Abstract
Lead-polluted agricultural soils are a serious problem for food safety, with organic amendment being a promising mitigation method from the environmental perspective. Therefore, the purpose of this study was to evaluate lead availability and the effectiveness of the application of compost of biosolid with wood shavings and yard trimmings in contaminated soils. The physicochemical (Pb distribution, organic matter, pH, electric conductivity, cation exchange capacity, nitrogen, phosphorus, carbon, carbonates, exchangeable cations, sodium) and biological parameters (the microbial activity obtained by fluorescein diacetate hydrolysis) in Pb-polluted and non-polluted agricultural soils were evaluated after the addition of biosolid with wood shavings and yard trimming compost. Topsoils (lead-polluted and control) were collected in the vicinity of a former battery-recycling plant, amended with compost (0%, 5%, and 10%), and incubated in controlled conditions for 118 days. The results showed that lead availability decreased significantly, and the nutritional quality of the soils increased in the soils amended with 10% of compost. Taken together, the results of the present study indicated that compost amendment could be an effective method for mitigating the negative effects of lead in agricultural soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic emissions of potentially toxic trace metals have accelerated considerably in recent decades (Wong et al. 2006). One of the most toxic metals for humans is lead, which can be found in the environment due to either natural processes or to a wide variety of anthropic activities, such as painting and enamels (Needleman 2004), mining and industrial activities (Navarro et al. 2008), lead shot hunting (Romano et al. 2016; Selonen et al. 2012), battery residues (Salazar et al. 2012), and fuel additives. Moreover, it can remain bioavailable in the soil compartment a long time after being introduced (Lavado et al. 1998; Mielke et al. 2011).
With regard to agricultural soils contaminated with heavy metals, adverse effects have been reported on the production and quality of crops, thereby generating risks for the health of the population (Chen et al. 2000; Zheng et al. 2007; Salazar et al. 2012; Rodriguez et al. 2014). The heavy metal remediation of agricultural soils represents a technological challenge since the conventional physicochemical techniques (e.g., vitrification, chemical remediation, encapsulation, soil washing), based on engineering usually employed for the remediation of industrial soils, could result in negative effects on crop quality, soil sustainability, and productivity. Despite high efficiency, most of these techniques have the disadvantage of impairing or even destroying the biological soil functionality, besides being costly (Ghosh and Singh 2005; Henry et al. 2015; Khalid et al. 2016). Therefore, it is necessary to develop an adequate soil remediation technology for agricultural soils contaminated with heavy metals, which can also preserve the quality of the soil in terms of its biological, chemical, and productivity properties. Related to this, despite phytoremediation being an economically environmentally friendly alternative (Khalid et al. 2016), its application involves the use of hyperaccumulator or phytostabilizator species over a long time, which prevents agricultural production during the treatment. Thus, soil remediation in situ methodologies that employ organic amendments to immobilize the contaminants and thereby decrease their bioavailability for the crops could be a promising remediation alternative. Recently, numerous studies have also reported that organic amendments (e.g., compost, biochar) can reduce the bioavailability of contaminants in soils through their ability to immobilize heavy metals as a result of their high organic matter content (Ahmad et al. 2014). Organic amendments in the soils interact with the metals and immobilize them through several mechanisms, including adsorption, ion exchange, and surface complexation, which can occur as a result of the highly porous microstructure, cation exchange capacity, and active surface functional groups of the organic amendment (Ahmad et al. 2014 and references therein). After their proper application, soil amendments can reduce pollution by restricting the availability of metals in the environment and plant uptake (EPA 2007). In this way, many studies carried out with compost produced from spontaneous microbial oxidation of agricultural straw, livestock manure, and organic waste under aerobic conditions indicate that the amendment was effective in the retention of heavy metals in polluted soils and at the same time improved the soil physicochemical properties (Beesley et al. 2010; Karami et al. 2011; Liang et al. 2017; Mackie et al. 2015; Soares et al. 2015). Despite this, other studies showed that the mobility of some metals may also increases, whereas others elements decrease in the same compost application (Huang et al. 2016). Moreover, another problem related with compost-amended soils is the potential release of heavy metals over time as a result of external environment changes (e.g., pH), decomposition of organic matter, and other factors (Henry et al. 2015; Huang et al. 2016 and references therein). Therefore, it is important to evaluate the changes on the distribution of the toxic metal among the soil fractions over time, in order to understand the processes which ultimately determine the bioavailability of the heavy metals (Blanco et al. 2016).
Considering the abovementioned issues, this study proposes to evaluate the effect on lead availability in contaminated agricultural soils amended with a biosolid with wood shavings and yard trimming compost over time, through the analysis of the physical, chemical, and biological parameters and subsequently determine the effectiveness of the application of this amendment in the reduction of bioavailable lead in these soils.
