Abstract
A series of MnOx/ACFN, Ce-MnOx/ACFN, and Fe-Ce-MnOx/ACFN catalysts on selective catalytic reduction (SCR) of NOx with NH3 at low-middle temperature had been successfully prepared through ultrasonic impregnation method, and the catalysts were characterized by SEM, XRD, BET, H2-TPR, NH3-TPD, XPS, and FT-IR spectroscopy, respectively. The results demonstrated that the 15 wt% Fe(1)-Ce(3)-MnOx(7)/ACFN catalyst achieved 90% NOx conversion (100~300 °C), good water resistance, and stability (175 °C). The excellent catalytic performance of the Fe(1)-Ce(3)-MnOx(7)/ACFN catalyst was mainly attributed to the interaction among Mn, Ce, and Fe. The doping of Fe promoted the dispersion of Ce and Mn and the formation of more Mn4+ and chemisorbed oxygen on the surface of a catalyst. This work laid a foundation for the successful application of active carbon fiber in the field of industrial denitrification, especially in the aspect of denitrification moving bed.

Graphic abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, the use of gas boilers with clean combustible natural gas or liquefied petroleum gas (as fuel source) is encouragingly used as replacement to gas boilers with coal during the winter-heating period in China and, practically, this led to the policy called “coal-to-gas policy.” The main aim of this substitution is to reduce emission of nitrogen oxides (NOx) and other pollutants from coal consumption. One of the main sources of stationary NOx is the gas boilers with higher water vapor content, which resulted in many environmental problems including ozone hole, photochemical smog, and acid rain (Fan et al. 2018; Gao et al. 2017a; Zhang et al. 2017). Selective catalytic reduction (SCR) of NOx with NH3 has been industrially proven as the predominant method for post-combustion abatement of NOx emissions. At present, commercial SCR catalysts (V2O5-WO3/TiO2) exhibit catalytic activity within a narrow temperature window (300~400 °C), and they are easily poisoned by a high concentration of SO2 and dust (Marani et al. 2017; Sun et al. 2018a). The flue gas temperature behind desulfurization and dust wiper is ordinarily below 200 °C, which is beneficial to recover sensible heat and latent heat of water vapor condensation in the flue gas, thus improving the efficiency of the gas boiler. However, the actual flue gas temperature fluctuates so greatly that the catalysts need to maintain high catalytic activity over a relatively wide temperature range. Therefore, it is necessary to design and explore low-middle-temperature SCR catalysts (100~300 °C) (Gao et al. 2017b; Meng et al. 2018).
TiO2, γ-Al2O3, molecular sieve, and carbon-based materials have been extensively studied as the supported catalyst carriers (Anthonysamy et al. 2018; Li et al. 2018a, 2018b; Wang et al. 2015; Wang et al. 2018). Compared to aluminum and titanium, carbon-based materials including activated carbon/coke, activated carbon fibers, carbon nanotubes, and graphenes have abundant oxygen-containing functional groups, which are beneficial for the adsorption of reactants such as NOx and NH3. And, large specific surface area makes the active and assistant components homogeneously dispersed on the surface, thereby providing more catalytic active sites (Fan et al. 2011; Grzybek et al. 2008). Activated carbon fibers (ACFs) are a flexible material in the form of silk, felt, cloth, etc. If it will be used as an industrial moving-bed denitration catalyst, the problem of wear and tear among the catalyst particles will be avoided. Transition metal oxides, especially manganese oxides (Sun et al. 2018b; Yan et al. 2018; Zhang et al. 2018) and cerium dioxide (Hu et al. 2018; Ma et al. 2018), have been proven to be effective for low-middle-temperature NH3-SCR.
Liu et al. (2013) prepared MnCe catalysts by the surfactant template method, and 100% NOx conversion (at 100~200 °C) was achieved by the best catalyst. The research proved that increasing the content of Mn is helpful to improve the low-temperature activity of the MnCe catalyst, and the doping of Ce can increase the N2 selectivity. The appropriate proportion of the MnCe catalyst has the high surface area and the small active sites, which can promote the adsorption and activation of NH3 and NOx. Liu et al. (2018a) also found that the strong interaction between the different metals is benefited to improve the catalytic activity, and Ce easily interacts with other metals. The synergetic effect between Ce and Sn is favorable to enhance the redox property and increase the Lewis acidity, thus improving the NH3-SCR performance of the Ce-Sn binary oxide catalyst. Moreover, a novel Ni-Ce-Ti–mixed oxide catalyst prepared by hydrothermal showed higher NH3-SCR activity in a wide temperature range (250~450 °C). The synergetic effect between Ni and Ce can account for the excellent NH3-SCR catalytic activity of the Ni0.3Ce0.1Ti0.6 catalyst because of more oxygen vacancies and high redox property by the redox cycles (Ni3+ + Ce3+ ↔ Ni2+ + Ce4+) (Liu et al. 2018b). Recently, ACFs as the carrier were studied widely for the low-middle-temperature NH3-SCR, such as MnOx/ACF (Lu et al. 2016; Wang et al. 2014), CeO2/ACF (Lu et al. 2010; Zhu et al. 2011), and MnOx-CeO2/ACF (Li et al. 2015a, 2015b; Lu et al. 2016). Zhu et al. (2011) prepared the CeO2/ACFN catalyst that exhibited good catalytic activity, which obtained 94.0% NO conversion in the range of 150~270 °C. The MnOx-CeOx/ACFN catalyst studied by Shen et al. (2008) showed the best catalytic activity (nearly 100% NO conversion) at 100~150 °C. It is worth noting that the modification of MnOx-CeOx/support catalysts by doping transition metals proved to improve catalytic activity such as Fe or Co doped Mn-Ce/TiO2 catalyst (Xu et al. 2017; Shen et al. 2018). However, to the best knowledge of the authors, this research is among the very few publications on Fe-doped MnOx-CeOx/ACFN catalysts for broadening its low-middle-temperature window.
