Abstract
Se can regulate Cd accumulation and translocation in plants; however, such effects can be controversial because of the differences in plant species and Se species. In this study, pak choi was cultured under hydroponic conditions, and the effects of selenite and selenate on Cd accumulation were investigated in the edible parts of this vegetable. The results showed gradual improvements in the effects of the two Se species on the Cd content in pak choi shoots at the four assessed growing stages. Selenite did not lead to significant changes in Cd accumulation in the shoots until day 40, when it significantly reduced the accumulation by 34%. Selenate was always found to increase the Cd content in the shoots, and the differences on days 19 and 40 were 16% and 45%, respectively, compared with those of the Cd (only) treatment. Accordingly, selenate invariably enhanced Cd translocation from the roots to the shoots, whereas selenite insignificantly reduced the translocation only on day 40. Generally, selenomethionine (SeMet) accounted for much larger proportions in selenite-treated plants, while SeO42− was the dominant Se species in selenate-treated plants. However, under both Se treatments, the SeMet proportion increased substantially from day 19 to day 40 when that of SeO42− exhibited a drastic decrease; therefore, the relative proportion of seleno-amino acids to SeO42− may be the key factor for the regulation of Cd accumulation in pak choi via treatment with selenite and selenate at the different growing stages.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cd contamination in soil represents a growing concern owing to its threat to the environment and human health. Cd, a highly toxic heavy metal, is not only able to induce renal and liver failure, osteoporosis, and gout, but it also interferes with processes of respiration and boosts oxidative stress and can thus potentially cause cancer in humans (Jarup 2002). Cd intake by humans mainly occurs via the food chain and is mostly attributed to the uptake by crops from the environment (Jarup 2002). Pak choi (Brassica chinensis L.) is one of the most consumed leafy vegetables in China; however, it is also a potential accumulator of Cd, among many other plant species (Bo and Chen 2013; Yang et al. 2009). Given the substantial vegetable consumption and, more importantly, the current situation of Cd soil contamination in vegetable-farming areas (Hu and Ding 2009; Sawut et al. 2018), it is of great urgency to reduce the Cd accumulation in pak choi.
Se has six valence states, but selenate (6+) and selenite (4+) are the most dominant species in the soil environment (Terry et al. 2000). Se is essential for several human enzymes (Bhabak and Mugesh 2010), and although it is not an essential element for plants, at optimal doses, Se can restore cellular structure and improve resistance, photosynthetic efficiency, and antioxidative defense in plants (Feng et al. 2013a, b; Haghighi and da Silva 2016; Yu et al. 2018), to which most researches attribute the antagonism between Se and Cd. Although numerous related studies have been conducted, variations in the doses of elements, plant species, applied Se species (mostly selenite and selenate), and exposure times can lead to controversial and even opposing results. For example, selenite and selenate both reduced the Cd content in Phaseolus mungo treated with 17.70 μmol L−1 of Cd under the hydroponic condition (Shanker et al. 1995); foliar application of selenite enhanced Cd accumulation in the leaves, stems, and roots of Nicotiana tabacum (Yu et al. 2017); however, contrasting effects were also achieved between the two inorganic Se species in other pak choi cultivars as well as in Spinacia oleracea under field conditions (Golubkina et al. 2017; Yu et al. 2018). Consequently, one of the aims of this study was to compare the effects of selenite and selenate on Cd accumulation and translocation in pak choi.
Because of the different charges and uptake transporters of Cd (Cd2+) and Se (SeO32−/ SeO42−), there is no uptake competition between the two elements (Smith et al. 1997; Li et al. 2008; Clemens et al. 2013); therefore, in vivo interactions are considered to be the only reason for Se affecting Cd accumulation in plants. As an analog to sulfur (S), Se is assimilated via the S pathways in plants, where selenate that is taken up is firstly activated to adenosine phosphoselenate before being reduced to selenite (Dilworth and Bandurski 1977; Burnell 1981; Pilon-Smits and Quinn 2010). After the reduction of selenate, Se is gradually reduced to selenide (− 2) and incorporated into seleno-amino acids, such as selenocysteine (SeCys) and selenomethionine (SeMet), and selenoproteins (Dilworth and Bandurski 1977; Ng and Anderson 1979; Pilon-Smits and Quinn 2010). Such organic Se species have distinct chemical structures and biochemical properties (Whanger 2002; Rayman 2008), and they may interact with and affect in vivo Cd disparately. Therefore, the different effects of selenite and selenate can be attributed to the differences in their assimilation, and thus, another goal of the present study was to analyze how Se transformation affects Cd accumulation in pak choi.
