Cr(VI) removal from real tannery effluent
Pretreated swine hair was tested in the Cr(VI) removal in a real wastewater from a tannery industry, to evaluate its efficiency in the presence of interferers. The results obtained were promising, yielding more than 99% removal of heavy metal from real effluent, using 8.7% (m v−1) of the biomaterial, in 1 h 45 min of contact time, for an initial concentration of 7.22 ± 1.78 mg Cr6+ L−1. The pH of the wastewater was maintained at 3.49.
The presence of secondary compounds of the tannery effluent did not reduce the efficiency of the biomaterial as a potential biofilter in the removal of Cr(VI) from wastewater. Ullah et al. (2013) evaluated the Cr(VI) removal in sugarcane bagasse residue in the tannery effluent, obtaining a maximum removal of 80.6% in optimized conditions (pH 2 and 24 h of contact). A biomaterial composed of the mixture of tea leaf ash and pumpkin seeds studied in the removal of hexavalent chromium from contaminated wastewater resulted in removal values similar to this study, approximately 98% in 60 min of treatment (Maingi et al. 2016; Jobby et al. 2018). Thus, it is possible to observe the process advantages related to the use of only one component biomaterial and in terms of contact time (1 h 45 min), Cr(VI) removal percentage (> 99%), and irrelevance of pH adjustment.
Evaluation of the cytotoxicity of the tannery effluent
Table 3 shows the results for mitotic index from Allium cepa meristem cells. The MI that was determined for the crude effluent presented induction in the cell division, that is, it portrayed a larger number of nuclei, a result of accelerated DNA transcription. This data is consistent with reports in the literature, since the Cr(VI) heavy metal has characteristics such as high solubility, non-biodegradation, and biomagnification, as well as having a high carcinogenic potential that, through the modification of the DNA transcription process, generates aberrations in chromosomes (Dai et al. 2015; Luo et al. 2017).
When analyzing the results obtained for MI of the meristem in the treated effluent, where removal occurred with application of the biofilter, it is possible to perceive that the data are statistically equal to those of the cellular control. This result is of extreme relevance, demonstrating that the treatment in the biofilter actually made the solution with lower cytotoxicity.
Conclusions
Pretreatment of swine hair using H2O2 caused changes in the keratin conformation, propitiating until 99% of Cr(VI) removal using 8.7% of swine hair in a large pH range (1–10), demonstrating its potential as sustainable biofilter for tannery wastewater treatment.
References
Albert A (1961) Design of chelating agents for selected biological activity. Fed Proc 20:137–147
Al-Homaidan AA, Al-Qahtani HS, Al-Ghanayem AA, Ameen F, Ibraheem BM (2018) Potential use of green algae as a biosorbent for hexavalent chromium removal from aqueous solutions. Saudi J Biol Sci 25:1733–1738
Ali BA, Van Zaten HHE, Berentsen P, Bastiaansen JWM, Bikker P, Lansink AO (2017) Environmental and economic impacts of using co-products in the diets of finishing pigs in Brazil. J Clean Prod 162:247–259
Alvarez-Vasco C, Zhang X (2017) Alkaline hydrogen peroxide (AHP) pretreatment of softwood: enhanced enzymatic hydrolysability at low peroxide loadings. Biomass Bioenergy 96:96–102
APHA - American public health association (1998) 3500-CR CHROMIUM: Standard methods for the examination of water and wastewater, 20th edn. Water Environmental Federation, Washington
Baias M, Demco DE, Istrate D, Popescu C, Blümich B, Möller M (2009) Morphology and molecular mobility of fibrous hard α-keratins by 1H, 13C, and 129Xe NMR. J Phys Chem 113:12136–12147
