Abstract
Tetrabromobisphenol A (TBBPA) is an emerging contaminant and exists widely in river and lake systems due to its widespread use. In natural water-sediment systems, hydrodynamic disturbances always exist. However, few studies have investigated the mechanism of TBBPA biodegradation under the influence of water disturbances. In this paper, using a specialized type of racetrack-style flumes, the TBBPA biodegradation in water-sediment systems was studied under the influence of three typical hydrodynamic disturbances. The results of 5-week experiments showed that strong hydrodynamic disturbances greatly accelerate the TBBPA biodegradation rate of the water-sediment systems. The half-lives (T1/2) under static condition (SC) were approximately 40.2 days, and the T1/2 was reduced to 16.0 days under strong hydrodynamic condition (SHC). Furthermore, the physicochemical properties and corresponding bacterial communities under these conditions were investigated to help explain the TBBPA biodegradation mechanism. The results showed that strong currents could promote dissolved oxygen (DO) levels, increase nutrient concentrations, and reduce the bacterial diversity in the sediment. Meanwhile, due to the increase in DO and nutrient concentrations, the aerobic bacterial genera conducting TBBPA biodegradation showed rapid growth with strong water disturbances, while the growth of anaerobic bacterial genera was inhibited. Citrobacter, which was the most dominant degrading bacterial genus (0.6%–14.9% in water and 3.5%–17.4% in sediment), was closely related to water disturbances and may be linked to enhanced TBBPA biodegradation. Other minor degrading bacterial genera, such as Bacillus, Sphingomonas, Anaeromyxobacter, Geobacter, Clostridium, and Flavobacterium, were also found in these water-sediment systems. The findings from this study showed the importance of considering hydrodynamic disturbance in understanding TBBPA biodegradation in aquatic environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tetrabromobisphenol A (TBBPA) is a highly effective and relatively low cost flame retardant. It has been mass-produced and used in reactive- and additive-treated products worldwide (Malkoske et al. 2016). Its production largely increased from 120 million kg in 2001 to over 200 million kg in 2013 (Li et al. 2015a; Mäkinen et al. 2009). Due to the lack of suitable substitutes, the TBBPA production and usage maintains a fairly high growth rate (Malkoske et al. 2016).
As a result of the production, use, and disposal of TBBPA-containing products, TBBPA is inevitably discharged into aquatic environments through anthropogenic effluents (Matsukami et al. 2015). TBBPA has been detected in a wide range of aquatic environments, including rivers, lakes, wetlands, among others (Lee et al. 2015; Malkoske et al. 2016; Wang et al. 2016). Unfortunately, TBBPA is known to be a persistent toxic pollutant showing immunotoxicity and neurotoxicity, especially among aquatic species (Lyche et al. 2015; Pittinger and Pecquet 2018). Thus, understanding TBBPA remediation in these aquatic systems is a key aspect of evaluating TBBPA exposure levels and concentrations.
TBBPA may be removed by photooxidation, chemical oxidation, and biodegradation (Liu et al. 2018; Nyholm et al. 2010). TBBPA biodegradation is considered to be the main means of TBBPA removal in aquatic environments (Liu et al. 2018). Previous studies showed that the bacterial species and community play vital roles in both the aerobic and anaerobic biodegradation of TBBPA. A series of TBBPA-degrading bacterial genera (e.g., Citrobacter, Bacillus and Sphingomonas) were identified to have ability contributing to TBBPA and its degradant products (Lefevre et al. 2016; Liu et al. 2018). To date, the known degrading bacterial genera, which have been identified to have the ability to contribute to the metabolite degradation of TBBPA, are listed in Table S1. Thus, clarifying the degrading bacterial community in water-sediment systems is needed to help explain the TBBPA biodegradation process.