Materials and methods
Topsoil collection
Topsoil samples were collected from the surrounding area of a former battery recycling plant in the town of Bouwer, Córdoba Province, Argentina (31° 33′ 34.02″ S; 64° 11′ 9.05″ W). The soil is an Entic Haplustoll, and the samples corresponded to two different levels (low and high) of lead content (pseudototal concentrations), which were chosen following a systematic sampling according to previous studies (Vergara Cid et al. 2016; Rodriguez et al. 2014; Salazar and Pignata 2014). The low level corresponded to control soil (ConSoil ~ 22 mg kg¬1 pseudototal content; latitude 31° 34′ 7.05″ S; longitude 64° 11′ 10.59″ W), and the high level corresponded to lead-contaminated soil (PbSoil ~ 737 mg kg-1 pseudototal content; latitude 31° 33′ 32″ S; longitude 64° 11′ 6.5″ W).
All soil samples were air dried, sieved to < 2 mm, homogeneously mixed, and stored under controlled conditions of temperature in a range of 23–27 °C before being amended with compost and incubated over time.
Chemical characteristics of compost and experimental conditions of the incubation
In this study, compost from biosolid with wood shavings and yard trimmings was used. This was provided by the Soil group of the National University of Comahue (CRUB), Bariloche, Argentina. Briefly, composting was performed according to a turning pile system using compost treated with biosolids, wood shavings, and yard trimmings, at a 1:1 ratio by volume (biosolids:wood shavings + yard trimmings). For more details, see Hang et al. (2015) and Laos et al. (2002). A summary of the chemical characterization, stability-maturity indicators, and heavy metal content (below the permitted levels according to EPA 1995) of the compost is shown in Table 1, which corresponds to a study of Hang et al. (2015). Is important to note that the compost was produced under strict international quality standards; therefore, the characterization of the compost used in this study was within the values of the reported range of Hang et al. (2015).
An incubation experiment was conducted between the years 2017 and 2018 with the purpose to assess the changes over time in the availability of lead of the amended soil treatments. Plastic containers were filled with 25 kg of soil (S) amended with different proportions of compost (C) w/w in triplicate: 0% C/100% S; 5% C/95% S; and 10% C/90% S. Containers were covered with a perforated cap to limit water evaporation while ensuring gas exchange and incubated in the dark under controlled temperature in a range of 23–27 °C for 118 days. For each experiment, the moisture content of each soil treatment was maintained at 70% water holding capacity (WHC) by adding the corresponding water volume calculated by weight difference, being thoroughly mixed to ensure the homogeneity of the soil performing mixtures using the quartering procedure (EPA 2014).
Physical, chemical, and biological analyses
Soil nutrient and texture analysis
The nutritional quality of the soils was analyzed for samples collected at the end of the incubation period, with the following parameters being measured: pH and EC (1:2.5 soil:water); OM% and oxidizable C % (Walkley and Black 1934); total N (Kjeldahl method); C/N; N–NO3− (Jackson 1958); S–SO42− ; extractable P (Bray and Kurtz 1945); exchangeable K; exchangeable cations (Ca2+, Mg2+, Na+, K+); exchangeable H+; CEC according to Lavkulich (1981); exchangeable sodium percentage (ESP); base saturation %; and carbonate content (Soil Survey Staff 1996). In addition, the particle size distribution was measured by laser-diffraction size analysis using a Horiba LA-950 particle size analyzer according to Gaiero et al. (2013). The texture was categorized as clay (< 0.2 μm), silt (two sizes: 2–20 and 20–50 μm), or sand (50–2000 μm), according to the U.S. Department of Agriculture.
Selective sequential extraction
Lead (Pb) content in different fractions of the amended soils was determined by selective sequential extraction (SSE) procedures. In order to analyze the changes in Pb concentration in the available fractions during the incubation period, soil samples were collected periodically after mixing the soil by the quarter method every 7 days in the first month; every 15 days in the next two months; and finally once a month after that. In these samples, the SSE of Maiz et al. (1997) was performed, with the fractions I and II being measured, since these fractions were often satisfactorily correlated with the uptake of heavy metals by plants (Filgueiras et al. 2002; Maiz et al. 1997). For the purpose of evaluating the Pb distribution of the more stable soil fractions, the SSE of Tessier et al. (1979) (fractions I′, II′, III′, IV′, and V′) was carried out in samples collected at the end of the incubation period in PbSoil.
