Abstract
Thermal power generating industries affect the surrounding environment in various ways. Fly ash escapes along with flue gases and can be found in undesirable quantities in soil and water sources in the region. The water quality of an area must be evaluated regularly to ensure the quality of potable water. The present study evaluates the pre-monsoon and post-monsoon concentrations of several important physico-chemical parameters and heavy-metal contents of groundwater samples collected from sites near the Koradi Thermal Power Plant, a major source of power generation in the Nagpur Region. The maximum amount of total dissolved solids observed during the two seasons studied were 1571 mg/l and 1591 mg/l which is within the desirable limit implying that fly ash contamination did not affect this water quality parameter. The total hardness of samples from GW-3, GW-5 and GW-9 were 844 mg/l, 775 mg/l and 675 mg/l during pre-monsoon season, while GW-3 and GW-5 along with GW-4 continued to show high levels of total hardness at 1015 mg/l, 741 mg/l and 650 mg/l, respectively. These values are higher than the permissible limit due to the high levels of ions of bicarbonate, calcium, sodium and sulphate derived from fly ash leachate. Statistical analysis showed that sulphides, total hardness, electrical conductivity and total dissolved solids were the significant water quality parameters of the region. The evaluation of the parameters found that the three water sources (GW-3, GW-5 and GW-9) out of 10 are the most affected groundwater sources of fly ash pollution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The amount of power generated in India has increased greatly over the last decade with the current demand standing at around 300,000 MW. Coal is one of the major sources of power generation in India. More than 70% of the power needs of the country are generated by burning coal. A majority of the coal used is low-grade coal with high ash content. It is required in huge amounts by thermal power generating industries (Khan and Umar 2019). Fly ash (FA) is generated in large quantities near thermal power plants. FA can contain an abundance of heavy metals including mercury, lead, arsenic and chromium depending on the source of the coal used. It poses a threat to human health because if consumed or inhaled in higher quantities, it can cause respiratory disorders, gastrointestinal diseases and act as a carcinogen (Maree et al. 1987; Landrigan et al. 2000; Adamu et al. 2015). It is estimated that 1 in 50 people develop cancer when the source of drinking water contains heavy metal pollution. Fly ash is one of the major wastes generated indirectly from the mining industry. FA is a product of coal combustion and is composed of very fine particles (Carlson and Adriano 1993). FA can be disposed in dry landfills or in ash ponds (Kanchan et al. 2015). Some of the FA is recycled either as building materials, road base or for agricultural uses (Ghosh et al. 2018; Jambhulkar et al. 2018). Low-cost adsorbents have been developed using FA which can be used to adsorb dyes, mercury, organic pollutants, NOx and SOX. FA can be transformed into zeolites for aiding ion exchange (Ahmaruzzaman 2010). FA can also be used in formulation to immobilize lead (Dermatas and Meng 1995). Ash ponds contain the risk of infiltration of leachate or leaking of slurry to nearby water sources (Du et al. 2018; Demir et al. 2019). Leaching of fly ash happens in the form of dilute slurry from ash ponds; the process depends on the pH of the slurry and the liquid to solid ratio (Lindahl and Bockstaller 2012; Lu et al. 2019). Ninety percent of the potable water supply of the population is sourced from groundwater (Karthika et al. 2018). The groundwater contamination with FA leads to the subsequent addition of metals in the biogeochemical cycle (Mirzabeygi et al. 2017). Water sources can also be polluted via the routes of soil erosion and surface runoffs (Atafar et al. 2010). Devices like electrostatic precipitators are used for capturing FA escaping along with flue gases in thermal power industries. FA can be classified as Class F FA and Class C FA depending upon the composition of elements in it. Class F fly ash is chiefly generated during the burning of aged anthracite and bituminous coal, while Class C is produced by lignite and sub-bituminous coal. Class C contains a major amount of CaO (Jeon et al. 2018). FA contamination increases the amount of toxic heavy metals in the groundwater resources. The groundwater quality can be affected by several factors, including natural and anthropogenic causes (Kumarathilaka et al. 2018). Anthropogenic causes behind the water quality deterioration of groundwater include mining activities, industrial waste generation, sewage and solid waste disposal and urbanization (Gadgil and Guha 2013). The pollutant levels in groundwater sources remain unaltered for a longer period of time than the pollutant levels in surface water because of the slow rate of movement of groundwater (Shrinivasa Rao and Venkateswaralu 2000; Phoungthong et al. 2018; Schomburg et al. 2018). The quality of groundwater is assessed by analyzing the physical, chemical and biological properties of the water (Kaliprasad and Narayana 2018). Apart from harming human life, contamination of groundwater with toxic pollutants can lead to the deterioration of the flora and fauna in the region. The presence of a high amount of toxic elements in the water can also lead to metabolic impairments.