Meanwhile, in true water-sediment systems in aquatic environments, hydrodynamic disturbances are ubiquitous, and they are the main physical force driving bacterial living environments in water and sediment. On one hand, flow shearing and turbulence can directly affect the physical structure of bacteria (Battin et al. 2003; Besemer et al. 2009; Li et al. 2015b). Battin et al. (2003) showed that flow velocity could affect biofilm structure and composition. Besemer et al. (2009) found that the spatial variation in hydrodynamics was closely related to the variation in community composition. In further experiments, Li et al. (2015b) demonstrated that hydrodynamic characteristics could be a set of reasonable predictors of bacterial community composition. On the other hand, water disturbances can induce sediment resuspension and interface exchange (Cheng and Hua 2016; Eggleton and Thomas 2004; Pascolo et al. 2018). The environmental factors would change dramatically, such as nutrients, DO and suspended solids concentration (SSC). Then, the evolution of bacterial communities can be affected indirectly. However, little is known about the role of water disturbances on the TBBPA-degrading bacterial community in water-sediment systems. The information on the relationship between the degrading bacterial community and TBBPA biodegradation was also limited and incomprehensive.
The objective of this study was to investigate the mechanism of TBBPA biodegradation in a water-sediment system under the influence of water disturbances. Three hydrodynamic conditions, including static, weak, and strong hydrodynamic conditions, were implemented using specialized racetrack-style flumes. The TBBPA concentrations in the water and sediment were determined periodically, and the corresponding physicochemical properties were also determined. Moreover, by using high-throughput sequencing, the variation characteristics of TBBPA-degrading bacterial community were analyzed to better understand the biodegradation process.
Materials and methods
Experimental materials
Lake Chaohu is the fifth largest freshwater lake in China. Due to large amount of TBBPA loading from industrial sewage, the sediment in Lake Chaohu has been seriously polluted by TBBPA (Yang et al. 2012). Nanfei River is the most polluted and the largest inflow river of Lake Chaohu, and the TBBPA concentrations in its estuary sediment were detected as high as 632.7 mg/kg, which was in the high value ranges reported for shallow lakes (Cheng and Hua 2016; Yang et al. 2012). The Nanfei River estuary is also subject to prolonged hydrodynamic action; sediment from this site (31° 41′ 51″ N, 117° 24′ 16″ E) was collected as experimental materials. Details regarding the sampling sites can be found in Fig. S1 (supplementary material). Surface sediments were collected using a Peterson grab sampler, transferred into sterile polyethylene bags and then transported to the laboratory. All sediments were mixed together, and gravel and large particles were removed. Then, the homogenized sediments were preserved at − 80 °C until the flume experiments were performed. The physicochemical properties of the experimental sediment are detailed in Table S2 (supplementary material). To prevent the influence of the complex water quality of raw water, ultrapure water was employed as the overlying water in the following experiment.
Hydrodynamic disturbance experiment and sampling
A hydrodynamic disturbance experiment was conducted in four parallel racetrack-style flumes. These flumes have been proven to be effective in simulating hydrodynamic characteristics in water-sediment systems (Hua et al. 2015). The flow conditions could be shaped by adjusting a rotational-speed screw propeller. A sketch of the flume is shown in Fig. 1. The homogenized sediment was divided into four parts. One was used as a sterile control after sterilization at 121 °C for 30 min. The sterile sediment and the other three sediment portions were individually placed flat on the bottom of the four flumes at a depth of 5 cm. Ultrapure water was slowly injected into a 20-cm layer to avoid disturbing the sediment. Then, these four water-sediment systems were allowed to stand for a 1-week stabilization period to approach field conditions.
Three typical levels of hydrodynamic conditions were simultaneously simulated in these flumes based on the actual velocity observed and previous studies in the sampling area (Chen and Liu 2015). They were static condition (SC), weak hydrodynamic condition (WHC), and strong hydrodynamic condition (SHC). The three mean flow velocities (\( \overline{U} \)) were 0 cm/s, 4.5 cm/s, and 20.8 cm/s, respectively. The \( \overline{U} \) of the sterile control was also 0 cm/s. The experiment for each condition was 5 weeks in duration according to the TBBPA biodegradation process. The steady flow structures in each condition were performed continuously and monitored daily with an acoustic Doppler velocimeter along the vertical direction in 1-cm increments. Simultaneously, the solution pH values and dissolved oxygen (DO) concentrations were determined with a HACH portable automatic monitor. The indoor temperature was controlled at 15 ± 0.3 °C and monitored with a thermometer.
