Abstract
A field survey was conducted to investigate metal(loid) concentration in soils and native plants in the Baoshan mining area for potential application in phytoremediation. Total concentrations of arsenic (As), cadmium (Cd), lead (Pb), and zinc (Zn) in soil varied from 125 to 6656, 5.10 to 1061, 568 to 49294, and 241 to 17296 mg kg−1, respectively, showing severe contamination. Among 20 species native to this area, Pteris ensiformis accumulated 1091 mg kg−1 As in the shoot, and its translocation factor (TF) was greater than 1, suggesting potential capacity for As phytoextraction. Boehmeria nivea, Aster prorerus, and Hydrocotyle sibthorpioides showed potential for phytoextraction of Cd due to their high accumulation of Cd in shoots (490.3, 175.4, and 128.5 mg kg−1, respectively) and high TFs (92.0, 22.1, and 6.7, respectively). Eleusine indica and P. ensiformis were found to contain high concentrations of Pb (7474 mg kg−1) and Zn (1662 mg kg−1) in roots, but with low TFs for Pb (0.4) and Zn (0.2), suggesting potential capability for phytostabilization. There was a positive correlation (p < 0.01, N = 25) of TFs between the metal(loid)s, indicating a synergic interaction in the uptake of metal(loid)s by these plants. According to metal(loid) concentrations in shoots, bioconcentration factors (BFs), and TFs, as well as the botanical features such as wide occurrence, high biomass yield, and rapid growth of the plants, the five native species identified above have the potential for phytoremediation in the Baoshan mining area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Currently, 16.1% of Chinese soil is polluted, and 82% of the contaminated soils are related to toxic inorganic pollutants, such as cadmium (Cd), mercury (Hg), arsenic (As), chromium (Cr), and lead (Pb), which are toxic to biota (Chen et al. 2014; MEP and MLR of P. R. China 2014). Soil remediation is required to reduce risks to human and the environment. As a cost-effective, environmentally friendly remediation method, phytoremediation is considered a viable technology and has been widely applied in remediating metal(loid)-contaminated soils (Yoon et al.2006; Pinto et al. 2015; Sarwar et al. 2017). For example, Eucalyptus globulus was reported to have a decontamination capacity of 0.006–0.010 t for Cd, 0.23–0.55 t for Pb, and 0.56–1.13 t for Cu per hectare in two years (Luo et al. 2018).
Phytoremediation technology includes phytoextraction, phytostabilization, phytofiltration, phytovolatilization, phytodegradation, rhizodegradation, and phytodesalination (Alkorta et al. 2004; Hemen 2011; Ali et al. 2013), of which phytoextraction and phytostabilization are more extensively studied. Phytoextraction is the use of live plants to remove metal(loid)s from soil by uptake. Its efficiency depends on the ability of plant roots to absorb, translocate, and accumulate metal(loid)s from soil to the aboveground harvestable parts (Khalid et al. 2017). Phytostabilization decreases the bioavailability and mobility of metal(loid)s in soil through root sorption, precipitation, complexation, or reduction in rhizosphere (Ali et al. 2013).
Metallophytes are plants that are specifically adapted to and thrive in heavy metal–rich soils, and good candidates for phytoextraction and phytostabilization. Metallophytes are divided into three categories: metal hyperaccumulators, metal excluders, metal indicators (Ali et al. 2013). Metal hyperaccumulators can be used for phytoextraction and metal excluders for phytostabilization.
According to van der Ent et al.’s (2013) reports, hyperaccumulation threshold criteria for different metal and metalloids in dried foliage are as follows: 100 mg kg−1 for Cd and Se; 300 mg kg−1 for Co, Cr, and Cu; 1,000 mg kg−1 for As, Ni, and Pb; 3,000 mg kg−1 for Zn; and 10,000 mg kg−1 for Mn, with plants growing in their natural habitats. Some other characteristics to be considered include the following: (1) bioconcentration factor > 1, (2) translocation factor > 1, and (3) extreme metal(loid) tolerance due to effective biochemical detoxification (Baker and Whiting 2002). There are more than 500 plant species from at least 101 families that have been reported to hyperaccumulate metal(loid)s, including members of Asteraceae, Brassicaceae, Caryophyllaceae, Cyperaceae, Cunoniaceae, Fabaceae, Flacourtiaceae, Lamiaceae, Poaceae, Violaceae, and Euphorbiaceae (Hemen 2011), and the number keeps increasing with further exploration and analysis. The best known metal(loid) hyperaccumulators are Noccaea caerulescens (formerly Thlaspi caerulescens) for Zn, which accumulate up to 26,000 mg kg−1 Zn without showing any injuries (Brown et al.1995) and Pteris vittata L. (Chinese brake fern) for As, which grow normally in soils of 50–4030 mg kg−1 As, and even in tailings with As of up to 23,400 mg kg−1 (Chen et al. 2007). Root compartments and excluders may be useful for phytostabilization. This strategy of phytoremediation does not remove the pollutants from soils but fixes metal(loid)s in the rhizosphere to limit the spread of metal(loid)s (Bech et al. 2012).