Materials and methods
Plant hydroponic culture
Pak choi (B. chinensis, “Hangzhouyoudonger”) was cultured in a greenhouse under the following conditions: 25/15 °C (day/night), a 14 h day−1 photoperiod, and 70% of relative humidity. Seeds were thoroughly washed with deionized water, soaked with saturated CaSO4 for 4 h, and then washed again before being germinated in vermiculite. After 10 days, uniform seedlings with three leaves were transplanted to 2.5-L plastic pots containing 1/5-strength Hoagland solution. The solution composition was as follows (mmol L−1): KNO3, 1.0; MgSO4, 0.4; NH4H2PO4, 0.5; Ca (NO3)2, 1.0; EDTA-Fe (III), 0.03; H3BO3, 3 × 10−3; MnSO4, 1 × 10−3; ZnSO4, 1 × 10−3; CuSO4, 2 × 10−4; and Na2MoO4, 2 × 10−4 (Hoagland and Arnon 1941). The nutrient solution was buffered with 1 mmol L−1 of MES (the pH was adjusted to 5.8 with KOH) and renewed every 3 days throughout the experiment.
Cd and Se exposure in four growing stages
At 6, 13, 20, and 27 days after the transplantation, 10 μmol L−1 of Cd (as Cd (NO3)2·4H2O) was added individually or with Se at the same dose (as either Na2SeO3 or Na2SeO4) to the nutrient solution. A control without the addition of either element was also prepared. Each treatment was replicated four times. Plants were harvested 3 days after the exposure (i.e., at 19, 26, 33, and 40 days after sowing, respectively), divided into shoots and roots, and thoroughly washed with deionized water. Roots were incubated in 150 mL of desorption solution of 1 mmol L−1 MES (pH 5.8) and CaSO4 to remove ions adsorbed on the root surface. After being washed with deionized water again, plant samples were weighed, frozen in liquid nitrogen, pulverized, and stored at − 25 °C for the element determination and Se speciation analyses.
Se speciation using HPLC-UV-AFS
Se extraction and the working conditions of the high-performance liquid chromatography coupled with atomic fluorescence spectrometry (HPLC-UV-AFS) were based on the procedures of Hu et al. (2018b), with some modifications. Pak choi samples that were collected 19 and 40 days after sowing (i.e., the first and the fourth samplings) were extracted with Streptomyces griseus protease (type XIV; Sigma Aldrich, USA), and only samples treated with both Cd and Se were included. Samples of shoots (1.00 g), selenite-treated roots (0.30 g), and selenate-treated roots (0.15 g) were ground in 5 mL of 8 mg mL−1 protease; the mixture was then oscillated at 150 rpm and 37 °C for 18 h before being centrifuged at 13000 rpm for 15 min. The supernatant was filtered (0.22 μm) for HPLC analysis.
Se speciation was performed using HPLC with an anion-exchange column (250.0 × 4.1 mm, 10 μm; PRP-X100; Hamilton, Switzerland) fitted in a guard column (25.0 × 2.3 mm, 10–20 μm; PRP-X100; Hamilton, Switzerland). Operating conditions for HPLC were as follows: 25 °C, 100 μL of injection volume, and 40 mmol L−1 of (NH4)2HPO4 as the mobile phase (the pH was adjusted to 6.10 with methanoic acid) at a flow rate of 1 mL min−1. To measure the concentration of a Se species at a certain retention time, the HPLC was coupled with UV-AFS (SA-20; Beijing Jitian Instrument Co. Ltd., PRC), and the operating conditions were set at 10 g L−1 of KI and 20 g L−1 of KBH4, both dissolved in 5 g L−1 of KOH and carried by 10% HCl (v/v; guaranteed reagent, GR).
Five selenocompounds: selenocystine (SeCys2), Se-methylselenocysteine (SeMeCys), SeO32− (Se (IV)), selenomethionine (SeMet), and SeO42− (Se (VI)), were mixed thoroughly as standard samples (i.e., GBW10087, GBW10088, GBW10032, GBW10034, and GBW10033, respectively; National Institute of Metrology, PRC). The retention times of these selenocompounds were approximately 172, 205, 250, 318, and 735 s, respectively.
Determination of Cd and Se
Samples of fresh shoots (0.30 g) and fresh roots (0.15 g) were microwave-digested (Mars 5; CEM, USA) with 65% HNO3 (w/v; GR). The concentrations of 111Cd and 78Se in the digestion were determined using ICP-MS (ICP-MS 7700; Agilent Technologies, USA). Blank digestion samples and a certificated material (GSB-26, celery; National Institute of Metrology, PRC) were included throughout the entire determination procedure. The elemental recovery for GSB-26 varied between 90 and 110%.
Data analysis
All the results are based on the fresh weights (FWs) and presented as means ± standard errors (SE; n = 4). The concentrations or contents of the selenocompounds are based on the amount of Se.
The translocation factor and uptake rate for Cd were calculated using Eqs. (1) and (2), respectively:
where CS, CR, WS, and WR represent the contents of Cd in shoots and roots (mg kg−1) and the fresh weights of shoots and roots (kg), respectively.
To evaluate the efficiency of Se extraction from the plant samples, the extraction rate was calculated using the following equation:
where E, C0, and C represent the extraction rate (%), the sum contents of five Se species in plant samples extracted with protease (mg kg−1), and the total Se content in plant samples that were microwave-digested with HNO3 (mg kg−1), respectively.