Both water and sediment samples were collected once a week from each flume for 5 weeks. The sampling sites in the flumes are shown in Fig. 1. Water samples were obtained using a layered hydrophore, with 300 mL collected per sampling site. Sediment samples were collected using a cylindrical sampler (5 cm I.D., 50 cm in length). The parallel samples of water and sediment were well homogenized and transferred into sterile bottles and tubes. Ultrapure water was added after sampling to maintain a constant water volume. TBBPA loss during water sampling was small (less than 1.0%), thus the dilution effect was neglected.
Chemical and bacterial community analysis
After sample collection, a 500 mL aliquot of water sample was coarse filtered through a 3-μm quartz fiber filter to remove the resuspended particles. Then, the filtrate was filtered through a 0.22-μm polyethersulfone membrane filter using a vacuum filter. Cell pellets on the polyethersulfone membranes were stored at − 80 °C for water bacterial community analysis (Kuang et al. 2013). The filtrates were used to determine the concentrations of nutrients and TBBPA in the water. Meanwhile, a 5-g sediment sample was also stored at − 80 °C for bacterial community analysis. The remaining sediment samples were freeze-dried and sieved with a mesh aperture of 0.1 mm. Then, 0.2 g (dry weight; d. w.) sediment samples were used to determine the TBBPA concentration.
For TBBPA concentration analysis, the extraction and cleanup method of water and sediment samples followed that described in our previous study (Cheng and Hua 2018; Cheng and Hua 2016). The TBBPA in the final extracts was analyzed using an Agilent 1290 ultra-high performance liquid chromatograph (UPLC; Agilent, Waldbronn, Germany). Detection was performed using an Agilent 6460 Triple Quad-liner mass spectrometer (MS) equipped with an electrospray ionization source. The pretreatment methods and UPLC-MS-MS operating conditions are detailed in the Supplementary Material. The TBBPA recoveries were 97% ± 10% for water and 93% ± 13% for sediment. The method detection limits for the water and sediment samples were 10 ng/mL and 20 ng/g, respectively. Three sample replicates were analyzed to assess the repeatability, and the average values were reported (standard deviations within 0.3%–9.4%). The details of quality control are shown in the Supplementary Material.
For the bacterial community analysis, genomic DNA from the water and sediment samples was extracted using a PowerWater Sterivex DNA Isolation Kit (MoBio, USA) and a PowerSoil DNA Isolation Kit (MoBio, USA). DNA integrity and purity were monitored on 1% agarose gels by spectrophotometric analysis with a NanoDrop ND-2000 (Thermo Fisher Scientific, Wilmington, USA). The V4 hypervariable region was amplified using the primer pairs 515F and 806R (Bates et al. 2011). The PCR programs were run on a 4 (Bio-Rad Laboratory, CA, USA). Samples were amplified in triplicate using the following protocol: 94 °C for 5 min, 31 cycles at 94 °C for 30 s (denaturation), 52 °C for 30 s (annealing), 75 °C for 45 s (extension), and a final extension of 72 °C for 10 min. The DNA was sequenced on an Illumina HiSeq 2500 platform by Guangdong Magigene Biotechnology Co. Ltd. (Guangzhou, China). The sequenced results were analyzed as in a previous study (Cui et al. 2019).
Results and discussion
Physicochemical properties in the water-sediment systems
Prolonged water disturbances did not cause discernible differences in the physicochemical properties of the different conditions. The average values from 0 to 5 weeks are shown in Table 1. The physicochemical properties in each condition were basically in equilibrium, similar to the results from a previous study (Cheng et al. 2019). These results indicated that water disturbance was a key factor that resulted in the differences among different treatment conditions.
As the mean flow velocity (\( \overline{U} \)) increased, dissolved oxygen (DO) levels increased from 6.32 mg/L in SC to 8.95 mg/L in SHC. This phenomenon was mainly caused by the different oxygen fluxes through thin boundary layers at the air-water interface. Previous studies showed that higher velocity and turbulent motions enhanced the waterside mixing and diffusion processes, and they brought in additional oxygen into the overlying water (McKenna and McGillis 2004; Raboni et al. 2015; Poindexter and Variano 2013). Meanwhile, both total phosphorus (TP) and total nitrogen (TN) obviously increased from 0.11 mg/L to 0.59 mg/L and from 1.93 mg/L to 6.45 mg/L, respectively, along with the enhanced water disturbance. The reason for this phenomenon was that more nutrients were resuspended and desorbed from the sediment during sediment disturbance events under the stronger bed shear stress (τ) (Eggleton and Thomas 2004). Similar phenomena were found in previous studies (Wang et al. 2015a, b).
