Abstract
Bacteria-derived biochars from Bucillus sp. biomass under different pyrolysis temperature (250 °C, 350 °C, 450 °C, and 550 °C, respectively) were prepared, forming polyptychial, mesoporous graphite-like structure. The adsorption and sequestration efficiencies of Cd2+ by these biochars were evaluated, and the underlying mechanisms were then discussed. Cd2+ sorption data could be well described by Langmuir mode while the pseudo-second-order kinetic model and Elovich model best fitted the kinetic data. The functional groups complexation, cation-π interactions, and interaction with minerals (including surface precipitation with phosphorus and ion exchange) jointly contributed to Cd2+ sorption and sequestration on biochar, but the interaction with minerals played a dominant role by forming insoluble cadmium salt composed by polycrystalline and/or amorphous phosphate-bridged ternary complex. The maximum sorption capacity of BBC350 in simulated water phase of soil for Cd2+ was 34.6 mg/g. Furthermore, the addition of bacteria-derived biochars (1%, w/w) decreased the fractions easily absorbed by plants for Cd in the test paddy soils by 1.9–26% in a 10-day time. Results of this study suggest that bacteria-derived biochar would be a promising functional material in environmental and agricultural application.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soils contaminated with heavy metals (HMs) could have adverse implications for food quality and safety and have attracted much public concern especially after the frightening media reports of cadmium (Cd) in Hunan-grown rice in China in 2013 (Yu 2016). Based on a 9-year survey of contaminants in soils from 2005 to 2013 conducted by China’s Ministry of Environmental Protection (MEP) and Ministry of Land and Resources (MLR), 19.4% agricultural soils surveyed was classified as being contaminated, of which HM contamination accounts for the majority (82.4%) (MEP 2016). Notably among the HMs, Cd ranks the first in the percentage of 7.0% soil samples exceeding the MEP limit (MEP 2016). A substantial amount of Cd in agricultural soil is bioavailable to crops, and the bioavailability was highly influenced by Cd mobility.
Sequestration of soil Cd would be a potential remedial strategy for reducing the health and environmental risk. Indeed, current remedial actions of HM-contaminated soil recur to two strategies (Liu et al. 2018a): (i) ex situ extracting and/or separating HMs from soil including flotation and washing, with good remediation efficiency but high costs, and (ii) in situ stabilization of HMs including passivation, immobilization, and bio-mineralization, and are widely applied due to easy operation, low cost, and reliable remediation efficiency (Li et al. 2018). Recently, HM stabilization has been attracting domestic and international researchers’ concerns in the field of soil remediation. Many stabilization sorbents have been studied to immobilize Cd in soil, including activated carbon, organic material, microbial biomass, and agricultural waste materials, but most of the sorbents have limitations of either low efficiency, re-release of HMs after stabilization, or disposal restrictions (Peng et al. 2009; Liu et al. 2018a). To solve this issue, a high efficiency and environment-friendly sorbents with strong passivation capacities for HM ions are urgently needed.
Biochar is C-rich residue of incomplete combustion of biomass under oxygen-limited conditions at relatively low temperatures (< 700 °C). It is a promising soil conditioner for agricultural and environmental field because of its high surface area, porosity, sufficient functional groups, and easy accessibility, which distinguishes it from other carbonaceous products such as activated C (AC) and charcoal (Ahmad et al. 2014). Furthermore, application of biochar to soil can also help improve soil properties and enhance soil quality by retaining higher moisture, elevating nutrients, and microbial activity, therefore increasing crop yields (O'Connor et al. 2018; Yu et al. 2018).