Physico-chemical properties of a water sample provide an in-depth analysis of the groundwater quality of a region apart from highlighting the important pollutants (Patil et al. 2012; Yadav et al. 2012). The evaluation of physico-chemical properties is necessary to determine the potability of a source of water (Kumar et al. 2018; Nzung'a 2018).
The accumulation of trace metals in FA occurs as a result of condensation and evaporation during different production operations (Dandautiya et al. 2018). During the monsoon season, the rate of leaching is enhanced leading to increased concentrations in GW sources. Coal contains traces of fluorine and chlorine. FA generated during combustion contains between 10 and 40% of the F and Cl content of coal (Deng et al. 2016).
The analysis of metals in groundwater is necessary to evaluate its possible impact on the environment and human health. FA is a heterogeneous mixture implying that the elemental composition is not evenly distributed. The distinguished components releasing from FA leachate include Ca and SO42−, Cl and Na. The leachability depends on factors like the combustion procedure, class of coal, redox conditions and pH (Izquierdo and Querol 2012). Fluorine condenses in minute proportions in FA, and its leaching level ranges from between 5 and 60 mg/kg (Piekos and Paslawska 1998; Álvarez-Ayuso and Querol 2008). These values are not very significant for consideration of FA leachate contamination. FA leachate can affect the composition of different metals depending on conditions like pH and temperature of coal combustion process and amount of sulphates and phosphates in the coal.
Arsenic (As) condenses on the surface of FA in the form of arsenate (Goodarzi et al. 2008). As has maximum solubility in the pH range of 7–11. This is attributed to the lower affinity of arsenic to metal oxides at higher pH. Arsenic uptake by plants is primarily dependent on the type of plant rather than the concentration of arsenic in groundwater or soil. The arsenic concentration in plants changes with different arsenic bearing phases of the plant life-cycle. The young tissues of the root and stem take up the maximum amount of arsenic during the vegetative phase. A considerable reduction of As concentration is observed during the reproductive phase. This can be attributed to the reduction of use of groundwater during this phase. Methylation by microorganisms present in the field also aids the removal of As. During the ripening phase, a considerable gain in As concentration is again observed due to crystallization of thermodynamically unstable Fe oxyhydroxide and the subsequent release of adsorbed As (Chowdhury et al. 2018). Arsenic is a known carcinogen in humans, and ingestion in even minute countries can be harmful. The groundwater sources of several regions of West Bengal are contaminated by As (Samadder 2011).
Cadmium is highly toxic to the aquatic ecosystem due to its high solubility. However, it is immobile in neutral and alkaline pH and hence does not usually cross its desirable limit.
Chromium (Cr) is found in bituminous coal and is a potential carcinogen in its hexavalent (Cr6+) form in aqueous environment. Cr+ remains in FA as trivalent chromium (Cr(III)) and is less dangerous due to its lower solubility in this form. Leachability of Cr is highly pH dependent with effective leaching occurring only at higher pH values (Dubikova et al. 2006).
Lead (Pb) is associated with sulphides in coal and is highly insoluble and immobile. Earlier studies suggested Pb leaching rates to be generally low and only effective at high alkaline values of pH. The leachability of elements like Zn and Ni varies highly in FA depending on the surrounding conditions. Zn is found in close association with sulphide in coal.
Mercury is a highly toxic metal found in coal in minute quantities. The sorption of g depends on the presence of Cl and S (Meij 1995). The leachability of Hg is very low in FA eliminating it as an environmental concern as FA leachate.
The electrical conductivity of the water can be used to estimate the amount of conductive ions present in it. The presence of ions of inorganic materials and salts in dissolved form results in a high conductivity (Tank and Chandel 2010). The pH measures the hydrogen ion concentration of the water, which in turn, is necessary for determining the alkalinity of the water source. High values of alkalinity suggest the presence of a large amount of carbonates and bicarbonates. Total dissolved solids can affect the taste of the water.
Another important parameter for measuring the water quality is the hardness of water. Excess amount of hardness is undesirable as it can have laxative effects upon drinking. Groundwater contains more calcium than surface water (Sienko and Plane 1974; Spielmeyer et al. 2017). Since fly ash contains a high amount of calcium oxide (CaO), the concentration of calcium is high in the FA polluted groundwater sources. Fluoride is present in water in ionic form and is important for dental health and bone density. However, a large quantity of fluoride is that values exceeding 1.5 mg/l can lead to occurrence of fluorosis. Presence of excess amount of sulphates in water can lead to scaling in boilers and alter the taste of drinking water (Wanganeo et al. 2018; Walaszek et al. 2018).