SC, static condition; WHC, weak hydrodynamic condition; SHC, strong hydrodynamic condition; \( \overline{U} \), mean flow velocity; τ, bed shear stress; DO, dissolved oxygen; TP, total phosphorus; TN, total nitrogen. All the data shown in the table are significant, p < 0.05. TP and TN were measured according to the molybdenum blue method and Kjeldahl method by spectrophotometry, respectively.
TBBPA degradation in the water-sediment systems
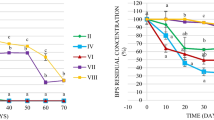
TBBPA degradation occurred in these water-sediment systems, with the different conditions showing statistically significant differences (Fig. 2a, b). The remaining percentage of TBBPA (RTBBPA) in the water and sediment in the sterile control was approximately 95% after a 5-week period. The small amount of TBBPA loss (5%) in sterile control may be a result of volatilization and sediment aging (Malkoske et al. 2016). In contrast, the RTBBPA in water and sediment both decreased significantly in non-sterile conditions, including static condition (SC), weak hydrodynamic condition (WHC), and strong hydrodynamic condition (SHC). This suggested that TBBPA dissipation in the non-sterile conditions was mainly caused by the combined action of bacteria and current.
The initial concentrations in the water and sediment of each system were 4.9 μg/L and 0.62 mg/kg. When the flow shearing and disturbance were induced, the TBBPA concentrations in the water (Cwater) at 0 weeks were 4.9, 8.1, and 15.6 μg/L for SC, WHC, and SHC when each system was stabilized (Fig. 2a). The difference was caused by the TBBPA release from the interstitial water and sediment during the resuspension process (Cheng and Hua 2016). Much of the research has showed that the sediment solute would be redistributed and resuspended into the overlying water with the water disturbances (Cheng and Hua 2016; Eggleton and Thomas 2004; Pascolo et al. 2018). After the 5-week period, CWater decreased to 2.7, 3.2 and 3.3 μg/L, and the RTBBPA in the water were 55.6%, 31.9% and 21.5%, respectively. Meanwhile, the TBBPA concentration in the sediment (Csediment) and RTBBPA in the sediment presented a similar trend (Fig. 2b). The Csediment decreased from 0.62 mg/kg to 0.34, 0.25 and 0.13 mg/kg in SC, WHC, and SHC, respectively; and RTBBPA in the sediment were 55.3%, 40.0%, and 22.5% in SC, WHC, and SHC, respectively in the end of 5 weeks, respectively. The loss of TBBPA in SHC was also faster. These results indicated that TBBPA biodegradation was significantly enhanced by water disturbance.
Table 2 summarizes the degradation kinetic parameters of the first-order kinetics (R2 > 0.98). The RTBBPA and half-lives (T1/2) of TBBPA in the water and sediment were basically consistent in each condition, indicating that TBBPA biodegradation in each form of the water-sediment systems was interdependent and closely connected. The average value of T1/2 in these systems were approximately 40.2 ± 0.1 days in SC, 25.5 ± 0.1 days in WHC and 16.0 ± 0.0 days in SHC. TBBPA was obviously more easily degraded under stronger hydrodynamic conditions. Comparing with our previous study (as shown in Fig. S2 in the Supplementary material), T1/2 were longer in this experiment with similar \( \overline{U} \) (Cheng and Hua 2018). This is most likely caused by the relatively shallower water depth (20 cm) used in this experiment as compared with the 50-cm water depth in the previous study. The kurtosis of vertical velocity distribution, which can be described as logarithmic-curve shaped, would be relatively smaller while \( \overline{U} \) was constant and water depth became shallower (Hua et al. 2013). This result also indicated that stronger current could improve the TBBPA biodegradation efficiency. Hydrodynamic disturbance and the changing environmental factors are physical forces and cannot affect TBBPA biodegradation directly. The TBBPA-degrading bacterial community might change in association water disturbances, so we analyzed the bacterial communities in SC, WHC, and SHC to help further understand the biodegradation process.