Baker DH, Czarnecki-Maulden GL (1987) Pharmacologie role of cysteine in ameliorating or exacerbating mineral toxicities. Am Instit Nutr 22:1003–1010
Cai C, Zheng X (2009) Medium optimization for keratinase production in hair substrate by anew Bacillus subtilis KD-N2 using response surface methodology. J Ind Microbiol Biotechnol 36:875–883
Calayag AMB, Paclibare PAP, Santos PDM, Bautista CAC, Rivera WL (2017) Molecular characterization and antimicrobial resistance of Salmonella enterica from swine slaughtered in two different types of Philippine abattoir. Food Microbiol 65:51–56
Cheong CW, Lee YS, Ahmad SA, Ooi PT, Phang LY (2018) Chicken feather valorization by thermal alkaline pretreatment followed by enzymatic hydrolysis for protein-rich hydrolysate production. Waste Manag 79:658–666
Cherubini E, Zanghelini GM, Alvarenga RAF, Franco D, Soares SR (2015) Life cycle assessment of swine production in Brazil: a comparison of four manure management systems. J Clean Prod 87:68–77
Croce S, Wei Q, Imporzano GD, Dong R, Adani F (2016) Anaerobic digestion of straw and corn stover: the effect of biological process optimization and pretreatment on total bio-methane yield and energy performance. Biotechnol Adv 34:1289–1304
Dai L, Cui L, Zhou D, Huang J, Yuan S (2015) Resource recovery of Cr (VI) from electroplating wastewater: laboratory and pilot-scale investigation using fibrous weak anion exchanger. J Taiwan Inst Chem Eng 54:170–177
Dusman E, Luzza M, Savegnago L, Lauxen D, Vicentini VE, Tonial IB, Sauer TP (2014) Allium cepa L. as a bioindicator to measure cytotoxicity of surface water of the Quatorze River, located in Francisco Beltrão, Paraná, Brazil. Environ Monit Assess 186:1793–1800
Feghelman M, Haly AR (1960) The mechanical properties of wool keratin and its molecular configuration. Colloid Polym Sci 168:107–115
Gould JM (1985) Studies on the mechanism of alkaline peroxide delignification of agricultural residues. Biotechnol Bioeng 27:225–231
Jiang R, Wang M, Chen W (2018) Characterization of adsorption and desorption of lawn herbicide siduron in heavy metal contaminated soils. Chemosphere. 204:483–491
Jobby R, Jha P, Yadav AK, Desai N (2018) Biosorption and biotransformation of hexavalent chromium [Cr(VI)]: a comprehensive review. Chemosphere. 207:255–266
Kan CC, Ibe AH, Rivera KKP, Arazo RO, Luna MDG (2017) Hexavalent chromium removal from aqueous solution by adsorbents synthesized from groundwater treatment residuals. Sustain Environ Res 27:163–171
Khelaifia FZ, Hazourli S, Nouacer S, Rahina H, Ziati M (2016) Valorization of raw biomaterial waste-date stones-for Cr (VI) adsorption in aqueous solution: thermodynamics, kinetics and regeneration studies. Int Biodeterior Biodegradation 114:76–86
Luo S, Qin F, Ming Y, Zhao H, Liu Y, Chen R (2017) Fabrication uniform hollow Bi 2S3 nanospheres via Kirkendall effect for photocatalytic reduction of Cr (VI) in electroplating industry wastewater. J Hazard Mater 340:253–262
Mabrouk MEM (2008) Feather degradation by a new keratinolytic Streptomyces sp MS-2. J Microbiol Biotechnol 24:2331–2338
Maingi FM, Mbuvi HM, Nganga MM (2016) Remediation of water contaminated with Cr6þ and Cd2þ using aluminophosphates derived from ashes of tea leaves and pumpkin seed. Chem Mater Res 8:94–102
Millington KR (2006) Bleaching and whitening of wool: photostability of whites. In: Lewis DM, Rippon JA (eds) The coloration of wool and other keratin fibres. Wiley, New Jersey, pp 131–155
Moussavi G, Barikbin B (2010) Biosorption of chromium (VI) from industrial wastewater onto pistachio hull waste biomass. Chem Eng J 162:893–900
Panda SK, Chouldhury S (2005) Chromium stress in plants. Braz J Plant Physiol 17:95–102