The soil samples were dried at room temperature, sieved at 2 mm with a stainless steel mesh, and then SSEs were performed with a few modifications as follows. Regarding the SSE of Maiz et al. (1997), the mobile/exchangeable extraction (I) was performed under continuous agitation for 2 h of soils suspended in a solution of CaCl2 0.1 M (1:10 w/v). Subsequently, the mobilizable fraction (II) was used with the remaining soil from the previous extraction and suspended under agitation in DTPA 0.005 M, CaCl2 0.01 M and TEA 0.1 M at pH 7.3 (1:2 w/v) solution for 4 h. For each extraction, the supernatant was obtained by centrifugation (3500 rpm) and then filtered with Whatman® grade 542 filters. All the extractions were analyzed using an AAS (Perkin-Elmer AA3110, flame atomic absorption spectrometer; Norwalk, CT, USA).
The five-step SSE of Tessier et al. (1979) was followed, with a modification in the last extraction: I′, exchangeable (MgCl2 1.0 M); II′, susceptible to changes in pH (NaOAc 1.0 M); III′, bound to Fe and Mn oxides (NH2OH–HCl 0.04 M in HOAc 25% v/v); IV′, bound to OM (HNO3 0.02 M + H2O2 30%, 85 °C); and V′, pseudo-residual (digestion with HNO3 65% for 24 h). The sum of all extractions corresponded to the pseudototal concentration of metals in the soil.
As a quality control, blanks, a Pb standard solution, and a certified reference material “BAM-U113 Soil” (heavy metal polluted soil, Germany), were measured to check the quality of the analytical procedures of the samples evaluated. These results were found to be between 88 and 95% of the certified value, with the data indicating a low error of typically less than 15%. The coefficient of variation of replicate analyses (n = 3) was calculated for each determination and was less than 10%.
The detection limit of the flame AAS was 1 mg kg−1 for Pb, which was calculated by considering the calibration curve of lead, the weight of the sample, and the volume of extraction.
Fluorescein diacetate
The soil microbial activity was quantified by measurement of the hydrolysis of fluorescein diacetate (FDA), according to Adam and Duncan (2001). To carry this out, soil samples were collected both at the beginning of the incubation process and at the end (4 months). Briefly, 2 g of soil was added to 15 mL of 60 mM potassium phosphate buffer (pH 7.6) in a conical flask. Then, the substrate (FDA, 1000 mg mL−1) was incorporated to start the reaction and flasks were placed in an orbital incubator at 30 °C (100 rpm; 20 min). Subsequently, 15 mL of chloroform/methanol (2:1 v/v) were added in order to stop the reaction. Samples were centrifuged at 447 g for 5 min, and finally, the supernatant was filtered and measured at 490 nm on a spectrophotometer (Perkin Elmer).
Data analyses
The data were subjected to general linear mixed models (GLMM) with repeated measures, using the post hoc test DGC for mean comparisons (Di Rienzo et al. 2002). The cumulative percentage was calculated for the lead content in the different fractions obtained in each sequential extraction procedure, for each soil treatment over time. In addition, the Pearson correlation coefficient was used with the purpose of identifying the relationships between the nutritional soil variables and microbial activity in the different amended soils (ConSoil and PbSoil). These analyses were performed using the software Infostat ® coupled with R (Di Rienzo et al. 2011), version 2012.
Results and discussion
Texture and nutritional status of the soil
The particle size distribution in the PbSoil is shown in Fig. 1, which indicates a higher composition of silt in the fraction 2–20 μm and a decrease in the sand fraction for the soil amended with 10% compost.
Soil particle size has been widely studied in relation to the distribution of metal concentrations in soils, due to its important role in retention and availability. Finer particles have been reported to have higher concentrations of heavy metals as a result of increased surface area, and also to be closely related to the soil particle compositions, such as OM, and Fe/Al oxides (Gong et al. 2014; Liu et al. 2018; Qian et al. 1996; Quenea et al. 2009). Quenea et al. (2009) performed an analysis using sodium pyrophosphate to dissolve soil OM in order to extract the metals bound to the OM, and also the organo-mineral forms. An important increase in Pb concentrations was observed by these authors in relation to particle size reduction, with the fractions 20–2 μm and < 2 μm being associated with the higher concentrations not only for Pb but also for Zn, Cu, and Cd.
Taking into account the above findings, the significant increase in finer particles in the 10% compost treatment may represent a higher Pb retention/immobilization due to the stable interactions of OM with the finer soil particle sizes and this metal.