RTBBPA, TBBPA remaining percentage; k, degradation rate constant; T1/2, half-life; R2, determination coefficient. All k and T1/2 values are the mean of duplicate samples, and all the data presented in the table are significant, p < 0.05. SC, static condition; WHC, weak hydrodynamic condition; SHC, strong hydrodynamic condition
Bacterial diversity in the water-sediment systems
The operational taxonomic units (OTUs) and diversity index values were obtained from the sequencing data from the water and sediment samples, with a 97% sequence identity threshold. As shown in Fig. 3, there were 554-1107 OTUs (mean value 744) in the water samples. This was far less than the number in the corresponding sediment samples, which varied from 1594 to 3990 (mean value 3051). Similar tendencies were also found for the AEC, Chao, and Shannon index values. These results showed that the species richness in the sediment was much higher than that in the water. This is explained by the higher adaptability in the sediment, which could provide more micro niches with higher substrate availability to support increased cell concentrations (Crump et al. 2012).
Because the experimental water and sediment were parallel in each condition, the hydrodynamic conditions were the crucial factor influencing the bacterial community structure. The OTU numbers in the sediment gradually decreased with water disturbance, and they were 3693, 2948 and 1594 in the 5th week for SC, WHC and SHC, respectively (Fig. 3a-1). The values of the AEC, Chao and Shannon index for the sediment also significantly decreased with enhanced water disturbances, showing a tendency to coincide with OTU (Fig. 3b-1, c-1, and d). These results indicated that strong currents inhibited the bacterial richness and diversity in sediment. Similar results were reported in existing literatures (Li et al. 2015b; Santos et al. 2011).
The OTU numbers and diversity estimators in water fluctuated with time (Fig. 3a-2, b-2, c-2, and d). It is worth noting that the AEC, Chao and Shannon index in WHC and SHC were higher than that in SC. For example, the mean AEC values in the water were 965.7, 1145.5 and 1130.3 in SC, WHC and SHC, respectively (Fig. 3b-2). Such tendency was different from that in the sediment. This might be caused by the fact that two habitats (water and sediment) were closely associated and coupled. In these water-sediment systems, stronger currents promoted the mixing and exchange of bacterial cells between the two habitats though the resuspension process (Keshri et al. 2018; Santos et al. 2011). Due to the decrease of richness and diversity in sediment, the AEC, Chao, and Shannon index in water of SHC were generally lower than that of WHC.
As shown in Fig. 4, rarefaction analysis also indicated that the habitat type caused significant differences in bacterial richness and diversity. The tendency of the rarefaction curves approached a saturation plateau, indicating an effective description of the entire bacterial community in the water and sediment samples.
Composition evolution of degrading bacterial community
In total, 16S rRNA gene sequences were obtained from the 18 water samples and 18 sediment samples by Illumina HiSeq sequencing. In the water samples, analysis with the RDP classifier revealed 48 phyla, 166 classes, 325 orders, 519 families, and 809 genera of known bacteria. In an attempt to identify the degrading bacterial genera that might be associated with TBBPA biodegradation, we individually examined each OTU of our dataset and found 3 OTUs in water samples that closely affiliated to genera previously described as aerobic/anaerobic degraders of TBBPA (listed in Table S1). As shown in Fig. 5a, there were three TBBPA-degrading bacterial genera with a large proportion of sequences (mean relative abundance > 0.1%), followed by Citrobacter, Bacillus, and Sphingomonas. These three bacterial genera aerobically degrade TBBPA and are commonly found in aquatic environments (Chang et al. 2012; Li et al. 2014; Peng et al. 2017). It is worth noting that the growth of Citrobacter was obviously stimulated by enhanced water disturbances. In SC, the initial relative abundance of Citrobacter was 7.8% at 0 weeks, gradually decreasing to 0.6% with incubation time. In contrast, the relative abundance in SHC increased from 9.8 to 14.9%. This may be attributed to the increased DO and nutrient levels (Table 1). Citrobacter species are aerobic and nutrient-loving microbes, and the Citrobacter growth rate could be promoted by higher DO and nutrient concentrations (Huang and Tseng 2001; Wu et al. 2019). Similarly, in SC, the relative abundance of Bacillus decreased from 0.5% at week 0 to 0.0% at week 5; the variation in Bacillus showed a slight increase from 0.3% to 0.5% in SC and 0.4% to 0.6% in SHC, benefiting from the elevated DO levels (Balleste and Blanch 2010). The variation in Sphingomonas was not significant in water under the influence of water disturbances.