Although there are current efforts to enhance phytoremediation with plant growth–promoting bacteria (Novo et al. 2018) and advanced genetic engineering techniques (Basharat et al. 2018), the screening of suitable plants for phytoremediation is still critical. Screening suitable native plants for phytoremediation is of primary importance because these plants are tolerant to metal(loid) pollution in specified soil conditions, and they also perform better in terms of survival, growth, and reproduction, as compared with plants introduced from other environments (Frérot et al. 2006; Yoon et al.2006; Antosiewicz et al. 2008).
The Baoshan mine is located in Chenzhou, southern Hunan province, and rich in lead, zinc, silver, tungsten, tin, and molybdenum ores. The long history of mining has caused severe pollution of metal(loid)s in the surrounding environment. The average concentrations of Cd and Pb in soybeans collected in the Baoshan mine were 1.59 and 13.08 mg kg−1, respectively (Zhou et al. 2013), which greatly exceeded the limited value of national standards for food safety of China (GB 2762-2017) (NHFPC and NFDASA 2017). In order to give recommendations for mine rehabilitation and reduce pollution to the surrounding environment from the Baoshan mine, a survey on the metal(loid) tolerance of native plants was conducted in this study; the specific objectives were (1) determining the concentrations of metal(loid)s in soil and plant samples, (2) examining the potential interactions between metal(loid)s during plant accumulation, and (3) assessing effectiveness of plants for phytoremediation.
Material and methods
Site characterization
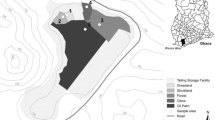
The study area is located in the Baoshan mine, Guiyang County, Hunan province, China, with longitude 112° 13′–112° 55′ E and latitude 25° 27′–26° 13′ N. This is a subtropical humid monsoon climate area, with the annual average temperature of 17.2 °C and the annual rainfall of 1385.2 mm. The region comprises a wide variety of strata spanning from the Sinian to the Quaternary except for the Silurian (Xie et al. 2013), and the strata of the mining area consists of mainly carboniferous carbonate rocks, enriched with non-ferrous metals such as Cu, Pb, Zn, Au, Ag, and Mo. The associated minerals are As, Cd, and S. Both open-cast mining and underground mining were operated in the Baoshan mine.
Sample collection
Samples of soil and plant were collected from two zones in the Baoshan mining area in August 2010, including 7 sites in the smelter area and 5 sites in the tailing pond area (Fig. 1). At each site, one to seven plant samples in an area of 1 m2 were collected. Soil samples from the rooting zone (0–20 cm) were collected from each site. In order to obtain accurate plant species information, plants were photographed and transported to the laboratory as soon as possible; a plant taxonomist was invited to identify each plant. In total, 20 species from 13 families were collected from 12 sites; their basic information including relative abundance, life form, and grown stage was listed in Table 1. The relative abundances were estimated visually and described as 1–5 (from very rare to abundant).
Soil and plant analysis
After large stones and plant debris were removed, the soil samples were air-dried at room temperature, and ground to < 0.15-mm powder prior to use. Plant samples were separated into shoots and roots, washed carefully with tap water, and then rinsed with deionized water 3 times to remove soil particles that adhered to the surfaces. The washed plant samples were oven-dried (at 105 °C for 30 min and then at 70 °C for 48 h) and ground to powder. Soil pH was measured using a pH meter with the 1:2.5 soil to water ratio. Soil samples were digested with concentrated HNO3 and H2O2 (USEPA 3050B). Plant samples were digested with a mixture of concentrated HNO3 and HClO4 (Chen et al. 2002). The concentrations of Cd, Pb, and Zn in digested soil or plant samples were then determined using an inductively coupled plasma optical emission spectrometer (ICP-OES, Optima 5300DV, PerkinElmer Co., USA). The concentration of As in the digested samples was determined using an atomic fluorescence spectrometer (AFS 9800, Haiguang Instrument Co., China). To ensure accuracy and precision, reagent blanks and appropriate standard reference materials (GBW07401 for soil, GBW07603 for plant) from the National Standard Substances Center of China were carried through the digestion and subsequent analysis. The recovery of As, Cd, Pb, and Zn was 90.4–102.7% for GBW07401 and 92.1–103.2% for GBW07603.