Among the nutritional parameters measured at the end of the incubation period, the CEC indicated a greater buffer capacity of the PbSoil in comparison with ConSoil, which was further increased by the addition of the amendment. Is important to note that a higher CEC indicates a greater ability of the soil to bind more cations to the exchange sites, as in the case of organic matter particle surfaces, which is directly related to nutrient availability. As mentioned above, the higher CEC of PbSoil observed may be due to the land use, leading to a higher content of OM since no intensive farming was carried out in soils contaminated with Pb in comparison with control soils, tending to a higher accumulation of OM and nutrients. Moreover, it was observed that the addition of an amendment increased the total N and extractable P in both soils, as a consequence of the content of the compost (Tables 1 and 2). However, PbSoil without the addition of the amendment (0%) revealed a higher total N and extractable P contents than ConSoil (Table 2), as a consequence of land use. In contrast, the nitrate content was higher in ConSoil than in PbSoil, but the addition of the amendment significantly increased these values in both soils. These results are consistent with the differential use of both soils, as explained above, since for example the PbSoil did not present any agricultural activity, whereas the ConSoil, whose main crop is soybean (main source of N is the biological fixation), in rotation with sorghum and corn, requires N fertilization leading to the accumulation of nitrate (Salvagiotti 2009).
Lead distribution in soil fractions
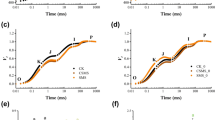
The results of Pb sequential extraction according to Maiz et al. (1997) for ConSoil and PbSoil amended soils over time are shown in Fig. 2. During the incubation period, Pb concentration in the mobile (I) in the ConSoil and mobilizable fraction (II) in the PbSoil were significantly different (p < 0.05) for the soils amended at 10% of compost. Stabilization of Pb concentration was obtained only for this fraction in PbSoil. However, a stable lead distribution in soil fractions over time is not easily achieved in comparison with other metals, as mentioned by de Santiago-Martín et al. (2015), who performed soil incubation spiked with different metals, indicating that Pb concentrations did not attain equilibrium during the incubation time. In addition, other studies have reported that soluble/exchangeable lead forms (Pb2+) in soils become part of more stable fractions over either short or long periods of time (Kabala et al. 2011; Arenas-Lago et al. 2014; Ferreyroa et al. 2014).
Pb content variations over time in control soils (ConSoil) in mobile fraction I (a) and mobilizable fraction II (b), and in lead-polluted soils (PbSoil) in mobile fraction I (c) and mobilizable fraction II (d). Different small letters indicate significant differences between mean Pb concentrations at 118 days of incubation time (ANOVA, p < 0.05). SEE used according to the modified method of Maiz et al. (1997)
At the end of the incubation period, the Pb distribution in the available and stable fractions was evaluated using the SEE of Tessier et al. (1979). ANOVA results (mean ± SEM) in different fractions are shown in supplementary Table S1, whereas Fig. 3 shows the cumulative percentage (mean ± SEM) of Pb content in different fractions of PbSoil amended with compost. These results showed that for PbSoil, no significant differences were observed among soil treatments for the mobile/exchangeable fraction (I′). On the other hand, the PbSoil amended with 5 or 10% of compost showed a significant decrease in the Pb content in soils susceptible to changes in pH (II′) (149 ± 8; 118 ± 2; and 90.3 ± 0.6 mg kg−1 for 0, 5, and 10%, respectively, at p < 0.05). Moreover, the fractions bound to the Mn and Fe oxides (III′), and OM-sulfites (IV′) revealed a significant decrease in the Pb content for the PbSoil amended with 10% of compost (60 and 36 mg kg−1 less than the unamended soil, respectively, for the fractions III′ and IV′). However, for the pseudo-residual (V′) fraction, the highest values of Pb content were found in the PbSoil amended with 10% of compost (83 ± 3 mg kg−1) in comparison with the control, and 5% of compost-amended soils, which showed that all the Pb decreases in the other fractions (II′, III′, IV′) at the highest concentration of compost, was strongly bound to the most stable components of the soil (Table S1; Fig. 3). In agreement, Manios et al. (2003) indicated that compost added in contaminated substrate can decrease the mobile and exchangeable metal fractions by organic binding. Moreover, similar results to those reported in our study were found by Zhou et al. (2017), who performed a remediation study of agricultural multi-heavy metal–contaminated soil, using red mud and compost, with a decrease of heavy metal bioavailability in the soil fractions bound to carbonates, OM, and Fe–Mn oxides in the amended soils. The increment of Pb concentration in fraction V′ for PbSoil 10% is also related to OM since in the soil mineral fraction (represented by SEE fraction V′) is also present and its different functional groups play an important role as ligands (Dube et al. 2001). Regarding this, Ferreyroa et al. (2014) performed a study about Pb incorporation to soils, finding that Pb was incorporated highly associated to carboxylic functional groups in the clay-silt mineral fraction, and that OM was covering different mineral surfaces. Thus, our results indicate the effectiveness of the application of biosolid with wood shavings and yard trimming compost for the reduction of the bioavailable Pb concentration in agricultural soils, since there was a change in the pollutant distribution by becoming part of the mineral fraction of the soil in a higher proportion in comparison to the unamended treatment.