In this work, the modified ACFs with nitric acid (40 wt%) which named ACFN was used to prepare a series of MnOx/ACFN, M-MnOx/ACFN (M = Ce, Cu, Co, Cr, Ni, Fe), and Fe-Ce-MnOx/ACFN catalysts. The relationship between the surface property and catalytic activity of the catalysts was studied by means of scanning electron microscopy (SEM), X-ray diffraction (XRD), BET, H2-TPR, NH3-TPD, and X-ray photoelectron spectroscopy (XPS). Fourier transform infrared (FT-IR) spectroscopy was used to explore the effect of H2O on the catalyst. The aim is to research the effect of transition metal on MnOx/ACFN and promote catalytic activity and water resistance of Ce-MnOx/ACFN catalysts by Fe doping at the different molar ratios of Fe/Ce for low-middle-temperature NH3-SCR of NOx. Thus, the successful application of activated carbon fibers in the field of industrial moving bed denitrification is realized.
Experimental section
Catalyst preparation
The rayon-based ACF (5 cm × 5 cm) pretreated with 40 wt% HNO3 solution (noted as ACFN) was used as the supporter, and the following analytical grade reagents: Mn(CH3COO)2·4H2O, Ce(NO3)3·6H2O, and Fe(NO3)3·9H2O were used as the precursors of metal oxides to modified ACFN. Firstly, the MnOx/ACFN was prepared via the ultrasonic impregnation method (Huang et al. 2008; Marbán et al. 2003; Shen et al. 2008). Meanwhile, the optimum mass load (0%, 10%, 15%, 20%) of the MnOx/ACFN and the calcination temperature (400 °C, 500 °C, 600 °C, in N2 stream) were studied. Then, the MnOx/ACFN was modified with Ce at different Ce/Mn molar ratios and tested for SCR activity at low-middle temperature. Different doping elements (Cu, Co, Cr, Ni, and Fe) were screened with reference to the Ce/Mn optimal molar ratio. Finally, a trace amount of Fe-doped Ce-MnOx/ACFN catalysts was prepared and the optimum molar ratio was screened. The typical catalysts were investigated for improved SCR performances (activity and water resistance) at low-middle temperature.
Catalytic performance
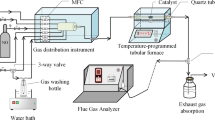
The catalytic performance was evaluated by a quartz fix-bed continuous-flow reactor (i.d. 7 mm) containing 0.1 g of the catalyst. Each flue gas component was accurately controlled by a mass flow controller to ensure the total flow rate of 200 mL min−1, corresponding to a mass hour space velocity that can be expressed as W/F = 0.5 mg (mL min−1)−1. Meanwhile, the filling height of the catalyst is controlled to ensure that the gas volume space velocity is constant at 20,000 h−1. The composition of reaction gas is as follows: 500 ppm NO, 500 ppm NH3, 5 vol% O2, and balance gas of N2. The concentration of NOx in inlet and outlet gas was analyzed by an online FT-IR spectrometer (iS50). The NOx conversion was calculated as follows:
Catalyst characterization
A SU8010 electron microscope was used for the SEM. The specific surface area of the samples was analyzed for the Brunauer-Emmett-Teller (BET) method with an Autosorb-iQ. The crystal structure of the samples was analyzed by powder XRD using a TTR-III system with CuKα (λ = 0.154 nm) radiation, and the scanning range was 10~90° (2θ) with a 10° min−1 sampling interval. Temperature-programmed reduction with hydrogen (H2-TPR) was performed using an Extrel MAX300 (Extrel, USA) apparatus, and the heating rate is 10 °C min−1. Temperature-programmed desorption of ammonia (NH3-TPD) was performed using the Extrel MAX300 (Extrel, USA) apparatus. The heating rate of the samples is 10 °C min−1. XPS was measured at ambient temperature by a Thermo Scientific Escalab 250Xi using Al Kα radiation (hv = 1486.6 eV). FT-IR spectroscopy spectra results of the samples were obtained from a FT-IR spectrometer (iS50).
Results and discussion
NH3-SCR performance
The NOx conversion of X wt% MnOx/ACFN catalysts (X = 0, 10, 15, and 20) is shown in Fig. 1 a. The pure ACFN was mostly inactive from 100 to 300 °C, where the best NOx conversion of only 20.1% was observed at 150 °C. The higher NOx conversion was about 60.0% at 175 °C for 10 wt% MnOx/ACFN. The 15 wt% MnOx/ACFN catalyst showed the best SCR activity, and the NOx conversion was about 40.4% at 100 °C and increased to 80.7% at 175 °C, while the activity was reduced to 48.2% at a middle temperature of up to 300 °C. As the mass load increased to 20 wt%, the best SCR activity of 66.0% NOx conversion was achieved at 175 °C. This phenomenon might result from the occupation of active sites and the blockage of surface pores over the overload MnOx/ACFN catalysts (Shen et al. 2008; Zhu et al. 2011). The SCR activity of 15 wt% MnOx/ACFN catalysts calcined at different temperature varied with the reaction temperature (100~300 °C) is shown in Fig. 1 b. The NOx conversion of the sample calcined at 500 °C was higher than that of the other samples. In the reaction, the main reason for the deactivation of MnOx/ACFN catalysts is due to the incomplete decomposition of metal salts or the agglomeration of active oxides. Therefore, the proper calcination temperature is especially important for the catalytic activity of catalysts.
Thus, in the further studies, the optimum total mass load of metal oxides was set as 15% on the ACFN catalyst, and the calcination temperature was optimized as 500 °C for 6 h in the stream of nitrogen.
Figure 2 a illustrates that the catalytic activity of Ce-MnOx/ACFN catalysts gradually increased with an increase of Mn/Ce ratio. The Ce(3)-MnOx(7)/ACFN catalyst showed good low-temperature activity at 100~200 °C, and up to 87.7% NOx conversion at 175 °C was achieved. As reported by Wang et al. (2012), the presence of CeO2 could not only promote the dispersion of MnOx but also result in different oxidation states of manganese, which could be beneficial to improve the activity of the NH3-SCR catalyst. Furthermore, the presence of CeO2 in Mn-based catalysts could improve the oxygen storage capacity and oxygen mobility, thus facilitating the catalytic process via the fast SCR.
Figure 2 b shows the NOx conversion of MnOx/ACFN catalysts doped with several elements based on the optimal molar ratio of Mn/Ce. As shown above, the Ce-MnOx/ACFN catalyst showed higher activity than other catalysts at low temperature, but its catalyst activity is poorer than that of others in the middle-temperature range. Fe-MnOx/ACFN and Cr-MnOx/ACFN catalysts had better SCR activity at middle temperature (200~300 °C). However, Cr is a highly toxic heavy metal, which has harmful effects on the human health and environment, so the use of chromium-containing catalysts is highly restricted.