Additionally, the proportions of each selenocompound were obtained using the following equation:
where Pi, Ci, and ∑Ci represent the proportions of a certain selenocompound among all five compounds (%), the concentration of the selenocompound in the extraction (mg Se kg−1), and the sum concentration of all five selenocompounds (mg Se kg−1), respectively.
Results
Biomass
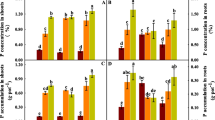
The growth rate of pak choi increased substantially with age (Fig. 1); the shoot biomass was 100 times higher at the first sampling than that at the last sampling (Fig. 1a). More importantly, the variation in the shoot biomass was more obvious on day 40 than on any other date (Fig. 1a). Although all the treatments slightly reduced the shoot biomass compared with the control, none of the differences in the first three samplings were significant. Only in the last sampling, the shoot fresh weight was found to have been significantly decreased by 24% (p < 0.05) in the individual Cd treatment, which then rose back to the control level under the influence of selenite and selenate (Fig. 1a).
Shoot (a) and root (b) biomass of pak choi (B. chinensis “Hangzhouyoudonger”) at four growing stages under different treatments (fresh weight). CK, the control; Cd, individual treatment of 10 μmol L−1 Cd; Cd + SeIV, 10 μmol L−1 of Cd combined with selenite; Cd + SeVI, 10 μmol L−1 of Cd combined with selenate. Results are presented as means + SEs (n = 4); different letters indicate significant differences in the biomass of shoots according to the LSD test (p < 0.05)
The differences in the root biomass between the treatments were much greater than those in the shoot biomass (Fig. 1b). Cd applied individually or in combination with selenite did not have any significant effects on the root biomass in the first three samplings, whereas Cd with selenate significantly reduced it by ~ 40% and ~ 60% on days 26 and 33, respectively, regardless if compared with the control or Cd alone (p < 0.05). On day 40, the root biomass declined by 42% with only Cd addition (p < 0.05), and those with selenite and selenate addition increased by 35% and 45%, respectively, compared with that of Cd alone; however, they were still under the level of the control (Fig. 1b).
Cd accumulation and translocation and Se uptake in pak choi
Obvious bio-dilution was observed in the Cd content in both shoots and roots; i.e., the Cd contents were 69% and 63% on day 40, respectively, less than those on day 19 (Fig. 2). Selenite enhanced the Cd content in pak choi shoots to a certain degree in the first two samplings, compared with that of the individual Cd treatment; however, the Cd content significantly decreased in the last two samplings (to 34% in the last sampling; p < 0.05). On the other hand, selenate always stimulated Cd accumulation in shoots, and the Cd contents in the combined Cd and selenate treatments were 16%, 50%, 15%, and 45% more than those in the Cd only treatment, in the four growing stages, respectively (Fig. 2a).
The Cd content in shoots (a) and roots (b) of pak choi (B. chinensis “Hangzhouyoudonger”) in four growing stages with different treatments (fresh weight). Cd, individual treatment of 10 μmol L−1 Cd; Cd + SeIV, 10 μmol L−1 Cd combined with selenite; Cd + SeVI, 10 μmol L−1 Cd combined with selenate. Results are presented as means + SEs (n = 4); different letters indicate significant differences in the Cd content in shoots according to the LSD test (p < 0.05)
The effect of selenite and selenate on the roots was consistent over the four samplings; i.e., they both reduced the Cd content in roots, and selenate was more effective (Fig. 2b). The addition of selenate diminished the Cd content in roots by 28%, 19%, 47%, and 22% in the four samplings, respectively, when compared with treatments with Cd only.
In contrast to the sharp decline in Cd content over the growth stages, the Cd uptake rate was essentially maintained at comparable levels (Fig. 3). Moreover, the uptake rate was invariably inhibited by selenite but increased by selenate; the differences between the uptake rates of plants treated with Cd only and in combination with selenite were no more than 16% before day 33, but increased to a significant extent to 27% on day 40 (p < 0.05). Compared with the Cd only treatment, selenate did not cause significant change, except on day 26 when the uptake rate was improved by roughly 25%.
The Cd uptake rate by pak choi (B. chinensis “Hangzhouyoudonger”) in four growing stages with different treatments (fresh weight). Cd, individual treatment of 10 μmol L−1 Cd; Cd + SeIV, 10 μmol L−1 Cd combined with selenite; Cd + SeVI, 10 μmol L−1 Cd combined with selenate. Results are presented as means + SEs (n = 4); different letters indicate significant differences in the uptake rate according to the LSD test (p < 0.05)
Both Se treatments enhanced the Cd translocation from the root to the shoot before day 33 (Table 1); the effect of selenite was non-significant, while that of selenate was greater and always significant (p < 0.05). The translocation factors for plants treated with selenate were 37%, 100%, 116%, and 97% higher than those only treated with Cd in the four stages, respectively. However, at the last sampling, selenite inhibited the rate by 33%, but the difference was still insignificant.