Cumulative percentage (mean ± SE) of Pb content in different fractions (I′, II′, III′, IV′, and V′) of lead-polluted soils (PbSoil) amended with compost (0, 5, and 10%). Different letters indicate significant differences between mean Pb concentrations corresponding to different soil treatments (ANOVA, p < 0.05). No significant differences were found for the I′ fraction; SEE used according to the modified method of Tessier et al. (1979)
Microbial activity in soils amended with compost over time
The microbial activity response revealed a soil effect, with the highest activity being associated to the PbSoil. In addition, a time effect was also observed, as the microbial activity at the end of the incubation period was higher, independent of the soil used or the amendment (Fig. 4). Regarding ConSoil, the increase in microbial activity towards the end of incubation corresponded to an increase in OM and other associated variables, such as N, C, sulfates, and P (Table 3). Lee et al. (2009, 2011) have also found a higher microbial activity after the application of organic amendments in soils. In contrast, in PbSoil, a negative association was observed between the microbial activity and EC, K, and CEC (Table 3). While some authors have found a negative correlation between microbial activity and heavy metal concentrations in soils (Kızılkaya et al. 2004; Lee et al. 2009), no significant correlations were found among Pb concentration in soils and microbial activity (FDA) in our work. This lack of correlation may be due to many years of pollution persistence in PbSoil, which could have arisen the development of resistant microorganisms to Pb toxicity (Blanco et al. 2016). Moreover, the higher microbial activity in PbSoil in comparison with ConSoil could also be due to land use since ConSoil has been exposed to an intensive agricultural production, including the use of pesticides, which can affect soil microorganisms.
Our findings showed that the microbial activity was associated to the content of organic matter and nutrients from the soil, either because of the amendment addition or the soils characteristics due to land use (see “Texture and nutritional status of the soil” section for PbSoil). However, it was not possible to find a clear pattern of response for incubation time in all soils, and between amendment treatments in PbSoil, which could be related to the variation of the microbial community of the studied soils, being probably different in abundance and richness. In this context, variations in the native microbial communities of soils have shown different effects on the chemistry of Pb in soils depending on numerous physicochemical characteristics, being not easily transferred the results from laboratory experiments to real soils (Henry et al. 2015 and references therein).
Conclusions
Our findings showed that lead distribution in the mobile and mobilizable fractions did not attain equilibrium during the incubation period. In addition, PbSoil revealed different physicochemical properties (higher OM, CEC, total N, and P) than ConSoil, which were directly related with the land use history of the polluted soils. These parameters were also increased with the incorporation of the biosolid with wood shavings and yard trimming compost as an amendment in both soils. The microbial activity of soils was higher in PbSoil, without a strong response being observed related to the incorporation of the amendment. However, our findings showed that the application of the amendment potentially decreased the available Pb content in the studied soils, with 10% of compost being the best. This treatment modified soil texture with an increase in fine silt (2–20 μm) as well as lead sorption in soil by changing its distribution among the different soil fractions, with an increase of this metal bound to the mineral fraction. These results may suggest that organo-mineral complexes were formed in 2–20-μm particles, which would represent stable Pb binding to soil. Hence, our results are a promising addition towards the goal of achieving the safe use of agricultural soils contaminated with lead. Nevertheless, further studies related to the potential of this toxic metal to interact and be absorbed by living organisms should be performed, in order to evaluate the toxicological risk of these contaminated soils amended with compost.