Many studies had found that FeOx as the main active component or auxiliary agent of SCR denitration catalyst can remarkably improve catalytic activity (Xu et al. 2017). Two ways were used to control the molar amount of doped Fe for broadening the temperature window of the Ce-MnOx/ACFN catalyst. One way was to adjust the doping amount of Ce and Fe on the basis of constant Mn molar number. As shown in Fig. 3 a, the change trend of Fe(0.5)-Ce(2.5)-MnOx(7)/ACFN, Fe(1)-Ce(2)-MnOx(7)/ACFN, and Fe(2)-Ce(1)-MnOx(7)/ACFN was similar, and as compared to Ce(3)-MnOx(7)/ACFN, the catalysts showed well-broadening catalytic activity which was about 85.4% at 200 °C. Another way was to add trace Fe on the premise that the doping amount of Mn and Ce remains unchanged; as shown in Fig. 3 b, Fe(0.5)-Ce(3)-MnOx(7)/ACFN has good low temperature performance, but its catalytic performance is poor at middle temperature. The Fe(1)-Ce(3)-MnOx(7)/ACFN catalyst showed 90.8% NOx conversion at 175 °C, which was only increased by 3.1% than Ce(3)-MnOx(7)/ACFN, but the catalytic performance at the middle-temperature (200~300 °C) level was significantly improved. On the basis of Fe(1)-Ce(3)-MnOx(7)/ACFN, any increase or decrease in the amount of Fe doping did not widen the catalyst temperature window. It is proved that Fe-modified Ce-MnOx/ACFN catalysts could enhance its catalytic activity at the low-middle temperature for NH3-SCR selectivity of NOx.
Figure 4 a compares the NOx conversion of MnOx/ACFN, Ce(3)-MnOx(7)/ACFN, and Fe(1)-Ce(3)-MnOx(7)/ACFN catalysts. It could be seen from Fig. 4 a that the addition of Ce availably promoted the activity of the MnOx/ACFN catalyst from 100 to 200 °C. Further doping of trace Fe could obviously enhance the NOx conversion of the Ce(3)-MnOx(7)/ACFN catalyst in the middle-temperature range (200~300 °C), thereby widening the temperature window of the Fe(1)-Ce(3)-MnOx(7)/ACFN catalyst. Figure 4 b shows the N2 selectivity of MnOx/ACFN, Ce(3)-MnOx(7)/ACFN, and Fe(1)-Ce(3)-MnOx(7)/ACFN catalysts. It was found that the N2 selectivity decreased with the increase of temperature. Throughout the temperature range, the Fe(1)-Ce(3)-MnOx(7)/ACFN catalyst showed good N2 selectivity. It can be concluded that the Fe(1)-Ce(3)-MnOx(7)/ACFN catalyst not only possesses high De-NOx property but also has high N2 selectivity.
SEM and XRD analysis
Figure 5 shows SEM images of the samples, in which the ACFN was uniformly decorated with metal oxides. As shown in Fig. 5 a, the surface of ACFN was smooth and clean, which proved that nitric acid treatment could remove impurities from the ACFN surface. After loading Mn, Ce, and Fe oxides, the ACFN surface became rougher (Fig. 5b–d). For MnOx/ACFN, the surface of ACFN had some agglomerations of MnOx, which might be relevant to incomplete modification (Fig. 5b) (Zhu et al. 2011). For Ce(3)-MnOx(7)/ACFN and Fe(1)-Ce(3)-MnOx(7)/ACFN, active phases were well dispersed on the surface of ACFN, which indicated that the doping of Ce and Fe contributed to dispersion of active components (Fig. 5c, d), but there were still some larger particles in the former. As compared to the MnOx/ACFN and Ce(3)-MnOx(7)/ACFN catalysts, the Fe(1)-Ce(3)-MnOx(7)/ACFN catalyst showed more apparent uniform and fine particles adhering to the surface of the catalyst, which resulted in the good catalytic activity of the catalyst.
The powder X-ray diffraction patterns of ACFN, MnOx/ACFN, Ce(3)-MnOx(7)/ACFN, and Fe(1)-Ce(3)-MnOx(7)/ACFN catalysts in the 2θ range of 10°–90° are shown in Fig. 6. For the ACFN, the intense and sharp diffractions at 23.9° and 43.8° could be primarily attributed to graphite charcoal (Zhu et al. 2011), which indicated that ACFN is highly graphitized. After loading MnOx, the peak intensity of graphite became small and flattened, which illustrated that there was a strong interaction of MnOx and ACFN (Lu et al. 2015). The interaction between MnOx and CeO2 was stronger, so the diffraction peaks of graphite were also detected on the Ce(3)-MnOx(7)/ACFN catalyst. The diffraction peaks corresponding to the CeO2 were detected on the surface of Ce(3)-MnOx(7)/ACFN and Fe(1)-Ce(3)-MnOx(7)/ACFN catalysts at 29.1° and 57.2°, respectively (Chang et al. 2012), but the MnOx and FeOx diffraction peaks did not appeared, which implies that the metal oxides were well distributed on the surface of the support. The above conclusion is also proved by SEM images (Fig. 5).
BET analysis
Figure 7 a shows the nitrogen adsorption-desorption isotherms of the samples. All the adsorption isotherm curves exhibited type I, and there was no obvious hysteresis loop on the basis of the IUPAC classification (Thommes et al. 2015), which suggested that they were predominantly microporous materials. The surface areas and pore parameters of ACF, ACFN, MnOx/ACFN, Ce(3)-MnOx(7)/ACFN, and Fe(1)-Ce(3)-MnOx(7)/ACFN catalysts are listed in Table 1, which shows that the surface area of all catalysts is above 1000 m2 g−1. Figure 7 b illustrates the pore diameter distribution of all the samples. As shown in Table 1, the nitric acid modification could increase the micropore surface area (Smicro) but reduce the external surface area (Sexternal) and mesopore surface area (Smeso) obviously. When ACFN supported the Mn, Ce, and Fe, the SBET and Smicro of MnOx/ACFN, Ce(3)-MnOx(7)/ACFN, and Fe(1)-Ce(3)-MnOx(7)/ACFN declined in different degrees, which might be due to the dispersion of metal oxide species on the surface of the carrier or micropore which was blocked by the metal species (Huang et al. 2008; Zhu et al. 2011), while the Fe(1)-Ce(3)-MnOx(7)/ACFN catalyst exhibited better low-middle-temperature SCR performance, probably due to the abundant micropores in the catalyst. As it can be seen from Table 1, doping of Fe enlarged the micropore-specific surface area and micropore volume of the Fe(1)-Ce(3)-MnOx(7)/ACFN catalyst, thereby improving the low-middle-temperature SCR activity.