Statistical analysis
The bioconcentration factor (BF) was defined as the ratio of a metal(loid) in the plant shoot to that in the soil. The translocation factor (TF) was defined as the ratio of a metal(loid) in the plant shoot to that in the plant root. All data were analyzed by Excel 2016 and SPSS 20.0 (IBM, USA). Data were standardized using log transformation and the statistical differences between the means of pH and metal(loid) concentration of soils from the smelter area and tailing pond area were determined by t test at p < 0.05 level. The correlation of metal(loid) concentration in soils and plants, and BF and TF in plants, was undertaken by the bi-variation method, with two-tailed significance, and Spearman’s correlation coefficients were determined.
Results
Soil properties and metal(loid) concentrations
Soil pH ranged from 5.78 to7.83. The total concentrations of potentially toxic elements in the soil samples ranged from 125 to 6656 mg kg−1 for As, 5.10–1061 mg kg−1 for Cd, 568–49294 mg kg−1 for Pb, and 241–17296 mg kg−1 for Zn (Table 2). To assess the level of contamination, concentrations of total As, Cd, Pb, and Zn in our soil samples were compared with the national soil environmental quality–risk control standard for soil contamination of agricultural land (GB 15618-2018) (MEE and SMSA 2018a) and risk control standard for soil contamination of development land (GB 36600-2018) (MEE and SMSA 2018b). Obviously, concentrations of As, Cd, Pb, and Zn of all sampling sites from the smelter area and tailing pond area exceeded the risk control standard for soil contamination of agricultural land, except for As in site no. 10 from the tailing pond area. They were 1.04–54.5, 1.55–265, 0.14–69.4, and 0.21–68.2 times higher for As, Cd, Pb, and Zn, respectively. According to the risk control standard for soil contamination of development land, all sites from the smelter area exceeded standards (class B) for As and Pb, while 80% and 40% sites from the tailing pond area were above the critical level for As and Pb, respectively. 85.7% sites from the smelter area exceeded the standard (class B) for Cd, but none of the sites from the tailing pond area exceeded that of the critical level of Cd (Table 2). There were significant differences (p < 0.05) in soil pH and concentrations of As, Pb, and Zn between the two sampling areas. The average concentration of Cd was significantly higher in the smelter area than in the tailing pond area (p < 0.05). These results indicated that Baoshan mine suffered a severe contamination of As, Cd, Pb, and Zn, with Cd contamination level being higher in the smelter than in the tailing pond area.
Metal(loid) concentrations in plants
The concentrations of As, Cd, Pb, and Zn in plants are presented in Table 3. The metal(loid) concentrations varied between plant samples and species. Total As concentration ranged from 7~1091 mg kg−1 in shoots and 6~1047 mg kg−1 in roots. The highest As concentration occurred in the shoot and root of Pteris ensiformis from the smelter area, which was the only plant species that accumulated more than 1000 mg kg−1 As in the shoot, meeting the criterion for a hyperaccumulator (Ent et al. 2013).
Total Cd concentration in shoot ranged from 4.7~490.3 mg kg−1 and 2.4~157.2 mg kg−1in root, with the highest Cd concentration measured in the shoot of Boehmeria nivea. In addition to B. nivea, the shoots of Aster prorerus, Hydrocotyle sibthorpioides, Chrysanthemum indicum, Euphorbia esula, and Rumex acetosa also contained significant amounts of Cd (112.5~215.0 mg kg−1), and they were all above 100 mg kg−1, the criterion for a Cd hyperaccumulator.
The Pb concentration ranged from 90~4462 mg kg−1 in the shoot and 23~7474 mg kg−1 in the root. The highest Pb concentrations occurred in the shoot of B. nivea and in the root of Eleusine indica. The criterion for a Pb hyperaccumulator is 1000 mg kg−1 in the shoot; 11 species among all the plant samples reached this value, including B. nivea, P. ensiformis, A. prorerus, Valeriana officinalis, H. sibthorpioides, C. indicum, D. morifolium ‘Xiaohuangju’ nom. ined., Saussurea japonica, E. esula, R. acetosa, and E. indica.
The Zn concentration in shoot ranged from 152~2506 mg kg−1, and 28~1662 mg kg−1 in roots. R. acetosa had the highest Zn concentration in the shoot, whereas P. ensiformis had the highest Zn concentration in the root. In addition to R. acetosa, the shoots of Polygonum bungeanum and E. indica also contained significant amounts of Zn (1133~1427 mg kg−1). However, none of the plant species accumulated Zn above 10000 mg kg−1 in the shoot, which is the criterion for a Zn hyperaccumulator.
Bioconcentration and translocation of metal(loid)s
In addition to metal(loid) concentrations suggested by van der Ent et al. (2013) to qualify a hyperaccumulator, bioconcentration factors (BFs) and translocation factors (TFs) are two other key factors used for the screening of potential metal(loid) hyperaccumulators. The BFs and TFs of the plant samples collected from the study sites are listed in Table 4.