References
Adam G, Duncan H (2001) Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol Biochem 33(7–8):943–951. https://doi.org/10.1016/S0038-0717(00)00244-3
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS (2014) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99:19–33. https://doi.org/10.1016/j.chemosphere.2013.10.071
Arenas-Lago D, Vega FA, Silva LF, Lago-Vila M, Andrade ML (2014) Lead distribution between soil geochemical phases and its fractionation in Pb-treated soils. Fresenius Environ Bull 23(4):1025–1035
Beesley L, Moreno-Jimenez E, Gomez-Eyles JL (2010) Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ Pollut 158(6):2282–2287. https://doi.org/10.1016/j.envpol.2010.02.003
Blanco A, Salazar MJ, Vergara Cid C, Pereyra C, Cavaglieri LR, Becerra AG, Pignata ML, Rodriguez JH (2016) Multidisciplinary study of chemical and biological factors related to Pb accumulation in sorghum crops grown in contaminated soils and their toxicological implications. J Geochem Explor 166:18–26. https://doi.org/10.1016/j.gexplo.2016.01.020
Bray R, Kurtz LT (1945) Determination of total, organic, and available forms of phosphorus in soils. Soil Sci 59:39–46. https://doi.org/10.1097/00010694-194501000-00006
Chen ZS, Lee GJ, Liu JC (2000) The effects of chemical remediation treatments on the extractability and speciation of cadmium and lead in contaminated soils. Chemosphere 41:235–242. https://doi.org/10.1016/S0045-6535(99)00416-6
de Santiago-Martín A, Quintana JR, Valverde-Asenjo I, Lafuente AL, González-Huecas C (2015) Temporal trends of metal extractability in calcareous soils affected by soil constituents and metal contamination levels. Int J Environ Res 9(1):323–332. https://doi.org/10.22059/ijer.2015.904
Di Rienzo JA, Guzmán AW, Casanoves F (2002) A multiple comparisons method based on the distribution of the root node distance of a binary tree. J Agric Biol Environ Stat 7(2):1–14. https://doi.org/10.1198/10857110260141193
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW (2011) InfoStat versión 2012. Grupo InfoStat, FCA. Universidad Nacional de Córdoba, Argentina URL http://www.infostat.com.ar
Dube A, Zbytniewski R, Kowalkowski T, Cukrowska E, Buszewski B (2001) Adsorption and migration of heavy metals in soil. Pol J Environ Stud 10:1–10
EPA (1995) Test methods for evaluating solid waste. Vol IA: laboratory manual physical/chemical methods. Environmental Protection Agency. Ed. U.S. Gov. Print. Office, Washington D.C
EPA (2007) Office of Superfund Remediation and Technology Innovation (OSRTI), The use of soil amendments for remediation, revitalization, and reuse. Environmental Protection Agency, pp 1–27. https://doi.org/10.1017/CBO9781107415324.004
EPA (2014) Soil sampling. Operating procedure SESDPROC-300-R3, Science and Ecosystem Support Division, Athens, Georgia, p 24
Ferreyroa GV, Montenegro AC, Tudino MB, Lavado RS, Molina FV (2014) Time evolution of Pb (II) speciation in Pampa soil fractions. Chem Speciat Bioavailab 26(4):210–218. https://doi.org/10.3184/095422914X14142516366997
Filgueiras AV, Lavilla I, Bendicho C (2002) Chemical sequential extraction for metal partitioning in environmental solid samples. J Environ Monit 4:823–857. https://doi.org/10.1039/b207574c
Gaiero D, Simonella L, Gasso S, Gili S, Stein A, Sosa P, Becchio R, Arce J, Marelli H (2013) Ground/satellite observations and atmospheric modeling of dust storms originating in the high Puna-Altiplano deserts (South America): implications for the interpretation of paleo-climatic archives. J Geophys Res Atmos 118:3817–3831. https://doi.org/10.1002/jgrd.50036
Ghosh M, Singh SP (2005) A review on phytoremediation of heavy metals and utilization of its byproducts. Appl Ecol Environ Res 3(1):18. https://doi.org/10.15666/aeer/0301001018
Gong C, Ma L, Cheng H, Liu Y, Xu D, Li B, Liu F, Ren Y, Liu Z, Zhao C, Yang K, Nie H, Lang C (2014) Characterization of the particle size fraction associated heavy metals in tropical arable soils from Hainan Island, China. J Geochem Explor 139:109–114. https://doi.org/10.1016/j.gexplo.2013.01.002
Hang S, Castán E, Negro G, Daghero A, Buffa E, Ringuelet A, Satti P, Mazzarino MJ (2015) Compostaje de estiércol de feedlot con aserrín/viruta: características del proceso y del producto final. Agriscientia 32(1):55–65
Henry H, Naujokas MF, Attanayake C, Basta NT, Cheng Z, Hettiarachchi GM, Maddaoni M, Schadt C, Scheckel KG (2015) Bioavailability-based in situ remediation to meet future lead (Pb) standards in urban soils and gardens. Environ Sci Technol 49(15):8948–8958. https://doi.org/10.1021/acs.est.5b01693