Paudel SR, Banjara SP, Choi OK, Park KY, Kim YM, Lee JW (2017) Pretreatment of agricultural biomass for anaerobic digestion: current state and challenges. Bioresour Technol 245:1194–1205
Pienpinijtham P, Thammacharoen C, Naranitad S, Ekgasit S (2018) Analysis of cosmetic residues on a single human hair by ATR FT-IR microspectroscopy. Spectrochim Acta A Mol Biomol Spectrosc 197:230–236
Rabelo SC, Amezquita Fonseca NA, Andrade RR, Maciel Filho R, Costa AC (2011) Ethanol production from enzymatic hydrolysis of sugarcane bagasse pretreated with lime and alkaline hydrogen peroxide. Biomass Bioenergy 35:2600–2607
Reddy MR, Reddy KS, Chouhan YR, Bee H, Reddy G (2017) Effective feather degradation and keratinase production by Bacillus pumilus GRK for its application as bio-detergent additive. Bioresour Technol 243:254–263
Rizzoni LB, Tobias ACT, Del Bianchi M, Garcia JAD (2012) Anaerobic digestion to treat swine manure. Rev Cient Eletr Med Vet 9:1–20
Robbins KR, Baker DH (1980) Effect of high-level copper feeding on the sulfur amino acid need of chicks fed corn soybean meal and purified crystalline amino acid diets. Poult Sci 59:1099–1108
Sanri V, Pant KK (2006) Removal of chromium from industrial waste by using eucalyptus bark. Bioresour Technol 97:15–20
Sato J, Ogawa CYL, Sandrini M, Medina AN, Sato F, Vasconcellos RS (2019) Study of keratin hair of domestic cat under methionine and cystine experimental diet using FT-Raman spectroscopy. Vib Spectrosc 100:1–5
Segat JC, Alves PRL, Baretta D, Cardoso EJBN (2015) Ecotoxicological evaluation of swine manure disposal on tropical soils in Brazil. Ecotoxicol Environ Saf 122:91–97
Sun C, Liu R, Cao W, Yin R, Mei Y, Zhang L (2015) Impacts of alkaline hydrogen peroxide pretreatment on chemical composition and biochemical methane potential of agricultural crop stalks. Energy Fuel 29:4966–4975
Thankaswamy SR, Sundaramoorthy S, Palanivel S, Ramudu KN (2018) Improved microbial degradation of animal hair waste from leather industry using Brevibacterium luteolum (MTCC 5982). J Clean Prod 189:701–708
Ullah I, Nadeem R, Iqbal M, Manzoor Q (2013) Biosorption of chromium onto native and immobilized sugarcane bagasse waste biomass. Ecol Eng 60:99–107
USDA-National Nutrient Database for Standard (2015) Reference Release 28. US Department of Agriculture. https://ndb.nal.usda.gov/ndb/
Venturin B, Camargo AF, Scapini T, Mulinari J, Bonatto C, Bazoti S, Siqueira DP, Colla LM, Alves SL Jr, Bender JP, Steimetz RLR, Kunz A, Fongaro G, Treichel H (2018) Effect of pretreatments on corn stalk chemical properties for biogas production purposes. Bioresour Technol 266:116–124
Wan Y, Bao, Zhou Q (2010) Simultaneous adsorption and desorption of cadmium and tetracycline on cinnamon soil. Chem. 80:807–812
Yang J, Yu M, Qiu T (2014) Adsorption thermodynamics and kinetics of Cr (VI) on KIP210 resin. Ind Eng Chem Res 20:480–486
Zhang Q, Shan G, Cao P, He J, Lin Z, Huang Y, Ao N (2015) Mechanical and biological properties of oxidized horn keratin. Mater Sci Eng 47:123–134
Zhao X, Luo K, Zhang Y, Zheng Z, Cai Y, Wen B, Cui Z, Wang X (2018) Improving the methane yield of maize straw: focus on the effects of pretreatment with fungi and their secreted enzymes combined with sodium hydroxide. Bioresour Technol 250:204–213
Acknowledgments
LCME/UFSC is acknowledged for the SEM images.
Funding
This work received financial support and scholarships from CNPq, CAPES, CAPES-PNPD, and FAPERGS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Angeles Blanco
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Paris Júnior, O., Scapini, T., Camargo, A.F. et al. Removal of chromium from wastewater by swine hair residues applied as a putative biofilter. Environ Sci Pollut Res 26, 33014–33022 (2019). https://doi.org/10.1007/s11356-019-06313-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06313-5