Se accumulation showed two distinct patterns in selenite and selenate-treated pak choi shoots (Table 2). The total Se content in the former was no more than 4% of that in the latter. However, comparable contents of Se were observed in the roots of plants subjected to the two Se treatments, with selenite causing a higher Se accumulation, except at the first sampling. On days 33 and 40, the Se contents in the roots of the selenite-treated plants were 85% and 73% higher, respectively, than those treated with selenite (p < 0.05).
Se speciation through HPLC
The extraction rates of Se species through protease were acceptable and ranging around 67% of the total Se (Table 3). To improve the separation of each Se species through HPLC, the pH of the mobile phase was slightly increased to 6.10. The retention times of SeCys2, SeMeCys, SeO32−, SeMet, and SeO42− were approximately 172, 205, 250, 318, and 735 s, respectively (Fig. 4). Under the current working conditions, the HPLC was able to separate the latter four selenocompounds excellently, but the resolution between SeCys2 and SeMeCys was not ideal with close retention times.
Because of the distinct levels between selenite and selenate taken up by pak choi (Table 2), the contents of every Se species in the selenate-treated plants (if detected) was higher than those in the selenite-treated plants. The contents of SeCys2, SeMeCys, SeMet, and SeO42− in selenate-treated shoots were 0.5, 6.0, 8.2, and 13.5 times higher than those in selenite-treated plants on day 19, respectively. On day 40, the differences in the contents of the latter three Se species were significant and became more obvious (p < 0.05; Table 4).
The variation in the contents of Se species in the roots was far minor, and the contents of SeCys2 and SeO32− were not significantly affected by either the treatments or the ages. On day 19, the SeMet content in the selenite-treated roots was no less than two times higher than that in the selenate-treated plants, but the difference was only 16% on day 40.
Besides, an important bio-dilution of each Se species was observed in the selenite-treated shoots. The contents of SeMeCys and SeMet on day 40 were no more than 25% of those on day 19, whereas the SeO42− content declined by 94%, and SeCys2 was even not detected. The dilution was less obvious in the roots or in the selenate-treated shoots (Table 4).
The proportions of the five selenocompounds in the Hangzhouyoudonger cultivar treated with selenite varied greatly over the two ages (Fig. 5). The HPLC revealed that SeO32− was not detected in the shoots, while all five species were found in the roots. SeO42− accounted for the highest proportion among five species in the shoots under both Se treatments at the two ages; i.e., at 19 days after sowing, no less than 60% of the Se was SeO42−, and the proportions of the other three detected species were relatively similar to each other (Fig. 5a). When the pak choi plants were 40 days old, the SeO42− proportion decreased by 37%, whereas those of SeMeCys and SeMet increased by 145% and 95%, respectively. The proportions of these three selenocompounds were all comparable at roughly 33% each. Plants treated with selenate did not exhibit much of a change in the proportions; i.e., that of SeO42− was higher than 2/3 at both ages, despite it having slightly decreased (by 13%) from day 19 to day 40. At the same time, the proportions of SeCys2, SeMeCys, and SeMet at the first sampling were 2.05, 1.00, and 6.89 percentage points higher than those on the last sampling day (Fig. 5a). In addition, the selenite treatment led to relatively high proportions of seleno-amino acids, such as SeMeCys and SeMet, while SeO42− was the predominant species in shoots under the influence of the selenate treatment.
The proportions of Se species contents in the shoots (a) and roots (b) of pak choi (B. chinensis “Hangzhouyoudonger”) at different ages (based on Se). Cd + SeIV 19, Cd + SeVI 19, Cd + SeIV 40, and Cd + SeVI 40 refer to treatments where selenite and selenate were applied with Cd when plants were 19 and 40 days old, respectively. SeCys2, SeMeCys, Se (IV), SeMet, and Se (VI) represent selenocystine, Se-methylselenocysteine, selenite, selenomethionine, and selenate, respectively
As the plants aged, the proportion of SeO42− in the selenite-treated roots drastically decreased by 82%, whereas that of SeMet increased by ~ 100%; those of the other three species were less than 10% and did not vary much (Fig. 5b). Similarly, the SeO42− proportion in roots treated with selenate decreased by 49% over the two ages, while that of SeMet increased by 61%. As observed in the shoots, the dominant species were SeO42− and SeMet in the selenate and selenite-treated roots, respectively, on day 19, and yet SeMet was found in the highest proportion under both Se treatments on day 40.
Discussion
Se treatments contrastingly affected Cd accumulation and translocation in pak choi
In this study, the two Se treatments, selenite and selenate, were observed to have gradually enhanced effects on the Cd content in shoots exposed to 10 μmol L−1 of Cd among the growing stages of pak choi (Fig. 2a). On day 40, selenite and selenate had opposite effects on the Cd content in shoots as well on as the translocation compared with those of the Cd only treatment, both to a significant extent.