Huang M, Zhu Y, Li Z, Huang B, Luo N, Liu C, Zeng G (2016) Compost as a soil amendment to remediate heavy metal-contaminated agricultural soil: mechanisms, efficacy, problems, and strategies. Water Air Soil Pollut 227(359). https://doi.org/10.1007/s11270-016-3068-8
Jackson ML (1958) Soil chemical analysis. Prentice Hall, Inc., Englewood Cliffs. https://doi.org/10.1002/jpln.19590850311
Kabala C, Karczewska A, Szopka K, Wilk J (2011) Copper, zinc, and lead fractions in soils long-term irrigated with municipal wastewater. Commun Soil Sci Plant Anal 42(8):905–919. https://doi.org/10.1080/00103624.2011.558960
Karami N, Clemente R, Moreno-Jimenez E, Lepp NW, Beesley L (2011) Efficiency of green waste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass. J Hazard Mater 191(1-3):41–48. https://doi.org/10.1016/j.jhazmat.2011.04.025
Khalid S, Shahid M, Niazi NK, Murtaza B, Bibi I, Dumat C (2016) A comparison of technologies for remediation of heavy metal contaminated soils. J Geochem Explor 182:247–268. https://doi.org/10.1016/j.gexplo.2016.11.021
Kızılkaya R, Aşkın T, Bayraklı B, Sağlam M (2004) Microbiological characteristics of soils contaminated with heavy metals. Eur J Soil Biol 40(2):95–102. https://doi.org/10.1016/j.ejsobi.2004.10.002
Laos F, Mazzarino MJ, Walter I, Roselli L, Satti P, Moyano S (2002) Composting of fish offal and biosolids in northwestern Patagonia. Bioresour Technol 81:179–186. https://doi.org/10.1016/S0960-8524(01)00150-X
Lavado RS, Rodríguez MS, Scheiner JD, Taboada MA, Rubio G, Alvarez R, Alconada M, Zubillaga MS (1998) Heavy metals in soils of Argentina: comparison between urban and agricultural soils. Commun Soil Sci Plant Anal 29(11-14):1913–1917. https://doi.org/10.1080/00103629809370081
Lavkulich LM (1981) Methods manual, pedology laboratory. Department of Soil Science, University of British Columbia, Vancouver, Canada
Lee SH, Lee JS, Jeong Choi Y, Kim JG (2009) In situ stabilization of cadmium-, lead-, and zinc-contaminated soil using various amendments. Chemosphere 77(8):1069–1075. https://doi.org/10.1016/j.chemosphere.2009.08.056
Lee SH, Park H, Koo N, Hyun S, Hwang A (2011) Evaluation of the effectiveness of various amendments on trace metals stabilization by chemical and biological methods. J Hazard Mater 188(1):44–51. https://doi.org/10.1016/j.jhazmat.2011.01.046
Liang J, Yang Z, Tang L, Zeng G, Yu M, Li X, Wu H, Qian Y, Li X, Luo Y (2017) Changes in heavy metal mobility and availability from contaminated wetland soil remediated with combined biochar-compost. Chemosphere 181:281–288. https://doi.org/10.1016/j.chemosphere.2017.04.081
Liu G, Wang J, Liu X, Liu X, Li X, Ren Y, Wang J, Dong L (2018) Partitioning and geochemical fractions of heavy metals from geogenic and anthropogenic sources in various soil particle size fractions. Geoderma 312:104–113. https://doi.org/10.1016/j.geoderma.2017.10.013
Mackie KA, Marhan S, Ditterich F, Schmidt HP, Kandeler E (2015) The effects of biochar and compost amendments on copper immobilization and soil microorganisms in a temperate vineyard. Agric Ecosyst Environ 201:58–69. https://doi.org/10.1016/j.agee.2014.12.001
Maiz I, Esnaola MV, Millan E (1997) Evaluation of heavy metal availability in contaminated soils by a short sequential extraction procedure. Sci Total Environ 206(2-3):107–115. https://doi.org/10.1016/S0048-9697(97)80002-2
Manios T, Stentiford EI, Millner P (2003) Removal of heavy metals from a metalliferous water solution by Typha latifolia plants and sewage sludge compost. Chemosphere 53(5):487–494. https://doi.org/10.1016/S0045-6535(03)00537-X
Mielke HW, Laidlaw MA, Gonzales CR (2011) Estimation of leaded (Pb) gasoline’s continuing material and health impacts on 90 US urbanized areas. Environ Int 37(1):248–257. https://doi.org/10.1016/j.envint.2010.08.006
Navarro MC, Pérez-Sirvent C, Martínez-Sánchez MJ, Vidal J, Tovar PJ, Bech J (2008) Abandoned mine sites as a source of contamination by heavy metals: a case study in a semi-arid zone. J Geochem Explor 96(2-3):183–193. https://doi.org/10.1016/j.gexplo.2007.04.011
Needleman H (2004) Lead poisoning. Annu Rev Med 55(1):209–222. https://doi.org/10.1146/annurev.med.55.091902.103653
Qian JIN, Shan XQ, Wang ZJ, Tu Q (1996) Distribution and plant availability of heavy metals in different particle-size fractions of soil. Sci Total Environ 187(2):131–141. https://doi.org/10.1016/0048-9697(96)05134-0