Higher diversity of bacterial communities was found in sediment samples. An examination of the database revealed 66 phyla, 241 classes, 455 orders, 694 families and 1065 genera of known bacteria. The top 6 known degrading bacterial genera were extracted from the 1065 genera (mean relative abundance > 0.1%), according to the TBBPA degraders listed in Table S1. They were followed by Citrobacter, Anaeromyxobacter, Geobacter, Clostridium, Flavobacterium, and Bacillus. As showed in Fig. 5b, Citrobacter species were also the most dominant functional bacterial genus. Due to the oxygen enrichment caused by hydrodynamic disturbances, similar tendencies in relative abundance of Citrobacter and Bacillus were found in the sediment, compared with water. The relative abundance of Citrobacter in SC fluctuated from 4.3 to 6.2%; the relative abundance gradually increased from 3.5 to 8.4% in WHC and from 4.9 to 17.4% in SHC. Meanwhile, the relative abundance of Anaeromyxobacter, Geobacter, and Clostridium gradually decreased with enhanced water disturbances. It was due to these three genera were anaerobic and had the link with DO (Moon et al. 2017; Perez-Fuentetaja et al. 2006). These results indicate that the addition of water disturbances may be an important physical driving force affecting the TBBPA-degrading bacterial community in water-sediment systems. The genus Citrobacter might have a key link with TBBPA biodegradation in water-sediment systems.
Conclusion
This study showed that the TBBPA biodegradation in the water-sediment system was prominently enhanced under the influence of water disturbances. Furthermore, this study investigated how water disturbances affected TBBPA biodegradation. The results showed that the bacterial diversity demonstrated a differentiated response to prolonged water disturbances and that the relative abundance of TBBPA-degrading bacteria increased with hydrodynamic disturbance. Citrobacter was the dominant functional bacteria in terms of TBBPA biodegradation in the water-sediment systems (0.6%–14.9% in the water and 3.5%–17.4% in the sediment). Other minor degrading bacterial genera, Bacillus and Sphingomonas, were found in the water, and the genera Anaeromyxobacter, Geobacter, Clostridium, Flavobacterium, and Bacillus were observed in the sediment. This study confirmed that water disturbance was the dominant driving force affecting the TBBPA biodegradation process in the water-sediment system of aquatic environments. Such information may contribute to optimization of water flow control and improving remediation of TBBPA-contaminated aquatic environments. More molecular paths and degradant products should be considered in the future studies in order to better understand the mechanism of TBBPA biodegradation in aquatic ecosystems.
References
Balleste E, Blanch AR (2010) Persistence of bacteroides species populations in a river as measured by molecular and culture techniques. Appl Environ Microbiol 76:7608–7616
Bates ST, Berg-Lyons D, Caporaso JG, Walters WA, Knight R, Fierer N (2011) Examining the global distribution of dominant archaeal populations in soil. ISME J 5:908–917
Battin TJ, Kaplan LA, Newbold JD, Cheng XH, Hansen C (2003) Effects of current velocity on the nascent architecture of stream microbial biofilms. Appl Environ Microbiol 69:5443–5452
Besemer K, Singer G, Hodl I, Battin TJ (2009) Bacterial community composition of stream biofilms in spatially variable-flow environments. Appl Environ Microbiol 75:7189–7195
Chang BV, Yuan SY, Ren YL (2012) Aerobic degradation of tetrabromobisphenol-A by microbes in river sediment. Chemosphere 87:535–541
Chen YY, Liu QQ (2015) Numerical study of hydrodynamic process in Chaohu Lake. J Hydrodyn 27:720–729
Cheng HM, Hua ZL (2016) Effects of hydrodynamic disturbances and resuspension characteristics on the release of tetrabromobisphenol A from sediment. Environ Pollut 219:785–793