Even as a non-essential element for higher plants, Se at optimal doses can still play an important role in regulating the uptake of essential elements and heavy metals (Feng et al. 2013a). There have been numerous reports of Se inhibiting Cd accumulation in plants; however, the findings can be controversial, when considering the different doses of the elements, Se species, plant species, exposure times, and even culture conditions. For example, the Cd accumulation in B. napus grown on a culture medium was significantly reduced by 2 μmol L−1 of selenite (Filek et al. 2008); selenite and selenate both inhibited the Cd uptake by Phaseolus mungo, and selenite was more effective (Shanker et al. 1995); similar to our findings, opposite effects were also achieved on Cd in other pak choi cultivars as well as Zn and Cd in S. oleracea under field conditions (Golubkina et al. 2017; Yu et al. 2018).
The disparate effects of the two forms of inorganic Se applied could be attributed to the differences in their assimilation in plants (Burnell 1981; Terry et al. 2000). It has been demonstrated that both selenite and selenate are assimilated through the S pathways in high plants, whereby selenate is first activated by ATP to adenosine phosphoselenate and then reduced to selenite by glutathione (GSH) in a non-enzymatic reaction (Dilworth and Bandurski 1977). More importantly, because of the significantly higher levels of Se and SeO42− (Table 2), it took substantial GSH to metabolize Se in selenate-treated plants (Terry et al. 2000). GSH is not only an electron donor that reduces heavy metal-induced reactive oxygen species (Pompella et al. 2003) but also the sole precursor of phytochelatins (PCs) under the catalysis of phytochelatin synthase in plants (Vatamaniuk et al. 2000). However, given that the level of GSH is generally much higher than that of Se (Table 2; Hasanuzzaman et al. 2017; Yu et al. 2018), GSH-mediated Cd sequestration in the root was unlikely impeded by Se assimilation in pak choi. Additionally, the activation of selenate is the rate-limiting step of the entire pathways (Dilworth and Bandurski 1977); therefore, even at appropriate doses, the reducing effect of the selenate treatment can be much later than that of the selenite treatment, as revealed by Wan et al. (2016) and our previous study on pak choi (Yu et al. 2018).
On the other hand, the Cd accumulation in and translocation to the shoots were both inhibited under the influence of selenite on day 40 (Fig. 2a, Table 1). As a chemical analog to S, Se can restore cellular structure and function such as the integrity of the endomembrane system and the antioxidative system (Feng et al. 2013a), which is of great significance for the restriction of Cd uptake. Considering the relatively lower total Se content and proportion of SeO42− (Tables 2 and 3), the GSH consumption in selenite assimilation was minor, and thus, it could not eliminate the reducing effect of Se on Cd accumulation and translocation in pak choi. However, when the doses of Cd and selenite increased, Se can still cause enhanced Cd accumulation and toxicity in plants (Feng et al. 2013b; Yu et al. 2018).
Se speciation in the first and last growing stages
Generally, Se4+ is reduced to Se2− through S assimilation, where the oxidation of Se is not involved, before being incorporated into seleno-amino acids and selenoproteins (Terry et al. 2000). This occurs in the chloroplast instead of the root under the catalysis of a set of enzymes (Dilworth and Bandurski 1977; Bruhl et al. 1996; Kim and Leustek 1996; Ng et al. 1979); however, after 3 days of exposure, respectable amounts of seleno-amino acids were found in pak choi roots with both Se treatments (Table 4). Not only did Huang et al. (2017) and Hu et al. (2018a) detect SeCys2, SeMeCys, and SeMet in selenite-treated wheat roots through X-ray absorption near-edge structure and HPLC-ICP-MS, respectively, but Li et al. (2008) reported that seleno-amino acids were the dominant species, whereas inorganic Se accounted for low amounts in the root xylem sap of selenite-treated wheat; they also found SeO42− in wheat exposed to Se4+ or Se0 (Huang et al. 2017; Hu et al. 2018a). Therefore, Se assimilation might take place in plant roots as well, and an unknown pathway for Se oxidation could exist in plants (Pronk et al. 1990).
Few studies have focused on how Se transformation affects metal accumulation in plants, and the mechanisms are still poorly understood. In this study, the Se species in pak choi were encompassed by the five standard selenocompounds with no unknown species found (Fig. 4). It is known that the function group in PCs is thiols (Vatamaniuk et al. 2000); Cd can even form more stable complexes with Se ligands than with S ligands in chemical reaction (Jalilehvand et al. 2012). Therefore, selenos (-SeH) might have similar functions to thiols with regard to the chelation of metal ions when S was substituted with Se in Cys/SeCys residues. The only seleno group–containing species detected in the present study was SeCys2, an oxidized dimer of SeCys via the linkage of two SeCys residues with a diselenide bond (Cys–Se–Se–Cys; Whanger 2002); the transformation between them happens spontaneously in the aqueous phase (Whanger 2002). These seleno groups were mostly found in roots with both Se treatments in this study, and given that PCs are more synthesized in the root, this might indicate the potential roles of SeCys/SeCys2 in immobilizing Cd in selenite/selenate-treated pak choi.