Quenea K, Lamy I, Winterton P, Bermond A, Dumat C (2009) Interactions between metals and soil organic matter in various particle size fractions of soil contaminated with waste water. Geoderma 149(3-4):217–223. https://doi.org/10.1016/j.geoderma.2008.11.037
Rodriguez JH, Salazar MJ, Steffan L, Pignata ML, Franzaring J, Klumpp A, Fangmeier A (2014) Assessment of Pb and Zn contents in agricultural soils and soybean crops near to a former battery recycling plant in Córdoba, Argentina. J Geochem Explor 145:129–134. https://doi.org/10.1016/j.gexplo.2014.05.025
Romano M, Ferreyra H, Ferreyroa GV, Molina FV, Caselli A, Barberis I, Beldoménico P, Uhart M (2016) Lead pollution from waterfowl hunting in wetlands and rice fields in Argentina. Sci Total Environ 545-546:104–113. https://doi.org/10.1016/j.scitotenv.2015.12.075
Salazar MJ, Pignata ML (2014) Lead accumulation in plants grown in polluted soils. Screening of native species for phytoremediation. J Geochem Explor 137(0):29–36. https://doi.org/10.1016/j.gexplo.2013.11.003
Salazar MJ, Rodriguez JH, Nieto GL, Pignata ML (2012) Effects of heavy metal concentrations (Cd, Zn and Pb) in agricultural soils near different emission sources on quality, accumulation and food safety in soybean [Glycine Max (L.) Merrill]. J Hazard Mater 233-234:244–253. https://doi.org/10.1016/j.jhazmat.2012.07.026
Salvagiotti F (2009) Manejo de soja de alta producción. Para Mejorar la Producción 42:57–62
Selonen S, Liiri M, Strömmer R, Setälä H (2012) The fate of lead at abandoned and active shooting ranges in a boreal pine forest. Environ Toxicol Chem 31(12):2771–2779. https://doi.org/10.1002/etc.1998
Soares MAR, Quina MJ, Quinta-Ferreira RM (2015) Immobilisation of lead and zinc in contaminated soil using compost derived from industrial eggshell. J Environ Manag 164:137–145. https://doi.org/10.1016/j.jenvman.2015.08.042
Soil Survey Staff (1996) Soil survey laboratory methods manual. Soil Survey Investigations Rep. 42. Version 3.0. U.S. Gov. Print. Washington, DC
Tessier A, Campbell PG, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51(7):844–851. https://doi.org/10.1021/ac50043a017
Vergara Cid C, Rodriguez JH, Salazar MJ, Blanco A, Pignata ML (2016) Effects of co-cropping Bidens pilosa (L.) and Tagetes minuta (L.) on bioaccumulation of Pb in Lactuca sativa (L.) growing in polluted agricultural soils. Int J Phytoremediation 18(9):908–917. https://doi.org/10.1080/15226514.2016.1156636
Walkley A, Black IA (1934) An examination of Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 37:29–37. https://doi.org/10.1097/00010694-193401000-00003
Wong CSC, Li X, Thornton I (2006) Urban environmental geochemistry of trace metals. Environ Pollut 142(1):1–16. https://doi.org/10.1016/j.envpol.2005.09.004
Zheng N, Wang Q, Zhang X, Zheng D, Zhang Z, Zhang S (2007) Population health risk due to dietary intake of heavy metals in the industrial area of Huludao city, China. Sci Total Environ 387:96–104. https://doi.org/10.1016/j.scitotenv.2007.07.044
Zhou R, Liu X, Luo L, Zhou Y, Wei J, Chen A, Tang L, Wu H, Deng Y, Zhang F, Wang Y (2017) Remediation of Cu, Pb, Zn and Cd-contaminated agricultural soil using a combined red mud and compost amendment. Int Biodeterior Biodegradation 118:73–81. https://doi.org/10.1016/j.ibiod.2017.01.023
Acknowledgments
We would especially like to thank to Dr. Mazzarino and Dr. Castán of the National University of Comahue (CRUB) for providing the compost. Special thanks are also due to Dr. Kowaljow regarding compost advice; to the landowner of the soil collection (M.R. Pavani and S. Herrera); and to Dr. Paul Hobson, native speaker, for language revision.
Funding
This work was partially supported by the Secretaría de Ciencia y Técnica de la Universidad Nacional de Córdoba, UNC, (30820150100435CB), Fondo para la Investigación Científica y Técnica (PICT 2013-0988), and Consejo de Investigaciones Científicas y Técnicas (11220120100402CO). The authors Dr. Ferreyroa, Dr. Dominchin, Dr. Verdenelli, and PhD student Biol-Vergara Cid were funded by CONICET through scholarships.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Ferreyroa, G.V., Vergara Cid, C., Verdenelli, R.A. et al. Availability of lead in agricultural soils amended with compost of biosolid with wood shavings and yard trimmings. Environ Sci Pollut Res 26, 30324–30332 (2019). https://doi.org/10.1007/s11356-019-06190-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06190-y