Cheng HM, Hua ZL (2018) Distribution, release and removal behaviors of tetrabromobisphenol A in water-sediment systems under prolonged hydrodynamic disturbances. Sci Total Environ 636:402–410
Cheng HM, Hua ZL, Wang L, Wang YL, Xie ZX, Zhu TY (2019) Relative effects of wind-induced disturbances and vegetation on tetrabromobisphenol A cycling in shallow lakes: direct and indirect effects. Environ Pollut 252:794–803
Crump BC, Amaral-Zettler LA, Kling GW (2012) Microbial diversity in arctic freshwaters is structured by inoculation of microbes from soils. ISME J 6:1629–1639
Cui P, Chen Z, Zhao Q, Yu Z, Yi ZG, Liao HP, Zhou SG (2019) Hyperthermophilic composting significantly decreases N2O emissions by regulating N2O-related functional genes. Bioresour Technol 272:433–441
Eggleton J, Thomas KV (2004) A review of factors affecting the release and bioavailability of contaminants during sediment disturbance events. Environ Int 30:973–980
Hua ZL, Wu D, Kang BB, Li QL (2013) Flow resistance and velocity structure in shallow lakes with flexible vegetation under surface shear action. J Hydraul Eng 139:612–620
Hua ZL, Tang ZQ, Xue B, Zhang JN, Yu L, Cheng HM (2015) Aggregation and resuspension of graphene oxide in simulated natural surface aquatic environments. Environ Pollut 205:161–169
Huang HK, Tseng SK (2001) Nitrate reduction by Citrobacter diversus under aerobic environment. Appl Microbiol Biotechnol 55:90–94
Keshri J, Ram ASP, Sime-Ngando T (2018) Distinctive patterns in the taxonomical resolution of bacterioplankton in the sediment and pore waters of contrasted freshwater lakes. Microb Ecol 75:662–673
Kuang JL, Huang LN, Chen LX, Hua ZS, Li SJ, Hu M, Li JT, Shu WS (2013) Contemporary environmental variation determines microbial diversity patterns in acid mine drainage. ISME J 7:1038–1050
Lee IS, Kang HH, Kim UJ, Oh JE (2015) Brominated flame retardants in Korean river sediments, including changes in polybrominated diphenyl ether concentrations between 2006 and 2009. Chemosphere 126:18–24
Lefevre E, Cooper E, Stapleton HM, Gunsch CK (2016) Characterization and adaptation of anaerobic sludge microbial communities exposed to tetrabromobisphenol A. PLoS One 11:1–20
Li FJ, Wang JJ, Nastold P, Jiang BQ, Sun FF, Zenker A, Kolvenbach BA, Ji R, Corvini PFX (2014) Fate and metabolism of tetrabromobisphenol A in soil slurries without and with the amendment with the alkylphenol degrading bacterium Sphingomonas sp strain TTNP3. Environ Pollut 193:181–188
Li FJ, Wang JJ, Jiang BQ, Yang X, Nastold P, Kolvenbach B, Wang LH, Ma YN, Corvini PFX, Ji R (2015a) Fate of tetrabromobisphenol A (TBBPA) and formation of ester- and ether-linked bound residues in an oxic sandy soil. Environ Sci Technol 49:12758–12765
Li Y, Wang C, Zhang WL, Wang PF, Niu LH, Hou J, Wang J, Wang LQ (2015b) Modeling the effects of hydrodynamic regimes on microbial communities within fluvial biofilms: combining deterministic and stochastic processes. Environ Sci Technol 49:12869–12878
Liu AF, Zhao ZS, Qu GB, Shen ZS, Shi JB, Jiang GB (2018) Transformation/degradation of tetrabromobisphenol A and its derivatives: a review of the metabolism and metabolites. Environ Pollut 243:1141–1153
Lyche JL, Rosseland C, Berge G, Polder A (2015) Human health risk associated with brominated flame-retardants (BFRs). Environ Int 74:170–180
Mäkinen MS, Mäkinen MR, Koistinen JT, Pasanen AL, Pasanen PO, Kalliokoski PJ, Korpi AM (2009) Respiratory and dermal exposure to organophosphorus flame retardants and tetrabromobisphenol A at five work environments. Environ Sci Technol 43:941–947
Malkoske T, Tang YL, Xu WY, Yu SL, Wang HT (2016) A review of the environmental distribution, fate, and control of tetrabromobisphenol A released from sources. Sci Total Environ 569:1608–1617
Matsukami H, Nguyen Minh T, Suzuki G, Someya M, Le Huu T, Pham Hung V, Takahashi S, Tanabe S, Takigami H (2015) Flame retardant emission from e-waste recycling operation in northern Vietnam: environmental occurrence of emerging organophosphorus esters used as alternatives for PBDEs. Sci Total Environ 514:492–499