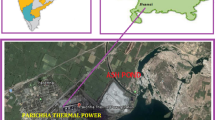
Koradi thermal power station (KTPS) located in Maharashtra is one of the major power stations of the region, which began its operation during the 1970s. It has a power generation capacity of 2400 MW. The nearby localities of Koradi power plant, Nagpur, were selected as the study area. Presently, the plant operates 8 units with plans of further increasing the power outputs. The present study investigates the role of FA leachate on groundwater contamination near thermal power plants before and after the monsoons and subsequently tries to recognize their possible effects on human health.
Materials and methods
Sampling site
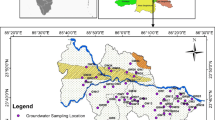
Nearby locality of Koradi Thermal Power Plant, Nagpur, was selected as the study area. Koradi Thermal Power Plant is situated in close proximity to Nagpur City and as the latitude and longitude of 21°14′56″ N and 79°5′56″ E, respectively. The plant had 2 units of 210 MW each at the early stages. Currently, its total capacity has increased to 2400 MW. The regional climate consists of hot summers with less humidity throughout the year except during the south-west monsoon season, i.e., June to September. The peak temperature during the seasons of summer and winter ranges between 45 and 12 °C, respectively, with annual rainfall of about 1200 mm. The three ash ponds in the study area receive ash from the two thermal power stations at Koradi and Khaparkheda. The ash generated in the power plant is eliminated by two different modes. Firstly, it is disposed of as fly ash in the atmosphere from the stacks and secondly the ash slurry containing the bottom ash is cast aside in ash ponds. The ash slurry is a mixture of the bottom ash with water and which is then transported to the pond through pipelines.
Collection of samples
Groundwater samples were collected from 10 different locations near Koradi Thermal Power Plant during pre-monsoon (April 2017) and post-monsoon (Nov 2017) season. The 10 locations were of nearby villages which could possibly be impacted by FA generation, and the details of the sample locations are given in Table 1. The sampling site lies between 21°15′ and 21°12′ in north latitudes and between 79°3′ and 79°11′ in east longitudes (Fig. 1). Each of the water samples was filtered with 0.45 μm membrane filters and collected in sterilized plastic bottles, which were pre-cleaned with concentrated nitric acid and distilled water in the laboratory. The samples were divided into two parts, of which one was acidified with concentrated nitric acid for cation estimation and the remaining was used for the estimation of other physico-chemical properties. The water was stored at 4 °C in the laboratory. The analysis of the water samples was done within 2 h of sampling.
Physico-chemical analysis
Physico-chemical parameters like pH, turbidity and conductivity were measured using a multi-parameter tester. Alkalinity was measured by acid titration, while total hardness, calcium hardness and magnesium hardness were determined by (ethylenediaminetetraacetic) EDTA titration. Chlorine was determined by argentometric titration. Fluoride was measured by colorimetry method. Standard reagents of analytical grade like KCl, CaCO3 EDTA and indicators were obtained from Merck® and were used for analysis, and the instruments were calibrated.
Analysis of heavy-metal compositions
Metals that have higher densities are categorized as heavy metals. They are found to have highly carcinogenic effects on human beings and animals (Alkarkhi et al. 2008). To determine the safety of a source of drinking water, its heavy metal analysis is necessary. The heavy metal composition of elements like arsenic, nickel, chromium, cadmium, zinc and nickel was found using standard methods of APHA (2012). ICP-MS analysis using PerkinElmer SCIEX ELAN DRCe was done for trace metals.
Statistical analysis and evaluation of source of ionic composition of groundwater
The overall ionic distribution of cations and anions in the samples and analysis of contributing elements was done using the Pipers Trilinear Diagram generated by the software Grapher 13. Statistical analysis was done using Excel 2007 for a detailed comparison of the different physico-chemical properties during the two seasons. The distribution pattern of sulphate ions was analyzed using Arcmap 10.3.
Results
Physico-chemical properties of groundwater
The pH was found to be between 7.1–7.7 during pre-monsoon and 6.8–7.5 during post-monsoon season. The pH of groundwater samples was found to be within the acceptable limit of BIS (2012). Electrical conductivity was found in the range of 540–2652 ms/cm in pre-monsoon season and 708–2618 ms/cm during post-monsoon season (Tables 2 and 4). Higher conductivity of groundwater is due to downward percolation of ions of chloride, sulphide and carbonates from ash ponds through soil strata. The total dissolved solid (TDS) varied between 324 and 1591 mg/l in pre-monsoon season and 425–1571 mg/l in post-monsoon season. Total dissolved solid is a measure of the organic and inorganic contents of substances in a liquid in molecular, ionized and suspended form. TDS value was found to be exceeding most of the time the acceptable limit of 500 mg/l (BIS 2012). Alkalinity of water may be due to either the presence of strong bases like sodium hydroxide or potassium hydroxide in water or the extreme low concentration of them. Maximum alkalinity of 303 mg/l was recorded in GW-3 in pre-monsoon and 420 mg/l in post-monsoon season which exceeds the standard value.