On the other hand, SeMeCys and SeMet are the main Se species in Brassica plants (Whanger 2002; Rayman 2008), as was the case in this study; however, the Se in these two selenocompounds is covered by a methyl group and therefore loses its reactive activity to metal ions (Rayman 2008). Nevertheless, the most important difference between the selenite- and selenate-treated pak choi was that SeMet was the dominant species in the former, while the SeO42− proportion was the highest in the latter. The Cd content in the selenite-treated shoot was significantly reduced only on day 40 when a sharp decline in SeO42− proportion was observed, but the proportions of SeMet and SeMeCys were significantly higher than those on day 19 (Fig. 2a, Table 4). Furthermore, enhanced production of SeMet in B. napus via the individual addition of both Cd and Pb was also reported elsewhere (Wu et al. 2016). SeMet is more redox-sensitive than methionine, a protein amino acid (Padmaja et al. 1996), and therefore, it can act as a more effective electron donor to eliminate ROS and prevent methionine oxidation (Assmann et al. 1998; Jing et al. 2015); this might explain why Se only increased the biomass of Cd-treated pak choi on day 40.
Another factor that could contribute to the differing effects of selenite and selenate was the distinct uptake transporters and transport patterns for selenite and selenate. Selenate uptake is regulated by high-affinity sulfate transporters, and the amount of selenate taken up by plants is much more than that of selenite (Terry et al. 2000). Furthermore, selenite is mostly retained in the root, while selenate is more readily transported to the shoot (Table 2; Terry et al. 2000; Wan et al. 2016). It has been reported that Se at lower doses can lead to a reduction in Cd accumulation; however, such effects can be reversed when higher doses of Se is applied, and the threshold for selenate is much lower (Feng et al. 2013b). Therefore, when taking the results for Se speciation into consideration, we can infer that SeMet is the key selenocompound for the reduction of Cd accumulation in pak choi, and SeCys2 can possibly be beneficial for Cd retention in roots; such effects could be abrogated by high levels of SeO42− and altered by the relative proportions of seleno-amino acids to SeO42− .
The effects of Se on plants are systemic, and Se may influence many processes involved in the uptake, transportation, and accumulation of metals in plants. For example, Se can affect transpiration, which is vital for metal uptake, especially when plants are subjected to heavy metal stress (Gao et al. 2018); we previously reported that Se altered Cd distribution in subcellular compartments in pak choi (Yu et al. 2019). Se may also stimulate the activity of antioxidative enzymes and alleviate Cd-induced oxidative stress to maintain the integrity of cellular barriers against Cd (Yu et al. 2019). Further work is still needed before we can fully understand the mechanism of the Se-mediated regulation of Cd accumulation in plants.
In conclusion, two inorganic Se species, selenite and selenate, conversely affected Cd accumulation and translocation in pak choi—selenite exhibited a significant antagonistic effect against Cd 40 days after sowing with regard to Cd accumulation and translocation—whereas selenate acted synergistically to the metal. Moreover, the effect of Se was observed to vary across growing stages in the present study. Neither Se treatment significantly affected either the biomass or the Cd content of shoots of pak choi seedlings as compared to Cd alone, but the effects of these treatments were significant on day 40. Such effects of Se on Cd accumulation resulted from the difference in the assimilation of selenite and selenate, which could be inferred from Se speciation. SeMet accounted for much larger proportions in selenite-treated pak choi, whereas SeO42− was the dominant species in selenate-treated pak choi. More importantly, a substantial increase in the SeMet proportion was observed from day 19 to day 40, at which time, a sharp decrease was noted in that of SeO42−. Therefore, the relative content and proportions of SeMet and SeO42− could be the key to determine whether Se functions as an antagonist to Cd in pak choi.
References
Assmann A, Briviba K, Sies H (1998) Reduction of methionine selenoxide to selenomethionine by glutathione. Arch Biochem Biophys 349(1):201–203. https://doi.org/10.1006/abbi.1997.0462
Bhabak KP, Mugesh G (2010) Functional mimics of glutathione peroxidase: bioinspired synthetic antioxidants. Acc Chem Res 43(11):1408–1419. https://doi.org/10.1021/ar100059g