Among the 20 plant species, P. ensiformis collected from the smelter area was the only species that had the BF higher than 1 for As (BF = 1.31). For 92% of the plant samples, the TFs of As were greater than 1, including P. ensiformis. P. ensiformis also showed a high accumulation of As (Table 3), and thus this species may be a potential As hyperaccumulator.
B. nivea showed the highest BF for Cd (BF = 20.0). Five other species, i.e., P. ensiformis, A. prorerus, Valeriana officinalis, H. sibthorpioides, and P. bungeanum, also had BFs greater than 1 for Cd. For 88% of the plant samples, the TFs of Cd were greater than 1, including the 6 plant species mentioned above. B. nivea also showed the highest TF for Cd (TF = 92.0). Three of the six plant species, i.e., B. nivea, A. prorerus, and H. sibthorpioides, accumulated Cd in excess of 100 mg kg−1 in the shoots, which suggested that they are suitable plants for the phytoextraction of Cd.
Similar to As and Cd, most of the plant species had a value of TFs for Pb and Zn greater than 1. However, only P. bungeanum had a BF of 5.91 for Zn. For Pb, E. indica had the lowest TF (TF = 0.4, Table 4) but with the highest concentration in the root. In addition, the corresponding soil sample at site 12 had the highest concentration of Pb (49294 mg kg−1, Table 3) with the normal growth of E. indica. These findings suggested that E. indica has the potential to stabilize Pb. P. ensiformis from the tailing pond area had similar characteristics as E. indica but for Zn, which included the accumulation of high amounts of Zn (1662 mg kg−1, Table 3) in the root with a low TF (TF = 0.2, Table 4) and normal growth in soil with a high concentration of Zn (8520 mg kg−1, Table 2). This result suggested that P. ensiformis is a suitable species for the phytostabilization of Zn.
Correlation analysis
To identify whether synergism or antagonism between heavy metal(loid)s existed in the plant absorption process, correlation analysis was conducted, and Spearman’s correlation coefficients are listed in Table 5. Significant correlations were found in soil samples between the concentrations of Cd and Pb (r = 0.699, p < 0.01, N = 12), Cd and Zn (r = 0.853, p < 0.01, N = 12), and Pb and Zn (r = 0.825, p < 0.01, N = 12). In the plant samples, strong correlations were found between the concentrations of As and Pb, Cd and Pb, Cd and Zn, and Pb and Zn (r = 0.628, 0.805, 0.633, 0.632, respectively, p < 0.01, N = 25), and a significant correlation was also found between the concentrations of As and Cd (r = 0.561, p < 0.01, N = 25). For BFs, highly significant correlations were found between the concentrations of Cd and Pb (r = 0.620, p < 0.01, N = 25), and Cd and Zn (r = 0.663, p < 0.01, N = 25). While for TFs, all of the four metal(loid)s had highly significant correlations, with correlation coefficients ranging from 0.507 to 0.769 (p < 0.01, N = 25).
Discussion
Contamination features of the Baoshan mining area
The present study clearly showed that concentrations of As, Cd, Pb, and Zn in the Baoshan mine greatly exceeded the national soil environmental quality–risk control standard for soil contamination of agricultural land (GB 15618-2018) and development land (GB 36600-2018). This result agreed with other reports for the same area (Liu et al. 2005; Zhou et al. 2013). There were no significant differences between the smelter area and tailing pond area in terms of As, Pb, and Zn concentrations, which may be a result of contaminant dispersion. Airborne particles and aerosols contributed most to As, Cd, Pb, Zn, and Cu inputs in the Shuikoushan mining area (Wei et al. 2009), which is also located in Zhuzhou city, Hunan province in China, with the same climate characteristics and a similar mining history and mining system as the Baoshan mining area. The deposition fluxes of As, Pb, and Zn in Zhuzhou city were 56.69, 1074.9, and 6295.1 mg (m2 year)−1 according to Ke (2015), which was much higher than those in other cities in China. The atmospheric As deposition could be an important source of As, as evidenced by the higher concentration of As in shoots than the roots of vegetables, since soil As was low (Liao et al. 2005). Therefore, atmospheric deposition may be an important pollution patterns in the Baoshan mining area, which resulted in no significant difference in the pollution levels between the smelter area and its adjacent tailing area for As, Pb, and Zn.
Characteristics of metal(loid) uptake and accumulation by plants
Plants grown in mining areas often show a strong tolerance to metal(loid)s. Normal heavy-metal contents of terrestrial plants growing in uncontaminated soils of Hunan province were 0.04 mg kg−1 for Cd, 0.09 mg kg−1 for Pb, and 18.0 mg kg−1 for Zn (Wang and Stuanes 2003), whereas in the present study, the concentrations of these metals in most of the plant species were much higher than those in the plants growing in uncontaminated soils. The high level of metal(loid) concentrations in soils was the main cause of elevated metal(loid) concentrations in plants, which could be demonstrated by the significant correlations between concentrations of As, Cd, and Pb in plants and soils (Table S1). Liang et al. (2016) also showed that metal(loid) concentrations in plants were proportional to their concentrations in soil, which fitted well a regression model with a high correlation coefficient (0.9417).