Total hardness is due to the presence of cations of calcium and magnesium and anions of carbonate and bicarbonate, chloride and sulphate in water. Hardness of groundwater in pre-monsoon was 212–844 mg/l and 207–1015 mg/l in post-monsoon season.
The anion chemistry showed that bicarbonate and sulphate were the dominant anions in groundwater with a minor contribution of chlorides. In the samples, the concentration of bicarbonate varies from a 108–370 mg/l in pre-monsoon season and 211–512 mg/l in post-monsoon season. Bicarbonates are derived mainly from the rhizospheric soil zone CO2 and dissolution of carbonates and reaction of silicates with carbonic acid. The soil in the rhizospheric zone is rich in CO2 pressure as a result of decay of organic matter and root respiration. This CO2-rich zone forms bicarbonates when infused with rain water. Bicarbonate may also be derived from the dissolution of carbonates and/or silicate minerals by the carbonic acid. Calcium was observed as 11–217 mg/l in pre-monsoon and 45–147 mg/l in post-monsoon season.
In most of the samples, calcium was found to be exceeding from the desirable range of 75 mg/l (BIS 2012). Magnesium was found to be exceeding the acceptable limit of BIS (2012) i.e. 30 mg/l in all samples except at GW-1, GW-8 and GW-10 in pre-monsoon season, and GW-1 and GW-10 in post-monsoon season. It was observed as 24–138 mg/l in pre-monsoon and 11–161 mg/l in post-monsoon.
Analysis of heavy-metal compositions
Heavy metal compositions were found to be within the permissible limits of WHO guidelines (WHO 2004). The concentration of iron, zinc and nickel reduced during the post-monsoon season to 0.237 mg/l, 0.131 mg/l and 0.012 mg/l, respectively (Tables 3 and 5). The concentration of these metals implied that the drinking water sources of the region did not possess the risk of cancer (Fig. 4).
Statistical analysis and evaluation of source of ionic composition of groundwater
The correlation of various water quality parameters showed that the most relevant parameters (> 0.8) were EC, TDS, total hardness, calcium and sulphate concentrations during both pre-monsoon and post-monsoon seasons (Supplementary Tables 1 and 2). The values marked in red highlight the significant parameters. Sulphate is a good indicator of pollution caused by industries. High concentrations of sulphate mark the groundwater source unfit for drinking. Similarly, excessive hardness can cause scaling in boilers, hindrances in industrial operations, and can impart an objectionable taste at higher concentrations. TDS and conductivity indicate the presence of ions of elements like nitrate, arsenic, copper, lead, etc. which can be detrimental for human health.
The distribution of anions and cations based on the source of ions is depicted in Figs. 2 and 3. Ca2+, Na+ and K+ were the main contributors of anions, while cations were mainly supplied by Cl− and HCO3−.
Discussion
Groundwater quality assessment based on important parameters
Groundwater is an important source of potable water. The values of physico-chemical parameters of groundwater remained within desirable limits of WHO (2004) and (BIS 2012), and it might be due to the samples not being affected by a source of significant pollution in the region. Much of the groundwater is used for domestic purposes (Karthika et al. 2018). The suitability of groundwater is analyzed by measuring the quantity of several physico-chemical parameters and heavy metal composition of the source. Industries have a significant effect on the groundwater and surface water sources of a region because of which it is necessary to assess the water quality parameters from time to time. Contamination due to fly ash occurs in the Nagpur Region due to the operations of several thermal power plants. Fly ash contains a significant amount of calcium carbonate and metals (Aubert et al. 2006). Excessive deposition fly ash constituents in groundwater via various routes lead to the unwanted increase of certain water quality parameters. The water quality parameters that were assessed in this study include pH, alkalinity, electrical conductivity, total hardness, calcium and magnesium concentration, nitrogen, phosphorus bicarbonate and several metal compositions (Fig. 4). Fly ash if not treated at the site of generation can travel a consequential distance and pollute the air, water and soil surface (Khan and Umar 2019). Fly ash components can percolate into groundwater sources. World Health Organization (WHO) has guidelines for the maximum permissible limits of various water quality parameters beyond which they are considered inconsumable (WHO 2004). pH of water can be altered by the presence of various chemical components. Electrical conductivity, which is a measure of the ionic constituents of water, indicates the presence of minerals in it. The total dissolved solid concentration in this study ranged from 324 to 1591 mg/l during the pre-monsoon period and from 425 to 1571 mg/l during the post-monsoon period. GW-5, GW-4 and GW-9 had greater values of TDS than the maximum permissible limit. The presence of dissolved solids in water sources can lead to changes in its taste (Vasanthavigar et al. 2012). GW-1 had minimum values for most of the water quality parameters indicating that it was one of the least affected groundwater sources in the region sampled. High TDS concentrations can often have adverse effects if ingested regularly. In the past, high TDS values in water resources have been suggested as a possible source of cardiovascular diseases, gastrointestinal disorders and cancer (Karthika et al. 2018).