H2-TPR analysis
The oxidation states of MnOx/ACFN, Ce(3)-MnOx(7)/ACFN, and Fe(1)-Ce(3)-MnOx(7)/ACFN catalysts are shown in Fig. 8. MnOx/ACFN showed three reduction peaks at 341 °C, 422 °C, and 480 °C, corresponding to MnO2 → Mn2O3, Mn2O3 → Mn3O4, and Mn3O4 → MnO, respectively (Chen et al. 2016; Ramesh et al. 2008; Tian et al. 2011). For the Ce(3)-MnOx(7)/ACFN catalyst, the peak at 335 °C could be regarded as the transformation of MnO2 → Mn2O3, the peak at 424 °C belonged to Mn2O3 → Mn3O4, and the peak around 489 °C was regarded as the reduction of Mn3O4 → MnO (Fang et al. 2017). And, the reduction peak from 520 to 670 °C was classified to CeO2 → Ce2O3, consisting with the description of Liu et al. (2014). For the Fe(1)-Ce(3)-MnOx(7)/ACFN catalyst, the peak at 337 °C and 415 °C could be attributed to the change of MnO2 → Mn2O3 and Mn2O3 → Mn3O4, respectively (Cao et al. 2015; Fang et al. 2017; Li et al. 2015a, 2015b). The peak centered at 708 °C was ascribed to the process of Fe3O4 → FeO (France et al. 2017). Compared with the other catalysts, the reduction peaks of MnOx shifted to lower-temperature regions, Zuo et al. (2009) thought that it was due to the good dispersion and tiny crystal size of MnOx. In addition, the Fe(1)-Ce(3)-MnOx(7)/ACFN catalyst showed the largest H2 consumption than the Ce(3)-MnOx(7)/ACFN catalyst followed by the MnOx/ACFN catalyst in the whole reaction temperature. It is concluded that the former exposed more reductive species, thus improving the redox ability of the catalyst (Gao et al. 2019). The doping of Fe can promote the formation of more surface-chemisorbed oxygen, thereby contributing to low-middle-temperature catalytic activity of the catalysts (Ma et al. 2018; Tang et al. 2016). This H2-TPR result is consistent with the XPS (Fig. 10).
NH3-TPD analysis
The adsorption and activation of NH3 on the surface acid sites of catalysts are viewed as a major process in NH3-SCR reaction (Zhang et al. 2013). The surface acidity and strength of the catalysts obtained from NH3-TPD curves are shown in Fig. 9. All the catalysts showed at least two desorption peaks. The NH3 desorption amounts of different catalysts are shown in Table 2. According to relevant literatures, the peaks around 100~200 °C were attributed to the ammonia which was physically adsorbed in the weak acid sites, the peaks at 200~500 °C led to NH3 desorption on the medium acidic sites, and the peaks above 500 °C were assigned to NH3 desorption on the strong acidic sites (Jiang et al. 2018; Xu et al. 2017). As shown in Table 2, the Fe(1)-Ce(3)-MnOx(7)/ACFN catalyst revealed the much larger area, especially at the high-temperature region, indicating the presence of abundant acid sites on the surface of the catalyst. The NH3-TPD results demonstrated that doping Fe enhanced strong acid amount, which may be favorable to the NH3-SCR reaction (Cao et al. 2015). Strong acid sites were dominant on the surface of Fe(1)-Ce(3)-MnOx(7)/ACFN catalysts.
XPS analysis
The different XPS spectra of Mn 2p, Ce 3d, Fe 2p, and O 1s are shown in Fig. 10, and the surface relative atomic ratios of Mn, Ce, Fe, and O are shown in Table 3.
From Fig. 10 a, Mn 2p spectra have two peaks including Mn 2p1/2 and Mn 2p3/2. By performing peak-fitting deconvolutions, the Mn 2p3/2 spectra were divided into Mn2+ (641.2 eV ± 0.1 eV), Mn3+ (642.5 eV ± 0.1 eV), and Mn4+ (645.0 eV ± 0.2 eV) (You et al. 2017; Zhou et al. 2016). From Table 2, the relative atomic ratio of Mn4+ increased from 47.6% of MnOx/ACFN to 48.6% of Ce(3)-MnOx(7)/ACFN. After doping with Fe, the relative atomic ratio of Mn4+ increased to 53.1% of Fe(1)-Ce(3)-MnOx(7)/ACFN. From the H2-TPR result shown in Fig. 8, it was generally thought that Mn4+ was propitious to the transformation of NH3 to NH2 (Wan et al. 2014). Cao et al. (2014) reflected that the coexistence of Mn4+ and Mn3+ could promote the catalytic activity of catalysts. It could prove that doping of Fe could provide a positive impact on increasing the relative content of Mn4+, thereby accelerating the NH3-SCR reaction.
The XPS spectra of Ce 3d are obtained in Fig. 10 b, which mainly contained the Ce 3d3/2 and Ce 3d5/2 spectra. The result indicated that Ce 3d orbit was represented by the multiple of v and u. The v, v″, and v′″ and u, u″, and u′″ peaks belonged to Ce4+ species, and v′ and u′ belonged to Ce3+ species (Sun et al. 2018c). From these peaks, it showed that the Fe(1)-Ce(3)-MnOx(7)/ACFN catalyst was mainly in the Ce4+ oxidation state and Ce4+ and Ce3+ coexist on the catalyst surface. It was reported that the Ce4+ could promote the oxygen storage and release by the valence state transformation of Ce3+ and Ce4+ (Lu et al. 2018). After doping of Ce, the content of Mn4+ and Ce4+ increased, while the doping of Fe had a little effect on the valence changes of Ce.