Most of the plants exhibited higher concentrations of As, Cd, Pb, and Zn in the shoots than in the roots, and their TFs were higher than one. Two reasons may explain this phenomenon. Firstly, native plants evolved a high transport ability for metals from the root to the shoot. Baoshan mining started in 1966 and has a long history of constant contamination. Plants grown in this area from generation to generation have evolved into metallophytes, which are specifically adapted to and thrive in metal(loid)-polluted environments (Sheoran et al. 2010; Sherameti and Varma 2011). The potential mechanisms may include a strong ability of metal uptake with large quantities of small organic molecules as metal-bonding ligands, and an enhanced xylem loading capacity for metal(loid)s (Rascio and Navariizzo 2011). Secondly, atmospheric deposition of metal(loid)s may be another factor that resulted in the high concentrations of aboveground plant parts. Several previous studies indicated that plants growing near smelters exhibited high foliar levels of metal(loid)s (Shahid M et al. 2013, 2016; Xiong et al. 2014), and the concentrations of metal(loid)s in leaves are significantly related to atmospheric deposition (De Temmerman et al. 2012, 2015). According to Hu et al. (2011), the approximate contributions of airborne Pb to levels found in leaves of plant (Aster subulatus) were 65.1~72.2%.
When the plants grow in multiple metal(loid)-contaminated soils, they usually show a characteristic of mutual promotion of translocation in metal(loid) accumulation. The concentrations in plants and enrichment coefficients indicated significant relationships between Pb and Cd, Pb and Zn, and Cd and Zn in a lead–zinc mine area (Zu et al. 2004). Zinc and Cu also interacted in terms of promoting accumulation in plants growing in a contaminated site in Florida (Yoon et al. 2006), while in this study it was found that the concentrations of As, Cd, and Pb and their TFs in plants were significantly related to each other (p < 0.01).
Potential for phytoremediation
Phytoextraction, the use of plants to extract toxic metal(loid)s from contaminated soil, has emerged as a cost-effective, environmentally friendly cleanup alternative (Pinto et al. 2015; Sarwar et al. 2017). Hyperaccumulators are often applied for phytoextraction due to their effective uptake of toxic metal(loid)s and accumulation of metal(loid)s in the harvestable parts of plants (Sainger et al.2011). The plants in the present study grew normally and exhibited strong tolerance to metal(loid)s. Four species accumulated metal(loid)s in the shoots above the thresholds defined by Ent et al. (2013), meeting the basic requirement for hyperaccumulation, i.e., P. ensiformis for As, and B. nivea, A. prorerus, and H. sibthorpioides for Cd. The results suggested that these species are potential hyperaccumulators for As and Cd, respectively. The “potential” means that some characteristics remain to be verified. As discussed above, atmospheric deposition might affect metal(loid) concentrations in plant aboveground. Hyperaccumulator should imply only active accumulation inside the plant leaf tissue, via the root; passive accumulation via airborne deposition on plant leaves should not be considered hyperaccumulation (Ent et al. 2013). An interesting contradiction was found when compared with other study. P. ensiformis was identified as a potential As hyperaccumulation in our study, but this species was considered a non-arsenic hyperaccumulator and sensitive to As by Singh and Ma (2006). For this reason, an experiment should be conducted to distinguish between As entering P. ensiformis via the root system and foliar absorption, under glasshouse conditions. Moreover, variation between different populations could be another explanation for this contradictory result. As described in Wan et al. (2013) study, four Pteris vittata populations showed differences in root As uptake and As species transformation. The As-tolerant populations of P. vittata displayed the tendency to sequester As by coordinating with a chelating agent (inactive form like As-GSH), while the As hyperaccumulators exhibited stronger affinities for As and were thus able to absorb As even at low concentrations. Different P. vittata populations can result in an approximately eightfold difference in terms of As accumulation (Wan et al. 2013). P. ensiformis in Singh and Ma’s research and this study were collected at different places and belonged to different populations, so we speculated that they may also have different abilities to accumulate As. But further experimental studies should be conducted to confirm this assumption. B. nivea was hypertolerant to Cd, which is consistent with a previous study that revealed B. nivea possesses a certain degree of constitutional metal(loid) tolerance to Pb, Zn, Cd, and As (Yang et al. 2010). Furthermore, the feasibility of phytoremediation of Cd-contaminated farmland by B. nivea has been proven (She et al. 2011). All of the data in the present study and the cited references suggested that B. nivea is a good choice for mining restoration.