McKenna SP, McGillis WR (2004) The role of free-surface turbulence and surfactants in air-water gas transfer. Int J Heat Mass Transf 47:539–553
Moon HS, Kim BA, Hyun SP, Lee YH, Shin D (2017) Effect of the redox dynamics on microbial-mediated As transformation coupled with Fe and S in flow-through sediment columns. J Hazard Mater 329:280–289
Nyholm JR, Lundberg C, Andersson PL (2010) Biodegradation kinetics of selected brominated flame retardants in aerobic and anaerobic soil. Environ Pollut 158:2235–2240
Pascolo S, Petti M, Bosa S (2018) On the wave bottom shear stress in shallow depths: the role of wave period and bed roughness. Water 10:19
Peng XX, Wang ZN, Huang JF, Pittendrigh BR, Liu SW, Jia XS, Wong PK (2017) Efficient degradation of tetrabromobisphenol A by synergistic integration of Fe/Ni bimetallic catalysis and microbial acclimation. Water Res 122:471–480
Perez-Fuentetaja A, Clapsadl MD, Einhouse D, Bowser PR, Getchell RG, Lee WT (2006) Influence of limnological conditions on Clostridium botulinum type E presence in eastern Lake Erie sediments (Great Lakes, USA). Hydrobiologia 563:189–200
Pittinger CA, Pecquet AM (2018) Review of historical aquatic toxicity and bioconcentration data for the brominated flame retardant tetrabromobisphenol A (TBBPA): effects to fish, invertebrates, algae, and microbial communities. Environ Sci Pollut Res 25:14361–14372
Poindexter CM, Variano EA (2013) Gas exchange in wetlands with emergent vegetation: The effects of wind and thermal convection at the air-water interface. J Geophys Res 118:1297–1306
Raboni M, Gavasci R, Viotti P (2015) Influence of denitrification reactor retention time distribution (RTD) on dissolved oxygen control and nitrogen removal efficiency. Water Sci Technol 72:45–51
Santos L, Santos AL, Coelho F, Gomes NCM, Dias JM, Cunha A, Almeida A (2011) Relation between bacterial activity in the surface microlayer and estuarine hydrodynamics. FEMS Microbiol Ecol 77:636–646
Wang JJ, Pang Y, Li YP, Huang YW, Luo J (2015a) Experimental study of wind-induced sediment suspension and nutrient release in Meiliang Bay of Lake Taihu, China. Environ Sci Pollut Res 22:10471–10479
Wang PF, Hu B, Wang C, Lei Y (2015b) Phosphorus adsorption and sedimentation by suspended sediments from Zhushan Bay, Taihu Lake. Environ Sci Pollut Res 22:6559–6569
Wang JZ, Jia XW, Gao ST, Zeng XY, Li HR, Zhou Z, Sheng GY, Yu ZQ (2016) Levels and distributions of polybrominated diphenyl ethers, hexabromocyclododecane, and tetrabromobisphenol A in sediments from Taihu Lake, China. Environ Sci Pollut Res 23:10361–10370
Wu YF, Lin H, Yin WZ, Shao SC, Lv SH, Hu YY (2019) Water quality and microbial community changes in an urban river after micro-nano bubble technology in situ treatment. Water 11:1–14
Yang SW, Wang SR, Liu HL, Yan ZG (2012) Tetrabromobisphenol A: tissue distribution in fish, and seasonal variation in water and sediment of Lake Chaohu, China. Environ Sci Pollut Res 19:4090–4096
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 51809226, 21607123 and 51709002), the China Postdoctoral Science Foundation funded project (Grant No. 2018M632390), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (Grant No. 18KJB610022), and the fund of Jiangsu Provincial laboratory for Water Environmental Protection Engineering (Grant No. W1804).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Robert Duran
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1442 kb)
Rights and permissions
About this article
Cite this article
Cheng, H., Wang, Y., Zhu, T. et al. Effects of hydrodynamic disturbances on biodegradation of tetrabromobisphenol A in water-sediment systems. Environ Sci Pollut Res 26, 31392–31400 (2019). https://doi.org/10.1007/s11356-019-06291-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06291-8