The Fe 2p spectra of the Fe(1)-Ce(3)-MnOx(7)/ACFN catalyst are shown in Fig. 10 c. By peak-fitting deconvolution, the spectrum peaks of Fe 2p3/2 and Fe 2p1/2 located at 710.5 eV, 715.1 eV, and 722.1 eV are attributed to Fe3+ (Li et al. 2015a, 2015b). In addition, the Fe2+ peaks (712.6 eV) are presented in the Fe 2p3/2 peak. As shown in Table 3, Fe3+ is the main present form of Fe element with a relative surface content of 76.7%. The result was consistent with H2-TPR profiles (Fig. 8). The strong interaction between Fe and Mn led to the change of Fe2+ and Fe3+, which promoted the generation of oxygen vacancy on the surface of the catalysts, thereby facilitating the low-middle SCR activity (Lu et al. 2018).
O 1s XPS spectrum is shown in Fig. 10 d. The peak at 529.8 eV ± 0.2 eV belonged to lattice oxygen (Oβ); the peak at 531.3 eV ± 0.1 eV was part of chemisorbed oxygen and weakly bonded oxygen species (Oα); the binding energy of 533.0 eV ± 0.1 eV was considered to be oxygen in surface-adsorbed water species (Oγ). Table 3 shows the relative atomic ratio of oxygen species calculated through the peak area. The content of Oβ in Ce(3)-MnOx(7)/ACFN (26.7%) was higher than that in MnOx/ACFN (12.3%). Ce doping not only improved the redox performance of the MnOx/ACFN catalyst but also facilitated the adsorption-desorption reversible cycle of Oβ. The concentration of Oα in Fe(1)-Ce(3)-MnOx(7)/ACFN (46.5%) was higher than that in MnOx/ACFN (39.0%) and Ce(3)-MnOx(7)/ACFN (35.8%), while the concentration of Oβ decreased to 14.6%. Studies have shown that surface-chemisorbed oxygen was more active owing to the higher mobility (Jiang et al. 2018; Zhang et al. 2013). The high concentration of chemisorbed oxygen can promote the redox cycle and enhance the oxidation of NO to NO2 (Cao et al. 2015; Shen et al. 2017). Through above analysis, it is found that Fe had a positive effect on the transformation of gas-phase oxygen to chemisorbed oxygen.
The water resistance and stability of the catalysts
The gas boiler is one of the main sources of stationary NOx, which has low smoke temperature and higher water vapor content. The exhaust flue gas emitted from the gas boiler containing water vapor (H2O) is one of the important factors leading to the poisoning of catalysts at low-middle temperature. Figure 11 shows the NOx conversion of MnOx/ACFN, Ce(3)-MnOx(7)/ACFN, and Fe(1)-Ce(3)-MnOx(7)/ACFN catalysts in the existence of 10 vol% H2O. Before getting into the H2O resistance test, the catalytic activity was maintained at 175 °C for 40 min. The NOx conversion of MnOx/ACFN, Ce(3)-MnOx(7)/ACFN, and Fe(1)-Ce(3)-MnOx(7)/ACFN catalysts was 80.0%, 87.2%, and 90.5%, respectively. When 10 vol% H2O was added into the flue gas, the NOx conversion immediately decreased to 60.0%, 68.1%, and 76.0% for the MnOx/ACFN, Ce(3)-Mn(7)/ACFN, and Fe(1)-Ce(3)-MnOx(7)/ACFN catalysts in 5.5 h, respectively. The H2O was removed from the flue gas after undergoing 5.5 h, and the catalytic activity of the catalysts was recovered to its the original level. The H2O exhibited a reversible effect on the activities of the catalysts. It is possible that H2O had the competitive adsorption with NH3, which lead to a decrease in catalytic performance (Tang et al. 2018). Figure 12 presents the difference of functional groups in the fresh Fe(1)-Ce(3)-MnOx(7)/ACFN catalyst and H2O-resisted Fe(1)-Ce(3)-MnOx(7)/ACFN catalyst. The band at 3440 cm−1 belonged to the surface –OH group, which proved the presence of H2O (Wu et al. 2007). The band around 1640 cm−1 was caused by the surface C=O species. The band around 1199 cm−1 was attributed to the coordinated NH3 on Lewis acid sites (Li et al. 2018a, 2018b). It is obviously found that the peak around 1199 cm−1 became weaker after the introduction of H2O, which indicated the competitive adsorption between Lewis NH3 and H2O. The band at 1400 cm−1 was assigned to NH4+ adsorbed on Brønsted acid sites (Yu et al. 2017). The intensity of NH4+ peak became strong when H2O was injected into the gas, indicating that the introduction of H2O brought about more NH3 absorbed in Brønsted acid sites. Meanwhile, it can be found that the peak intensity at 1400 cm−1 just increased slightly, so it is inferred that the addition of Fe slowed down the interaction between H2O and Brønsted acid sites, thus improving the water resistance of the Fe(1)-Ce(3)-MnOx(7)/ACFN catalyst.
The stability of catalysts has an important application value in the field of practical industrial denitration, so we conducted the stability test of the Fe(1)-Ce(3)-MnOx(7)/ACFN catalyst, and Fig. 13 depicts the test result at 175 °C. The NOx conversion maintained a stable level of about 90.0% during the tested 10 h and then decreased slightly. It is speculated that the stability was closely related to the surface properties of catalysts.
Conclusion
The metal doping type and molar ratio have a significant impact on the NH3-SCR activity of catalysts. For Ce-MnOx/ACFN catalysts, the doping of Fe significantly broadened the temperature window of catalysts. Excellent catalytic performance of the Fe(1)-Ce(3)-MnOx(7)/ACFN catalyst mainly attributed to the interaction among Mn, Ce, and Fe. A high concentration of Mn4+, Ce4+, Fe3+, and chemisorbed oxygen can enhance catalytic activity. The characterization results exhibited that Fe-doped Ce(3)-MnOx(7)/ACFN could promote the dispersion of active components, improve the redox performance, and increase the acid amount. Meanwhile, the 15 wt% Fe(1)-Ce(3)-MnOx(7)/ACFN catalyst showed good water resistance and stability at 175 °C. This research is of great significance to the application of activated carbon fibers in the field of industrial denitrification, especially the denitrification of gas-fired boilers.