Bo HY, Chen BC (2013) The dynamic growth exhibition and accumulation of cadmium of pak choi (Brassica campestris L. ssp. chinensis) grown in contaminated soils. Int J Env Res Public Health 10(11):5284–5298. https://doi.org/10.3390/ijerph10115284
Bruhl A, Haverkamp T, Gisselmann G, Schwenn JD (1996) A cDNA clone from Arabidopsis thaliana encoding plastidic ferredoxin:sulfite reductase. BBA-Protein Struct M 1295(2):119–124. https://doi.org/10.1016/0167-4838(96)00066-0
Burnell JN (1981) Selenium metabolism in Neptunia-amplexicaulis. Plant Physiol 67(2):316–324. https://doi.org/10.1104/pp.67.2.316
Clemens S, Aarts MGM, Thomine S, Verbruggen N (2013) Plant science: the key to preventing slow cadmium poisoning. Trends Plant Sci 18(2):92–99. https://doi.org/10.1016/j.tplants.2012.08.003
Dilworth GL, Bandurski RS (1977) Activation of selenate by adenosine 5′-triphosphate sulfurylase from Saccharomyces-cerevisiae. Biochem J 163(3):521–529. https://doi.org/10.1042/bj1630521
Feng RW, Wei CY, Tu SX (2013a) The roles of selenium in protecting plants against abiotic stresses. Environ Exp Bot 87:58–68. https://doi.org/10.1016/j.envexpbot.2012.09.002
Feng RW, Wei CY, Tu SX, Ding YZ, Song ZG (2013b) A dual role of Se on Cd toxicity: evidences from the uptake of Cd and some essential elements and the growth responses in paddy rice. Biol Trace Elem Res 151(1):113–121. https://doi.org/10.1007/s12011-012-9532-4
Filek M, Keskinen R, Hartikainen H, Szarejko I, Janiak A, Miszalski Z, Golda A (2008) The protective role of selenium in rape seedlings subjected to cadmium stress. J Plant Physiol 165(8):833–844. https://doi.org/10.1016/j.jplph.2007.06.006
Gao M, Zhou J, Liu HL, Zhang WT, Hu YM, Liang JN, Zhou J (2018) Foliar spraying with silicon and selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. Sci Total Environ 631-632:1100–1108. https://doi.org/10.1016/j.scitotenv.2018.03.047
Golubkina NA, Kosheleva OV, Krivenkov LV, Dobrutskaya HG, Nadezhkin S, Caruso G (2017) Intersexual differences in plant growth, yield, mineral composition and antioxidants of spinach (Spinacia oleracea L.) as affected by selenium form. Sci Hortic 225:350–358. https://doi.org/10.1016/j.scienta.2017.07.001
Haghighi M, da Silva JAT (2016) Influence of selenium on cadmium toxicity in cucumber (Cucumis sativus cv. 4200) at an early growth stage in a hydroponic system. Commun Soil Sci Plan 47(2):142–155. https://doi.org/10.1080/00103624.2015.1109650
Hasanuzzaman M, Nahar K, Anee TI, Fujita M (2017) Glutathione in plants: biosynthesis and physiological role in environmental stress tolerance. Physiol Mol Biol Plants 23(2):249–268. https://doi.org/10.1007/s12298-017-0422-2
Hoagland DR, Arnon DI (1941) Physiological aspects of availability of nutrients for plant growth. Soil Sci 51(1):431–444. https://doi.org/10.1097/00010694-194106000-00002
Hu X, Ding ZH (2009) Lead/cadmium contamination and lead isotopic ratios in vegetables grown in peri-urban and mining/smelting contaminated sites in Nanjing, China. Bull Environ Contam Toxicol 82(1):80–84. https://doi.org/10.1007/s00128-008-9562-y
Hu T, Li HF, Li JX, Zhao GS, Wu WL, Liu LP, Wang Q, Guo YB (2018a) Absorption and bio-transformation of selenium nanoparticles by wheat seedlings (Triticum aestivum L.). Front Plant Sci 9. https://doi.org/10.3389/fpls.2018.00597
Hu T, Liu LP, Chen SZ, Wu WL, Xiang CG, Guo YB (2018b) Determination of selenium species in cordyceps militaris by high-performance liquid chromatography coupled to hydride generation atomic fluorescence spectrometry. Anal Lett 51(14):2316–2330. https://doi.org/10.1080/00032719.2017.1414827
Huang QQ, Wang Q, Wan YN, Yu Y, Jiang RF, Li HF (2017) Application of X-ray absorption near edge spectroscopy to the study of the effect of sulphur on selenium uptake and assimilation in wheat seedlings. Biol Plant 61(4):726–732. https://doi.org/10.1007/s10535-016-0698-z
Jalilehvand F, Amini Z, Parmar K (2012) Cadmium(II) complex formation with selenourea and thiourea in solution: an XAS and Cd-113 NMR study. Inorg Chem 51(20):10619–10630. https://doi.org/10.1021/ic300852t
Jarup L (2002) Cadmium overload and toxicity. Nephrol Dial Transplant 17:35–39. https://doi.org/10.1093/ndt/17.suppl_2.35
Jing CL, Dong XF, Wang ZM, Liu S, Tong JM (2015) Comparative study of DL-selenomethionine vs sodium selenite and seleno-yeast on antioxidant activity and selenium status in laying hens. Poult Sci 94(5):965–975. https://doi.org/10.3382/ps/pev045
Kim JS, Leustek T (1996) Cloning and analysis of the gene for cystathionine gamma-synthase from Arabidopsis thaliana. Plant Mol Biol 32(6):1117–1124. https://doi.org/10.1007/Bf00041395