The concentration of chloride in groundwater sources can also indicate the prevailing amount of pollution in the region. High chloride concentration is often caused by surface runoff and industrial discharges into water sources. Chloride ion values ranged from 70 to 378 mg/l during post-monsoon season and 78–433 mg/l during the pre-monsoon season. GW-4 showed a high presence of chloride in both cases. The chloride concentration of the groundwater samples was within the permissible limits and showed declines in their concentration during the post-monsoon season while the heavy-metal compositions were also within desired limits in the given period. (Tables 4 and 5).
Sulphates can be present in water sources in various forms, including magnesium sulphate and calcium sulphate. The concentration of sulphate ranged from 67 to 629 mg/l during post-monsoon season and 113 to 721 mg/l during pre-monsoon season. The concentration of sulphates was considerably high in many of the samples. The presence of a high amount of sulphate can be toxic. It can also alter the pH and make it more acidic.
Phosphate values ranged from 0.39 to 1.48 mg/l during the pre-monsoon period and 0.08 to 0.38 mg/l during the post-monsoon period. Phosphate concentration was considerably low in the groundwater sources of the region. The phosphate values also declined during the post-monsoon season. Hardness of water is one of the most important parameters based on which we deem it fit or unfit for drinking. The total hardness varied from 212 to 844 mg/l during pre-monsoon season, while it ranged from 207 to 1015 mg/l during post-monsoon period. The total hardness was also found to be exceeding the permissible values in many samples. GW-3, GW-4 and GW-5 showed maximum values for total hardness during post-monsoon season. It was also observed that the values of total hardness increased during the latter season. While the reduction of some parameters during the post-monsoon season could be attributed to the dilution caused by the rainfall, the increase in certain other parameters implies that the percolation of pollutants also increases during monsoon.
The metal compositions of the groundwater samples were found to be in check. Metals in excess quantities can be harmful to the digestive system while heavy metals can be carcinogenic even in small quantities (Covelo et al. 2007).
Fly ash and its effect on groundwater
Most of the power requirements of developing nations are met with coal fuelled power plants. Generation of fly ash is inevitable during such processes. Fly ash composition depends on the composition and type of coal from which it is generated (Wang 2018). Certain varieties of coal produce less fly ash than the others. Fly ash has found various productive uses in the building material industry. It is often added to enhance the strength of concrete (Wang and Lu 2018). Fly ash can be captured at source level by electrostatic precipitators and other particle filtration equipments to reduce the impact of fly ash pollution (Pandey et al. 2011). Thermal power industries require various devices to capture the escaping fly ash. They can be deposited in ponds. If the deposition ponds are not properly layered with impermeable material, some amount of fly ash can be intermixed with leachant (water) and percolate to groundwater (Pal et al. 2016). Many a times toxic elements are also involved with the fly ash leachate thus making water sources harmful (Wang et al. 2016). It can be referred to as a secondary source of pollution.
The ionic concentration of the region was due to high concentration of elements like bicarbonate, calcium, sodium and sulphate ions. The Piper’s trilinear diagram shows that the majority of the cations were supplied by calcium, sodium and potassium ions, while the anion was supplied by bicarbonate ions. The ionic concentration can increase the electrical conductivity of the water (Khan and Umar 2019). The present study showed that the leading sources of cation supply during both the pre-monsoon and post-monsoon season were made by sodium ions followed by calcium, magnesium and potassium ions (Figs. 5 and 6). The significant amount of calcium ions can also be linked with the presence of fly ash pollution in the region because fly ash contains a high amount of calcium (Wang 2018).
Interpretation of groundwater quality in Koradi Region
Interpretations of groundwater quality reveal its suitability for human use (Bhakar and Singh 2018). Ash ponds are man-made ponds situated near power plants where the FA and bottom ash are disposed as sludge. The ash ponds contain significantly higher quantities of FA when compared to the quantity of bottom ash. Studies conducted earlier in the Koradi region suggest that limited interactions take place between the ash pond and groundwater (Spadoni et al. 2014; Voltaggio et al. 2015). Several regulatory reforms have been initiated in developing countries like India to curb excessive pollution caused by Industries and power plants. Ghosh and Kathuria (2016) obtained the relative efficiency score (RTE) of several power plants around India with Koradi TPS receiving an RTE score of 88.2, while the most efficient plant scored 92.9. Plant size, plant age, the type of fuel used and amount of power generated are plant level factors affecting their environment and overall efficiency (See and Coelli 2012). Larger plant size can be linked to higher efficiency (Meibodi 1998). KTPS generates Class F FA which have low CaO content and a high percent of Al2O3 (37.72%) and Fe2O3 (7.41%) (Spadoni et al. 2014).