E. indica and P. ensiformis were found to be enriched with Pb (7474 mg kg−1) and Zn (1662 mg kg−1) in the roots, and the corresponding soils contained high concentrations of Pb (49294 mg kg−1) and Zn (8520 mg kg−1), respectively. And their TF values were less than 1. The above findings suggested that E. indica and P. ensiformis are hypertolerant, and they accumulated metal(loid)s from soil in their roots but restricted metal(loid) transporting to aerial parts. Plants with these features are considered to be an effective material for phytostabilization purposes (Mendez and Maier 2008; Ali et al.2013). Other features such as a dense and tough root system is also important (Pinto et al. 2015). E. indica has a notoriously tough root system (Zhang et al. 2016); this botanical characteristic makes it a good candidate for Pb phytostabilization.
In addition to the characteristics of enrichment or fixation of metal(loid)s by plants, there are some other botanical features, such as abundance, a high growth rate, and good biomass yields (McGrath and Zhao 2003; Ali et al. 2013) that should be considered for determining whether a plant is suitable for mine restoration. The five indigenous plant species, including P. ensiformis, B. nivea, A. prorerus, H. sibthorpioides, and E. indica, have high abundance in the Baoshan mining area; their abundance ranked 4 or 5, which means frequent or abundant distribution. Moreover, B. nivea is a vigorous, high-yielding plant (Wang et al. 2008); A. prorerus and H. sibthorpioides are perennial herb that can be mowed in succession; and E. indica has a high reproductive capacity and a developed root system (Zhang et al. 2016). They should have good potential for phytoremediation in mining restoration. In addition, the plants that showed greater fitness for phytoextraction should be subjected to further investigation regarding their potential for phytomining of valuable metal(loid)s (Novo et al. 2017).
Conclusion
This study was conducted to investigate metal(loid) concentrations in soils and native plant species from the Baoshan mining area and to determine the potential capacity of these plants for phytoremediation. The concentrations of As, Cd, Pb, and Zn in all the study sites exceeded the soil environmental quality–risk control standard in China, indicating severe pollution. According to metal(loid) concentrations in shoots, BFs, and TFs, of the 20 plant species examined, as well as their botanical features such as high abundance, good biomass yielding, and high growth rate, P. ensiformis is identified as potentially useful for phytoextraction of As and B. nivea, A. prorerus, and H. sibthorpioides potentially useful for phytoextraction of Cd, while E. indica and P. ensiformis can be considered for the potential application to phytostabilization of Pb and Zn, respectively.
References
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals—concepts and applications. Chemosphere 91(7):869–881
Alkorta I, Hernández-Allica J, Becerril JM, Amezaga I, Albizu I, Garbisu C (2004) Recent findings on the phytoremediation of soils contaminated with environmentally toxic heavy metals and metalloids such as zinc, cadmium, lead, and arsenic. Rev Environ Sci Biotechnol 3(1):71–90
Antosiewicz DM, Escudĕ-Duran C, Wierzbowska E, Skłodowska A (2008) Indigenous plant species with the potential for the phytoremediation of arsenic and metals contaminated soil. Water Air Soil Pollut 193(1-4):197–210
Baker AJM, Whiting SN (2002) In search of the holy grail: a further step in understanding metal hyperaccumulation. New Phytol 155:1–7
Basharat Z, Novo L, Yasmin A (2018) Genome editing weds CRISPR: what is in it for phytoremediation. Plants 7:51. https://doi.org/10.3390/plants7030051
Bech J, Abreu MM, Pérez-Sirvent C, Poschenrieder C, (2012) Phytoremediation of polluted soils. J Geochem Explor 123:1–2
Brown SL, Chaney RL, Angle JS, Baker AJM (1995) Zinc and cadmium uptake by hyperaccumulator Thlaspi caerulescens and metal tolerant Silene vulgaris grown on sludge-amended soils. Environ Sci Technol 29(6):1581–1585
Chen TB, Wei CY, Huang ZC, Huang QF, Lu QG, Fan ZL (2002) Arsenic hyperaccumulator Pteris vittata L. and its arsenic accumulation. Sci Bull 47(11):902–905
Chen TB, Liao XY, Huang ZC, Lei M, Li WX, Mo LY, An ZZ, Wei CY, Xiao XY, Xie H (2007) Phytoremediation of arsenic-contaminated soil in China. In: Willey N (ed) Phytoremediation: Methods and Reviews. Humana Press, NJ, pp 393–404