Li HF, McGrath SP, Zhao FJ (2008) Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol 178(1):92–102. https://doi.org/10.1111/j.1469-8137.2007.02343.x
Ng BH, Anderson JW (1979) Light-dependent incorporation of selenite and sulfite into selenocysteine and cysteine by isolated pea chloroplasts. Phytochemistry 18(4):573–580. https://doi.org/10.1016/S0031-9422(00)84263-6
Padmaja S, Squadrito GL, Lemercier JN, Cueto R, Pryor WA (1996) Rapid oxidation of DL-selenomethionine by peroxynitrite. Free Radic Biol Med 21(3):317–322. https://doi.org/10.1016/0891-5849(96)00132-3
Pilon-Smits EAH, Quinn CF (2010) Selenium metabolism in plants. Cell Biol Metal Nutr 17:225–241. https://doi.org/10.1007/978-3-642-10613-2_10
Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF (2003) The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol 66(8):1499–1503. https://doi.org/10.1016/S0006-2952(03)00504-5
Pronk JT, Meulenberg R, Hazeu W, Bos P, Kuenen JG (1990) Oxidation of reduced inorganic sulfur-compounds by Acidophilic Thiobacilli. FEMS Microbiol Lett 75(2–3):293–306. https://doi.org/10.1016/S0006-2952(03)00504-5
Rayman MP (2008) Food-chain selenium and human health: emphasis on intake. Br J Nutr 100(2):254–268. https://doi.org/10.1017/S0007114508939830
Sawut R, Kasim N, Maihemuti B, Hu L, Abliz A, Abdujappar A, Kurban M (2018) Pollution characteristics and health risk assessment of heavy metals in the vegetable bases of Northwest China. Sci Total Environ 642:864–878. https://doi.org/10.1016/j.scitotenv.2018.06.034
Shanker K, Mishra S, Srivastava S, Srivastava R, Dass S, Prakash S, Srivastava MM (1995) Effect of selenite and selenate on plant uptake of cadmium by kidney bean (Phaseolus mungo) with reference to Cd-Se interaction. Chem Speciat Bioavailab 7(3):97–100. https://doi.org/10.1080/09542299.1995.11083251
Smith FW, Hawkesford MJ, Ealing PM, Clarkson DT, VandenBerg PJ, Belcher AR, Warrilow GS (1997) Regulation of expression of a cDNA from barley roots encoding a high affinity sulphate transporter. Plant J 12(4):875–884. https://doi.org/10.1046/j.1365-313X.1997.12040875.x
Terry N, Zayed AM, de Souza MP, Tarun AS (2000) Selenium in higher plants. Annu Rev Plant Physiol Plant Mol Biol 51:401–432. https://doi.org/10.1146/annurev.arplant.51.1.401
Vatamaniuk OK, Mari S, Lu YP, Rea PA (2000) Mechanism of heavy metal ion activation of phytochelatin (PC) synthase - blocked thiols are sufficient for PC synthase-catalyzed transpeptidation of glutathione and related thiol peptides. J Biol Chem 275(40):31451–31459. https://doi.org/10.1074/jbc.M002997200
Wan YN, Yu Y, Wang Q, Qiao YH, Li HF (2016) Cadmium uptake dynamics and translocation in rice seedling: influence of different forms of selenium. Ecotoxicol Environ Saf 133:127–134. https://doi.org/10.1016/j.ecoenv.2016.07.001
Whanger PD (2002) Selenocompounds in plants and animals and their biological significance. J Am Coll Nutr 21(3):223–232. https://doi.org/10.1080/07315724.2002.10719214
Wu ZL, Yin XB, Banuelos GS, Lin ZQ, Liu Y, Li M, Yuan LX (2016) Indications of selenium protection against cadmium and lead toxicity in oilseed rape (Brassica napus L.). Front Plant Sci 7. https://doi.org/10.3389/fpls.2016.01875
Yang Y, Zhang FS, Li HF, Jiang RF (2009) Accumulation of cadmium in the edible parts of six vegetable species grown in Cd-contaminated soils. J Environ Manag 90(2):1117–1122. https://doi.org/10.1016/j.jenvman.2008.05.004
Yu Y, Wan YN, Wang Q, Li HF (2017) Effect of humic acid-based amendments with foliar application of Zn and Se on Cd accumulation in tobacco. Ecotoxicol Environ Saf 138:286–291. https://doi.org/10.1016/j.ecoenv.2017.01.011
Yu Y, Yuan SL, Zhuang J, Wan YA, Wang Q, Zhang JS, Li HF (2018) Effect of selenium on the uptake kinetics and accumulation of and oxidative stress induced by cadmium in Brassica chinensis. Ecotoxicol Environ Saf 162:571–580. https://doi.org/10.1016/j.ecoenv.2018.07.041
Yu Y, Fu PN, Huang QQ, Zhang JS, Li HF (2019) Accumulation, subcellular distribution, and oxidative stress of cadmium in Brassica chinensis supplied with selenite and selenate at different growth stages. Chemosphere 216:331–340. https://doi.org/10.1016/j.chemosphere.2018.10.138
Funding
This work was supported by the China Agriculture Research System (CARS-23-B16) and National Natural Science Foundation of China (No. 41471271).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, Y., Zhuang, Z., Luo, Ly. et al. Difference between selenite and selenate in selenium transformation and the regulation of cadmium accumulation in Brassica chinensis. Environ Sci Pollut Res 26, 24532–24541 (2019). https://doi.org/10.1007/s11356-019-05705-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05705-x