Numerous FA-based brick industries are situated near the villages included in the study. The groundwater in the region is high in salinity and TDS. The results of this study are synonymous with a previous study conducted by Spadoni et al. (2014) which suggested that the levels of Mg2 +, Ca2 +, NO3−, SO42 − and TDS levels of the groundwater sources exceed the desirable limits for potable water recommended by BIS (2012). The levels of SO42− in samples GW-4 and GW-5 collected from the villages Khairy and Mhasala were notably high and needs to be checked (Fig. 7). These sources could have been influenced by the chemical and brick manufacturing industries in the region as well. In 2015, regulatory norms for sulphur emissions were imposed for the first time in India which limits the emission level of SO2 to 200 mg/Nm3 and 600 mg/Nm3 for large and small power plant boilers, respectively (MoEFCC 2015). Such measures will reduce the chances of sulphur toxicity originating from drinking water.
Conclusion
The ash residues remaining after coal combustion generally contain significantly higher levels of ions of calcium, magnesium, chloride and bicarbonates. This can cause the contamination of groundwater. Contamination occurs due to leaching effect of FA when it is disposed of in ash ponds. The groundwater sources which were sampled from bore wells located nearby the ash disposal site of thermal power plant generally showed higher concentrations of electrical conductivity, total dissolved solids, alkalinity, hardness, bicarbonates and magnesium ions exceeding the desirable limits prescribed by regulatory authorities. From the present study, it can be concluded that there is a significant impact of ash pond on groundwater contamination. The statistical analysis of the physico-chemical parameters suggests that the concentrations of sulphur, TDS, total hardness and electrical conductivity were dominant factors in governing the groundwater quality of Koradi Region. However, trace metal concentrations were within desirable limits. This can be attributed to new environmental regulations introduced by the Government. The utility of advanced technological tools to curb environmental pollution was not accounted for in this study. Further investigations in the coming years will display the role of regulatory norms in reducing pollution.
References
Adamu C, Nganje T, Edet A (2015) Heavy metal contamination and health risk assessment associated with abandoned barite mines in Cross River State, southeastern Nigeria. Environ Nanotechnol Monit Manage 3:10–21
Ahmaruzzaman M (2010) A review on the utilization of fly ash. Prog Energy Combust Sci 36(3):327–363
Alkarkhi FA, Ismail N, Easa AM (2008) Assessment of arsenic and heavy metal contents in cockles (Anadara granosa) using multivariate statistical techniques
Álvarez-Ayuso E, Querol X (2008) Study of the use of coal fly ash as an additive to minimise fluoride leaching from FGD gypsum for its disposal. Chemosphere 71(1):140–146
APHA, AWWA, WEF (2012) Standard methods for examination of water and waste water, 22nd edn. American Public Health Association, Washington DC
Atafar Z, Mesdaghinia A, Nouri J (2010) Effect of fertilizer application on soil heavy metal concentration. Environ Monit Assess 160(1–4):83–89
Aubert JE, Husson B, Sarramone N (2006) Utilization of municipal solid waste incineration (MSWI) fly ash in blended cement: part 1: processing and characterization of MSWI fly ash. J Hazard Mater 136(3):624–631
Bhakar P, Singh AP (2018) Groundwater quality assessment in a hyper-arid region of Rajasthan, India. Natural Resources Research:1–18
Bureau of Indian Standards (2012) Specification for drinking water IS 10500: 2012. New Delhi
Carlson CL, Adriano DC (1993) Environmental impacts of coal combustion residues. J Environ Qual 22(2):227–247
Chowdhury NR, Das R, Joardar M, Ghosh S, Bhowmick S, Roychowdhury T (2018) Arsenic accumulation in paddy plants at different phases of pre-monsoon cultivation. Chemosphere 210:987–997
Covelo EF, Vega FA, Andrade ML (2007) Heavy metal sorption and desorption capacity of soils containing endogenous contaminants. J Hazard Mater 143(1–2):419–430
Dandautiya R, Singh AP, Kundu S (2018) Impact assessment of fly ash on ground water quality: an experimental study using batch leaching tests. Waste Manag Res 36(7):624–634