Chen RS, De Sherbinin AD, Ye C, Shi GQ (2014) China’s soil pollution: farms on the frontline. Science 344(6185):691–691
De Temmerman L, Ruttens A, Waegeneers N (2012) Impact of atmospheric deposition of As, Cd and Pb on their concentration in carrot and celeriac. Environ Pollut 166(11):187–195
De Temmerman L, Waegeneers N, Ruttens A, Vandermeiren K (2015) Accumulation of atmospheric deposition of As, Cd and Pb by bush bean plants. Environ Pollut 199:83–88
Ent AVD, Baker AJM, Reeves RD, Pollard AJ, Schat H (2013) Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant Soil 362(1-2):319–334
Frérot H, Lefèbvre C, Gruber W, Collin C, Santos AD, Escarré J (2006) Specific Interactions between local metallicolous plants improve the phytostabilization of mine soils. Plant Soil 282(1-2):53–65
Hemen S (2011) Metal hyperaccumulation in plants: a review focusing on phytoremediation technology. J Environ Sci Technol 4(2):118–138
Hu X, Zhang Y, Luo J, Xie MJ, Wang TJ, Lian HZ (2011) Accumulation and quantitative estimates of airborne lead for a wild plant (Aster subulatus). Chemosphere 82(10):1351–1357
Ke XS (2015) Pollution characteristics and source analysis of heavy metals in atmospheric deposition in Chang-Zhu-Tan area. Dissertation, Ocean university of China
Khalid S, Shahid M, Niazi NK, Murtaza B, Bibi I, Dumat C (2017) A comparison of technologies for remediation of heavy metal contaminated soils. J Geochem Explor 182:247–268
Liang SX, Gao N, Li ZC, Shen SG, Li JB (2016) Investigation of correlativity between heavy metals concentration in indigenous plants and combined pollution soils. Chem Ecol 32(9):872–883
Liao XY, Chen TB, Xie H, Liu YR (2005) Soil as contamination and its risk assessment in areas near the industrial districts of chenzhou city, southern china. Environ Int 31(6):791–798
Liu H, Probst A, Liao B (2005) Metal contamination of soils and crops affected by the Chenzhou lead/zinc mine spill (Hunan, China). Sci Total Environ 339(1):153–166
Luo J, He M, Qi S, Wu J, Gu X (2018) Effect of planting density and harvest protocol on field-scale phytoremediation efficiency by Eucalyptus globulus. Environ Sci Pollut Res 7:1–8
McGrath SP, Zhao FJ (2003) Phytoextraction of metals and metalloids from contaminated soils. Curr Opin Biotechnol 14(3):277–282
Mendez MO, Maier RM (2008) Phytostabilization of mine tailings in arid and semiarid environments-an emerging remediation technology. Environ Health Perspect 116(3):278–283
Ministry of Ecology and Environment and State Market Supervision and Administration (2018a) Soil environmental quality: risk control standard for soil contamination of agrcultrial land (GB 15618-2018)
Ministry of Ecology and Environment and State Market Supervision and Administration (2018b) Soil environmental quality: risk control standard for soil contamination of development land (GB 36600-2018)
Ministry of Environmental Protection and Ministry of Land and Resources of P.R. China (2014) “Reports on China’s Soil Pollution Survey” https://www.zhb.gov.cn/gkml/hbb/qt/201404/t20140417_270670.htm. Accessed 17 Apr 2014
National Health and Family Planning Commission and National Food and Drug Administration Supervisory Authority (2017) National standards for food safety of China: the limited value of contaminants in food (GB 2762-2017)
Novo LAB, Castro PML, Alvarenga P, da Silva EF (2017) Phytomining of Rare and Valuable Metals. In: Ansari AA, Gill SS, Gill R et al (eds) Phytoremediation - management of environmental contaminants, vol 5. Springer International Publishing, Cham, pp 469–486
Novo LAB, Castro PML, Alvarenga P, da Silva EF (2018) Plant growth-promoting rhizobacteria-assisted phytoremediation of mine soils. In: Bio-Geotechnologies for Mine Site Rehabilitation. Elsevier, pp. 281–295
Pinto AP, Varennes AD, Fonseca R, Teixeira DM (2015) In: Ansari AA et al (eds) Phytoremediation: Management of Environmental ContaminatsPhytoremediation of soils contaminated with heavy metals: techniques and strategies, vol 2015. Springer, pp 133–155
Rascio N, Navariizzo F (2011) Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting. Plant Sci 180(2):169–181
Sainger PA, Dhankhar R, Sainger M, Kaushik A, Singh RP (2011) Assessment of heavy metal tolerance in native plant species from soils contaminated with electroplating effluent. Ecotox Environ Safe 74(8):2284–2291