References
Anthonysamy SBI, Binti AS, Mehrnoush K, Mohamed ARB (2018) A review of carbon-based and non-carbon-based catalyst supports for the selective catalytic reduction of nitric oxide. Beilstein J. Nanotechnol 9:740–761. https://doi.org/10.3762/bjnano.9.68
Cao F, Xiang J, Su S, Wang PY, Sun LS, Hu S, Lei SY (2014) The activity and characterization of MnOx-CeO2-ZrO2/γ-Al2O3 catalysts for low temperature selective catalytic reduction of NO with NH3. Chem Eng J 243:347–354. https://doi.org/10.1016/j.apcatb.2013.10.049
Cao F, Su S, Xiang J, Wang PX, Hu S, Sun LS, Zhang AC (2015) The activity and mechanism study of Fe-Mn-Ce/γ-Al2O3 catalyst for low temperature selective catalytic reduction of NO with NH3. Fuel 139:232–239. https://doi.org/10.1016/j.fuel.2014.08.060
Chang HZ, Li JH, Chen XY, Ma L, Yang SJ, Schwank JW, Hao JM (2012) Effect of Sn on MnOx-CeO2 catalyst for SCR of NOx by ammonia: enhancement of activity and remarkable resistance to SO2. Catal Commun 27:54–57. https://doi.org/10.1016/j.catcom.2012.06.022
Chen QL, Guo RT, Wang QS, Pan WG, Wang WH, Yang NZ, Lu CZ, Wang SX (2016) The catalytic performance of Mn/TiWOx catalyst for selective catalytic reduction of NOx with NH3. Fuel 181:852–858. https://doi.org/10.1016/j.fuel.2016.05.045
Fan XY, Yang HS, Tian W, Nie AM, Hou TF, Qiu FM, Zhang XB (2011) Catalytic oxidation of chlorobenzene over MnOx/Al2O3-carbon nanotubes composites. Catal Letter 141(1):158–162. https://doi.org/10.1007/s10562-010-0450-9
Fan ZY, Shi JW, Gao C, Gao G, Wang BR, Wang Y, He C, Niu CM (2018) Gd-modified MnOx for the selective catalytic reduction of NO by NH3: the promoting effect of Gd on the catalytic performance and sulfur resistance. Chem Eng J 348:820–830. https://doi.org/10.1016/j.cej.2018.05.038
Fang NJ, Guo JX, Shu S, Luo HD, Chu YH, Li JJ (2017) Enhancement of low-temperature activity and sulfur resistance of Fe0.3Mn0.5Zr0.2 catalyst for NO removal by NH3-SCR. Chem Eng J 325:114–123. https://doi.org/10.1021/am5038164
France LJ, Yang Q, Li W, Chen ZH, Guang JY, Guo DW, Wang LF, Li XH (2017) Ceria modified FeMnOx-enhanced performance and sulphur resistance for low-temperature SCR of NOx. Appl Catal B Environ 206:203–215. https://doi.org/10.1016/j.apcatb.2017.01.019
Gao FY, Tang XL, Yi HH, Li JY, Zhao SZ, Wang JG, Chu C, Li CL (2017a) Promotional mechanisms of activity and SO2 tolerance of Co- or Ni-doped MnOx-CeO2 catalysts for SCR of NOx with NH3 at low temperature. Chem Eng J 317:20–31. https://doi.org/10.1016/j.cej.2017.02.042
Gao FY, Tang XL, Yi HH, Zhao SZ, Li CL, Li JY, Shi YR, Meng XM (2017b) A review on selective catalytic reduction of NOx by NH3 over Mn-based catalysts at low temperatures: catalysts, mechanisms, kinetics and DFT calculations. Catalysts 7(7):199. https://doi.org/10.3390/catal7070199
Gao FY, Tang XL, Yi HH, Zhao SZ, Wang JG, Gu T (2019) Improvement of activity, selectivity and H2O&SO2-tolerance of micro-mesoporous CrMn2O4 spinel catalyst for low-temperature NH3-SCR of NOx. Appl Surf Sci 466:411–424. https://doi.org/10.1016/j.apsusc.2018.09.227
Grzybek T, Klinik J, Motak M, Papp H (2008) Nitrogen-promoted active carbons as catalytic supports. Catal Today 137(2-4):235–241. https://doi.org/10.1016/j.cattod.2007.11.006
Hu G, Yang J, Tian YM, Kong BW, Liu QC, Ren S, Li JL, Kong M (2018) Effect of Ce doping on the resistance of Na over V2O5-WO3/TiO2 SCR catalysts. Mater Res Bull 104:112–118. https://doi.org/10.1016/j.materresbull.2018.04.009
Huang HC, Ye DQ, Huang BC, Wei ZL (2008) Vanadium supported on viscose-based activated carbon fibers modified by oxygen plasma for the SCR of NO. Catal Today 139(1-2):100–108. https://doi.org/10.1016/j.cattod.2008.08.028
Jiang LJ, Liu QC, Zhao Q, Ren S, Kong M, Yao L, Meng F (2018) Promotional effect of Ce on the SCR of NO with NH3 at low temperature over MnOx supported by nitric acid-modified activated carbon. Res Chem Intermed 44(3):1729–1744. https://doi.org/10.1007/s11164-017-3194-y
Li P, Lu P, Zhai Y, Li C, Chen T, Qing R, Zhang W (2015a) Low temperature SCR of NO with catalysts prepared by modified ACF loading Mn and Ce: effects of modification method. Environ Technol 36(18):2390–2400. https://doi.org/10.1080/09593330.2015.1031829
Li J, Yang CW, Zhang QQ, Li Z, Huang W (2015b) Effects of Fe addition on the structure and catalytic performance of mesoporous Mn/Al-SBA-15 catalysts for the reduction of NO with ammonia. Catal Commun 62:24–28. https://doi.org/10.1016/j.catcom.2015.01.003
Li YL, Han XJ, Hou YQ, Guo YP, Liu YJ, Cui Y, Huang ZG (2018a) Role of CTAB in the improved H2O resistance for selective catalytic reduction of NO with NH3 over iron titanium catalyst. Chem Eng J 347:313–321. https://doi.org/10.1016/j.cej.2018.04.107
Li G, Wang BD, Wang ZC, Li ZH, Sun Q, Xu WQ, Li YL (2018b) Reaction mechanism of low-temperature selective catalytic reduction of NOx over Fe-Mn oxides supported on fly-ash-derived SBA-15 molecular sieves: structure-activity relationships and in situ DRIFT analysis. J Phys Chem C 122(35):20210–20231. https://doi.org/10.1021/acs.jpcc.8b03135