Demir AEA, Dilek FB, Yetis U (2019) A new screening index for pesticides leachability to groundwater. J Environ Manag 231:1193–1202
Deng S, Shu Y, Li S, Tian G, Huang J, Zhang F (2016) Chemical forms of the fluorine, chlorine, oxygen and carbon in coal fly ash and their correlations with mercury retention. J Hazard Mater 301:400–406
Dermatas D and Meng X (1995) A leaching protocol to evaluate flyash reuse potential. In: Innovation Technologies for Soil Remediation and Hazardous Waste Management. ASCE, New York, pp 694–701
Du W, Zhang CY, Kong XM, Zhuo YQ, Zhu ZW (2018) Mercury release from fly ashes and hydrated fly ash cement pastes. Atmos Environ 178:11–18
Dubikova M, Jankowski J, Ward CR. & French D(2006) Modelling element mobility in water-flyash interactions. QCAT Technology Transfer Centre
Gadgil M & Guha, R (2013) Ecology and equity: the use and abuse of nature in contemporary India. Routledge
Ghosh R, Kathuria V (2016) The effect of regulatory governance on efficiency of thermal power generation in India: a stochastic frontier analysis. Energy Policy 89:11–24
Ghosh A, Ghosh A, Neogi S (2018) Reuse of fly ash and bottom ash in mortars with improved thermal conductivity performance for buildings. Heliyon 4(11):e00934
Goodarzi F, Huggins FE, Sanei H (2008) Assessment of elements, speciation of As, Cr, Ni and emitted Hg for a Canadian power plant burning bituminous coal. Int J Coal Geol 74(1):1–12
Izquierdo M, Querol X (2012) Leaching behaviour of elements from coal combustion fly ash: an overview. Int J Coal Geol 94:54–66
Jambhulkar HP, Shaikh SMS, Kumar MS (2018) Fly ash toxicity, emerging issues and possible implications for its exploitation in agriculture; Indian scenario: a review. Chemosphere 213:333–344
Jeon D, Yum WS, Jeong Y, Oh JE (2018) Properties of quicklime (CaO)-activated class F fly ash with the use of CaCl 2. Cem Concr Res 111:147–156
Kaliprasad CS, Narayana Y (2018) Mineralogy and physico-chemical parameters on the behavior of natural radionuclides in the riverine environs of Hemavathi. Radiation Physics and Chemistry, South India
Kanchan S, Kumar V, Yadav KK, Gupta N, Arya S, Sharma S (2015) Effect of fly ash disposal on ground water quality near Parichha thermal power plant, Jhansi-a case study. Curr World Environ 10(2):572–580
Karthika IN, Thara K, Dheenadayalan MS (2018) Physico-chemical study of the ground water quality at selected locations in Periyakulam, Theni district, Tamilnadu, India. Mater Today: Proc 5(1):422–428
Khan I, Umar R (2019) Environmental risk assessment of coal fly ash on soil and groundwater quality, Aligarh, India. Groundw Sustain Dev 8:346–357
Kumar P, Hegde K, Brar SK & Cledon M (2018) Physico-chemical treatment for the degradation of cyanotoxins with emphasis on drinking water treatment-how far have we come? J Environ Chem Eng
Kumarathilaka P, Seneweera S, Meharg A, Bundschuh J (2018) Arsenic speciation dynamics in paddy rice soil-water environment: sources, physico-chemical, and biological factors-a review. Water Res 140:403–414
Landrigan PJ, Boffetta P, Apostoli P (2000) The reproductive toxicity and carcinogenicity of lead: a critical review. Am J Ind Med 38(3):231–243
Lindahl AM, Bockstaller C (2012) An indicator of pesticide leaching risk to groundwater. Ecol Indic 2:95–108
Lu J, Bai Z, Velthof GL, Wu Z, Chadwick D, Ma L (2019) Accumulation and leaching of nitrate in soils in wheat-maize production in China. Agric Water Manag 212:407–415
Maree JP, Gerber A & Hill E (1987) An integrated process for biological treatment of sulfate-containing industrial effluents. J Water Pollut Control Fed 1069–1074
Meibodi AE (1998) Efficiency considerations in the electricity supply industry: the case of Iran. University of Surrey
Meij R (1995) The distribution of trace elements during the combustion of coal. In: Environmental aspects of trace elements in coal. Springer, Dordrecht, pp 111–127
Mirzabeygi M, Abbasnia A, Yunesian M, Nodehi RN, Yousefi N, Hadi M, Mahvi AH (2017) Heavy metal contamination and health risk assessment in drinking water of Sistan and Baluchistan, Southeastern Iran. Hum Ecol Risk Assess Int J 23(8):1893–1905
MoEFCC, 09 (2015) Ministry of environment, forest and climate change notification