Sarwar N, Imran M, Shaheen MR, Ishaque W, Kamran MA, Matloob A, Rehin A, Hussain S (2017) Phytoremediation strategies for soils contaminated with heavy metals: modifications and future perspectives. Chemosphere 171:710–721
Shahid M, Xiong T, Castrecrouelle M, Leveque T, Dumat C (2013) Water extraction kinetics of metals, arsenic and dissolved organic carbon from industrial contaminated poplar leaves. J Environ Sci-China 25(12):2451–2459
Shahid M, Dumat C, Khalid S, Schreck E, Xiong T (2016) Foliar heavy metal uptake, toxicity and detoxification in plants: a comparison of foliar and root metal uptake. J Hazard Mater 325:36–58
She W, Jie YC, Xing HC, Luo ZQ, Kang WL, Huang M, Zhu SJ (2011) Absorption and accumulation of cadmium by ramie (Boehmeria nivea) cultivars: A field study. Acta Agric Scand Sect B Soil Plant 61(7):641–647
Sheoran V, Sheoran AS, Poonia P (2010) Role of hyperaccumulators in phytoextraction of metals from contaminated mining sites: a review. Crit Rev Environ Sci Technol 41(2):168–214
Sherameti I, Varma A (2011) Detoxification of heavy metals. Ser: Soil Biol 30:448
Singh N, Ma LQ (2006) Arsenic speciation, and arsenic and phosphate distribution in arsenic hyperaccumulator Pteris vittata L. and non-hyperaccumulator Pteris ensiformis L. Environ Pollut 141(2):238–246
Wan XM, Lei M, Liu YR, Huang ZC, Chen TB, Gao D (2013) A comparison of arsenic accumulation and tolerance among four populations of Pteris vittata, from habitats with a gradient of arsenic concentration. Sci Total Environ 442(442):143–151
Wang HY, Stuanes AO (2003) Heavy metal pollution in air-water-soil-plant system of Zhuzhou City, Hunan Province, China. Water Air Soil Pollut 147(1-4):79–107
Wang X, Liu YG, Zeng GM, Chai LY, Song XC, Min ZY, Xiao X (2008) Subcellular distribution and chemical forms of cadmium in Bechmeria nivea (L.) Gaud. Environ Exp Bot 62(3):389–395
Wei CY, Wang C, Yang LS (2009) Characterizing spatial distribution and sources of heavy metals in the soils from mining-smelting activities in Shuikoushan, Hunan Province, China. J Environ Sci 21(9):1230–1236
Xie YC, Lu JJ, Ma DS, Zhang RQ, Gao JF, Yao Y (2013) Origin of granodiorite porphyry and mafic microgranular enclave in the Baoshan Pb-Zn polymetallic deposit, southern Hunan Province: zircon U-Pb chronological, geochemical and Sr-Nd-Hf isotopic constraints. Acta Petrol Sin 29(12):4186–4214 In Chinese
Xiong TT, Leveque T, Austruy A, Goix S, Schreck E, Dappe V, Sobanska S, Foucault Y, Dumat C (2014) Foliar uptake and metal(loid) bioaccessibility in vegetables exposed to particulate matter. Environ Geochem Health 36(5):897–909
Yang B, Zhou M, Shu WS, Lan CY, Ye ZH, Qiu RL, Jie YC, Cui GX, Wong MH (2010) Constitutional tolerance to heavy metals of a fiber crop, ramie (Boehmeria nivea), and its potential usage. Environ Pollut 158(2):551–558
Yoon J, Cao X, Zhou Q, Ma LQ (2006) Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Environ 368(2):456–464
Zhang H, Hall N, Mcelroy JS, Lowe EK, Goertzen LR (2016) Complete plastid genome sequence of goosegrass ( eleusine indica ) and comparison with other poaceae. Gene 600:36–43
Zhou H, Zeng M, Zhou X, Liao BH, Liu J, Lei M, Zhong QY, Zeng H (2013) Assessment of heavy metal contamination and bioaccumulation in soybean plants from mining and smelting areas of southern Hunan Province, China. Environ Toxicol Chem 32(12):2719–2727
Zu Y, Li Y, L, Schvartz C, Langlade, L, Liu F (2004) Accumulation of Pb, Cd, Cu and Zn in plants and hyperaccumulator choice in Lanping lead–zinc mine area, China, Environ Int, 30(4): 567–576
Acknowledgments
We thank Professor Zhenli He and Dr. Beibei Liu from the University of Florida for the linguistic assistance during the preparation of this manuscript.
Funding
This study was funded by the Hainan Provincial Natural Science Foundation of China (319QN274), the key Science and Technology Program of Hainan Province (ZDKJ2017002), and the Central Public-interest Scientific Institution Basal Research Fund for Chinese Academy of Tropical Agricultural Sciences Environment and Plant Protection Institute (no. 2018hzs1J004, no. 2016hzs1J008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Table S1
(DOCX 12 kb)
Rights and permissions
About this article
Cite this article
Pan, P., Lei, M., Qiao, P. et al. Potential of indigenous plant species for phytoremediation of metal(loid)-contaminated soil in the Baoshan mining area, China. Environ Sci Pollut Res 26, 23583–23592 (2019). https://doi.org/10.1007/s11356-019-05655-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05655-4