Liu ZM, Yi Y, Zhang SX, Zhu TL, Zhu JZ, Wang JG (2013) Selective catalytic reduction of NOx with NH3 over Mn-Ce mixed oxide catalyst at low temperatures. Catal Today 216:76–81. https://doi.org/10.1016/j.cattod.2013.06.009
Liu ZM, Zhu JZ, Li JH, Ma L, Woo SI (2014) Novel Mn-Ce-Ti mixed-oxide catalyst for the selective catalytic reduction of NOx with NH3. Acs Appl Mater Inter 6:14500–14508. https://doi.org/10.1021/am5038164
Liu ZM, Feng X, Zhou ZZ, Feng YJ, Li JH (2018a) Ce-Sn binary oxide catalyst for the selective catalytic reduction of NOx by NH3. Appl Surf Sci 428:526–533. https://doi.org/10.1016/j.apsusc.2017.09.175
Liu ZM, Liu HY, Feng X, Ma LL, Cao XZ, Wang BY (2018b) Ni-Ce-Ti as a superior catalyst for the selective catalytic reduction of NOx with NH3. Mol Catal 445:179–186. https://doi.org/10.1016/j.mcat.2017.11.028
Lu P, Li CT, Zeng GM, He LJ, Peng DL, Cui HF, Li SH, Zhai YB (2010) Low temperature selective catalytic reduction of NO by activated carbon fiber loading lanthanum oxide and ceria. Appl Catal B-Environ 96:157–161. https://doi.org/10.1016/j.apcatb.2010.02.014
Lu XL, Zou HQ, Zhang YB, Qiu HF, Zheng YY (2015) Low-temperature selective catalytic reduction of NO over carbon nanotubes supported MnO2 fabricated by co-precipitation method. Micro Nano Lett 10(11):666–669. https://doi.org/10.1515/pac-2014-1117
Lu P, Xing Y, Li CT, Qing RP, Su W, Liu NA (2016) A reusable material with high performance for removing NO at room temperature: performance, mechanism and kinetics. Catal Sci Technol 6(10):3520–3528. https://doi.org/10.1039/c5cy01562f
Lu P, Li R, Xing Y, Li YR, Zhu TY, Yue HF, Wu WR (2018) Low temperature selective catalytic reduction of NOx with NH3 by activated coke loaded with FexCoyCezOm: the enhanced activity, mechanism and kinetics. Fuel 233:88–199. https://doi.org/10.1016/j.cej.2017.02.148
Ma L, Seo CY, Nahata M, Chen XY, Li JH, Schwank JW (2018) Shape dependence and sulfate promotion of CeO2 for selective catalytic reduction of NOx with NH3. Appl Catal B Environ 232:246–259. https://doi.org/10.1016/j.apcatb.2018.03.065
Marani D, Silva RH, Dankeaw A, Norrman K, Werchmeister RML, Ippolito D, Gudik-Sørensen M, Hansen KK, Esposito V (2017) NOx selective catalytic reduction (SCR) on self-supported V-W-doped TiO2 nanofibers. New J Chem 41(9):3466–3472. https://doi.org/10.1039/C6NJ03205B
Marbán G, Antu AR, Fuertes AB (2003) Low-temperature SCR of NOx with NH3 over activated carbon fiber composite-supported metal oxides. Appl Catal B Environ 41(3):323–338. https://doi.org/10.1016/S0926-3373(02)00170-4
Meng DM, Xu Q, Jiao YL, Guo Y, Guo Y, Wang L, Lu GZ, Zhan WC (2018) Spinel structured CoaMnbOx mixed oxide catalyst for the selective catalytic reduction of NOx with NH3. Appl Catal B Environ 221:652–663. https://doi.org/10.1016/j.apcatb.2017.09.034
Ramesh K, Chen LW, Chen FX, Liu Y, Wang Z, Han YF (2008) Re-investigating the CO oxidation mechanism over unsupported MnO, Mn2O3 and MnO2 catalysts. Catal Today 131(1-4):477–482. https://doi.org/10.1016/j.cattod.2007.10.061
Shen BX, Liu T, Shi ZL, Shi JW, Yang TT, Zhao N (2008) Low-temperature selective catalytic reduction of NO with NH3 based on MnOx-CeOx/ACFN. Front Chem Eng China 2(3):325–329. https://doi.org/10.1007/s11705-008-0053-9
Shen Q, Zhang LY, Sun NN, Wang H, Zhong LS, He C, Wei W, Sun YH (2017) Hollow MnOx-CeO2 mixed oxides as highly efficient catalysts in NO oxidation. Chem Eng J 322:46–55. https://doi.org/10.1016/j.cej.2017.02.148
Shen BX, Zhu SW, Zhang X, Chi GL, Patel D, Si M, Wu CF (2018) Simultaneous removal of NO and Hg0 using Fe and Co co-doped Mn-Ce/TiO2 catalysts. Fuel 224:241–249. https://doi.org/10.1016/j.fuel.2018.03.080
Sun X, Guo RT, Liu SW, Liu J, Pan WG, Shi X, Qin H, Wang ZY, Qiu ZZ, Liu XY (2018a) The promoted performance of CeO2 catalyst for NH3-SCR reaction by NH3 treatment. Appl Surf Sci 462:187–193. https://doi.org/10.1016/j.apsusc.2018.08.114
Sun P, Huang SX, Guo RT, Li MY, Liu SM, Pan WG, Fu ZG, Liu SW, Sun X, Liu J (2018b) The enhanced SCR performance and SO2 resistance of Mn/TiO2 catalyst by the modification with Nb: a mechanistic study. Appl Surf Sci 447:479–488. https://doi.org/10.1016/j.apsusc.2018.03.245
Sun CZ, Liu H, Chen W, Chen DZ, Yu SH, Liu AN, Dong L, Feng S (2018c) Insights into the Sm/Zr co-doping effects on N2 selectivity and SO2 resistance of a MnOx-TiO2 catalyst for the NH3-SCR reaction. Chem Eng J 347:27–40. https://doi.org/10.1016/j.cej.2018.04.029
Tang AD, Hu LQ, Yang XH, Jia YR, Zhang Y (2016) Promoting effect of the addition of Ce and Fe on manganese oxide catalyst for 1,2-dichlorobenzene catalytic combustion. Catal Commun 82:41–45. https://doi.org/10.1016/j.catcom.2016.04.015
Tang XL, Li CL, Yi HH, Wang LF, Yu QJ, Gao FY, Cui XX, Chu C, Li JY, Zhang RC (2018) Facile and fast synthesis of novel Mn2CoO4@rGO catalysts for the NH3-SCR of NOx at low temperature. Chem Eng J 333:467–476. https://doi.org/10.1016/j.cej.2017.09.179
Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing KS (2015) (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl. Chem. 87(9-10):1051–1069. https://doi.org/10.1515/pac-2014-1117
Tian W, Yang HS, Fan XY, Zhang XB (2011) Catalytic reduction of NOx with NH3 over different-shaped MnO2 at low temperature. J Hazard Mater 188(1-3):105–109. https://doi.org/10.1016/j.jhazmat.2011.01.078
Wan YP, Zhao WR, Tang Y, Li L, Wang HJ, Cui YL, Gu JL, Li YS, Shi JL (2014) Ni-Mn bi-metal oxide catalysts for the low temperature SCR removal of NO with NH3. Appl Catal B Environ 148-149:114–122. https://doi.org/10.1016/j.apcatb.2013.10.049
Wang YL, Ge CZ, Zhan L, Li C, Qiao WM, Ling LC (2012) MnOx-CeO2/activated carbon honeycomb catalyst for selective catalytic reduction of NO with NH3 at low temperatures. Ind Eng Chem Res 51(36):11667–11673. https://doi.org/10.1021/ie300555f
Wang MX, Liu HN, Huang ZH, Kang FY (2014) Activated carbon fibers loaded with MnO2 for removing NO at room temperature. Chem Eng J 256:101–106. https://doi.org/10.1016/j.cej.2014.06.108
Wang WT, Herreros JM, Tsolakis A, York APE (2015) Increased NO2 concentration in the diesel engine exhaust for improved Ag/Al2O3 catalyst NH3-SCR activity. Chem Eng J 270:582–589. https://doi.org/10.1016/j.cej.2015.02.067
Wang DH, Yao Q, Hui SE, Niu YQ (2018) N2O and NO formation from NH3 oxidation over MnO2/TiO2 catalysts. Fuel 234:650–655. https://doi.org/10.1016/j.fuel.2018.07.073
Wu ZB, Jiang B, Liu YQ, Wang HQ, Jin RB (2007) DRIFT study of manganese/titania-based catalysts for low-temperature selective catalytic reduction of NO with NH3. Environ Sci Technol 41:5812–5817. https://doi.org/10.1021/es0700350
Xu Q, Su RJ, Cao L, Li YQ, Yang CY, Luo Y, Street J, Jiao PC, Cai LL (2017) Facile preparation of high-performance Fe-doped Ce-Mn/TiO2 catalysts for the low-temperature selective catalytic reduction of NOx with NH3. RSC Adv. 7(77):48785–48792. https://doi.org/10.1039/C7RA07854D
Yan QH, Chen SN, Zhang C, O’Hare D, Wang Q (2018) Synthesis of Cu0.5Mg1.5Mn0.5Al0.5Ox mixed oxide from layered double hydroxide precursor as highly efficient catalyst for low-temperature selective catalytic reduction of NOx with NH3. J Colloid Interface Sci 526:63–74. https://doi.org/10.1016/j.jcis.2018.04.099
You XC, Sheng ZY, Yu DQ, Yang L, Xiao XX, Wang SS (2017) Influence of Mn/Ce ratio on the physicochemical properties and catalytic performance of graphene supported MnOx-CeO2 oxides for NH3-SCR at low temperature. Appl Surf Sci 423:845–854. https://doi.org/10.1016/j.apsusc.2017.06.226
Yu CL, Huang BC, Dong LF, Chen F, Liu XQ (2017) In situ FT-IR study of highly dispersed MnOx/SAPO-34 catalyst for low-temperature selective catalytic reduction of NOx by NH3. Catal Today 281:610–620. https://doi.org/10.1016/j.cattod.2016.06.025
Zhang L, Zhang DS, Zhang JP, Cai SX, Fang C, Huang L, Li HR, Gao RH, Shi LY (2013) Design of meso-TiO2@MnOx-CeOx/CNTs with a core-shell structure as DeNOx catalysts: promotion of activity, stability and SO2-tolerance. Nanoscale 5(20):9821–9829. https://doi.org/10.1039/c3nr03150k
Zhang SG, Zhang BL, Liu B, Sun SL (2017) A review of Mn-containing oxide catalysts for low temperature selective catalytic reduction of NOx with NH3: reaction mechanism and catalyst deactivation. RSC Adv 7(42):26226–26242. https://doi.org/10.1039/c7ra03387g
Zhang SB, Zhao YC, Yang JP, Zhang JY, Zheng CG (2018) Fe-modified MnOx/TiO2 as the SCR catalyst for simultaneous removal of NO and mercury from coal combustion flue gas. Chem Eng J 348:618–629. https://doi.org/10.1016/j.cej.2018.05.037
Zhou LL, Li CT, Zhao LK, Zeng GM, Gao L, Wang Y, Yu ME (2016) The poisoning effect of PbO on Mn-Ce/TiO2 catalyst for selective catalytic reduction of NO with NH3 at low temperature. Appl Surf Sci 389:532–539. https://doi.org/10.1016/j.apsusc.2016.07.136
Zhu LL, Huang BC, Wang WH, Wei ZL, Ye DQ (2011) Low-temperature SCR of NO with NH3 over CeO2 supported on modified activated carbon fibers. Catal Commun 12(6):394–398. https://doi.org/10.1016/j.catcom.2010.10.028
Zuo SF, Huang QQ, Li J, Zhou RX (2009) Promoting effect of Ce added to metal oxide supported on Al pillared clays for deep benzene oxidation. Appl Catal B-Environ 91(1-2):204–209. https://doi.org/10.1016/j.apcatb.2009.05.025
Funding
This study was funded by the National Key R&D Program of China (2017YFC0210303-01), the National Natural Science Foundation of China (21806009), the project funded by the China Postdoctoral Science Foundation (2018M631344), the Fundamental Research Funds for the Central Universities (FRF-TP-18-019A1), and the program “Promoting the Cooperative Innovation Among Beijing, Tianjin, and Hebei” (Z161100002716025).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gu, T., Gao, F., Tang, X. et al. Fe-modified Ce-MnOx/ACFN catalysts for selective catalytic reduction of NOx by NH3 at low-middle temperature. Environ Sci Pollut Res 26, 27940–27952 (2019). https://doi.org/10.1007/s11356-019-05976-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05976-4