Nzung'a SO (2018) Physico-chemical and bacteriological quality of water sources in rural settings, a case study of Kenya, Africa. Scientific African, p e00018
Pal S, Mahato S, Sarkar S (2016) Impact of fly ash on channel morphology and ambient water quality of Chandrabhaga River of Eastern India. Environ Earth Sci 75(18):1268
Pandey VC, Singh JS, Singh RP, Singh N, Yunus M (2011) Arsenic hazards in coal fly ash and its fate in Indian scenario. Resour Conserv Recycl 55(9–10):819–835
Patil PN, Sawant DV, Deshmukh RN (2012) Physico-chemical parameters for testing of water-a review. Int J Environ Sci 3(3):1194
Phoungthong K, Shao LM, He PJ, Zhang H (2018) Phytotoxicity and groundwater impacts of leaching from thermal treatment residues in roadways. J Environ Sci 63:58–67
Piekos R, Paslawska S (1998) Leaching characteristics of fluoride from coal fly ash. Fluoride 31:188–192
Samadder SR (2011) Impact of arsenic pollution on spatial distribution of human development index. KSCE J Civ Eng 15(6):975–982
Schomburg A, Schilling OS, Guenat C, Schirmer M, Le Bayon RC, Brunner P (2018) Topsoil structure stability in a restored floodplain: impacts of fluctuating water levels, soil parameters and ecosystem engineers. Sci Total Environ 639:1610–1622
See KF, Coelli T (2012) An analysis of factors that influence the technical efficiency of Malaysian thermal power plants. Energy Econ 34(3):677–685
Shrinivasa Rao B, Venkateswaralu P (2000) Physicochemical analysis of selected groundwater samples. Indian J Environ Prot 20(3):161
Sienko MJ & Plane RA (1974) Chemical principles and property. McGraw-Hill
Spadoni M, Voltaggio M, Sacchi E, Sanam R, Pujari PR, Padmakar C, Wate SR (2014) Impact of the disposal and re-use of fly ash on water quality: the case of the Koradi and Khaperkheda thermal power plants (Maharashtra, India). Sci Total Environ 479:159–170
Spielmeyer A, Höper H, Hamscher G (2017) Long-term monitoring of sulfonamide leaching from manure amended soil into groundwater. Chemosphere 177:232–238
Tank DK, Chandel CS (2010) Analysis of the major ion constituents in groundwater of Jaipur City. Nat Sci 8(10):1–7
Vasanthavigar M, Srinivasamoorthy K, Prasanna MV (2012) Evaluation of groundwater suitability for domestic, irrigational, and industrial purposes: a case study from Thirumanimuttar river basin, Tamilnadu, India. Environ Monit Assess 184(1):405–420
Voltaggio M, Spadoni M, Sacchi E, Sanam R, Pujari PR, Labhasetwar PK (2015) Assessment of groundwater pollution from ash ponds using stable and unstable isotopes around the Koradi and Khaperkheda thermal power plants (Maharashtra, India). Sci Total Environ 518:616–625
Walaszek M, Bois P, Laurent J, Lenormand E, Wanko A (2018) Urban stormwater treatment by a constructed wetland: seasonality impacts on hydraulic efficiency, physico-chemical behavior and heavy metal occurrence. Sci Total Environ 637:443–454
Wang J (2018) Statistical study on distribution of multiple dissolved elements and a water quality assessment around a simulated stackable fly ash. Ecotoxicol Environ Saf 159:46–55
Wang W, Lu C (2018) Time-varying law of rebar corrosion rate in fly ash concrete. J Hazard Mater 360:520–528
Wang J, Liu G, Liu Y, Zhou C, Wu Y, Zhang Q (2016) Mobilization of substance around stackable fly ash and the environmental characteristics of groundwater: with particular reference to five elements: B, Ba, Pb, Sb and Zn. Fuel 174:126–132
Wanganeo A, Wanganeo RRN, Srivastava RKN, Kumar P (2018) Variations in physico-chemical characteristics of water bodies placed at different geographical coordinates in Antarctica. Pol Sci 18:48–56
World Health Organization (2004) World Health Organization guidelines for drinking water quality. World Health Organization, Geneva
Yadav KK, Gupta N, Kumar V, Arya S, Singh D (2012) Physico-chemical analysis of selected ground water samples of Agra city, India. Recent Res Sci Technol 4(11)
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 28.0 kb)
Rights and permissions
About this article
Cite this article
Pandey, V., Ray, M. & Kumar, V. Assessment of water-quality parameters of groundwater contaminated by fly ash leachate near Koradi Thermal Power Plant, Nagpur. Environ Sci Pollut Res 27, 27422–27434 (2020). https://doi.org/10.1007/s11356-019-06167-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06167-x