The underlying mechanisms for Cd immobilization on biochar included (i) metal-ligand complexation; (ii) metal ion exchange with Cd2+; (iii) interaction between Cd2+ and π electrons (e.g., C=C, C=O); (iv) co-precipitation with mineral groups (e.g., PO43−, CO32−) (Zhang et al. 2015; Qiao et al. 2018; Xiao et al. 2018). The above mechanisms were enslaved by the components in raw materials (e.g., hemicellulose, cellulose, and lignin in plant-derived feedstocks) and pyrolysis temperature (Yang et al. 2007). In case of raw materials, bacterial biomass materials are good precursors because of their natural polyptychial spacial structure such as peptidoglycan layer. Peptidoglycan layer is a three-dimension network stereostructure and composed by linear glycan strands cross-linked by short peptides (Matias and Beveridge 2005). Thus, the polyptychial mesoporous graphite-like structure would probably occur in biochar derived by bacteria biomass, which would be expected to promote Cd immobilization. In addition, phosphorus in bacterial can be transformed from organic phosphorus to inorganic phosphorus during carbonization process. The transformation might also strengthen Cd sequestration by the elevated sorption sites for Cd2+ (Zhao et al. 2016). Interestingly, microcrystalline graphitic sheets (disordered graphene layers) within biochar stacked together and the graphitic sheets grew with increasing carbonization temperature beyond 600 °C. Consequently, the formation of graphitic sheets within biochar increased the π/cation-π interactions and thereby improved sorption of heavy metals on biochar (Harvey et al. 2011; Klupfel et al. 2014).
In order to evaluate the impacts of the morphology and structure of bacteria-derived biochars on adsorption and sequestration of Cd2+, this study thus investigated the adsorption isotherms (such as Langmuir modle and Freundlich model) and adsorption kinetics (including pseudo-first-order model, pseudo-second-order model, and Elovich model) of Cd2+ using biochars derived from Bacillus sp. biomass by the different pyrolysis temperature (250 °C, 350 °C, 450 °C, and 550 °C, respectively). Sequestration effectiveness was also assessed by determining the leaching ratio based on the pH values of soil in the range from 5.0 to 6.0. Mechanisms responsible for Cd sequestration were illuminated using field emission scanning electron microscopy (SEM), transmission electron microcopy (TEM), energy dispersive X-ray analysis (EDX), select area electron diffraction (SAED), TEM mapping, and X-ray photoelectron spectroscopy (XPS). The results of this study would be expected to deepen the comprehension and broaden insights of the sequestration behavior of Cd in soils in the future remediation soils contaminated by HMs.
Materials and methods
Chemicals and materials
3CdSO4·8H2O (ultrapure grade) and other inorganic reagents (analytical grade) were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai. Ultrapure water was used for all the experiments. Soils were collected from the top layer (0–20 cm) of the paddy soil located in YangJi village, Xiangtan, China. The content of Cd in collected paddy soil was 0.7 mg/kg and soil pH was 6.3, which was significantly higher than the Chinese environmental quality standard for agricultural soils (GB 15618–2018, 0.4 mg/kg at 5.5 < pH < 6.5).
Biochar preparation
Gram-positive bacterial cells of Bacillus sp. were cultivated in LB medium at 30 °C for 36 h before centrifugation (4000 rpm for 15 min) and were subsequently freeze-dried (LGJ-10, Henan Brother Equipment Co., Ltd., China). The powdered biomass was tightly placed in a ceramic pot, and then pyrolyzed in a tube furnace (OTF-1200X, HF-Kejing, China) under N2 atmosphere (200 mL/min, 0.025 MPa), the pyrolysis was programmed to raise the internal biomass chamber temperature to the desired temperatures (250, 350, 450, and 550 °C, respectively) at a rate of 10 °C/min for 2 h (Luo et al. 2018). The bacteria-derived biochars were denoted as BBC250, BBC350, BBC450, and BBC550 in accordance with the desired pyrolysis temperature. All the biochar samples were ground to pass through a 0.25-mm sieve prior to use.
Bacteria-derived biochar characterizations
The special surface area and pore size distribution of bacteria-derived biochars (including BBC250, BBC350, BBC450, and BBC550) were analyzed by a NOVA-2200e Surface Area and Pore Size analyzer (Quantachrome, Boynton Beach, Florida, USA), respectively. After degassification under vacuum for 9 h at 150 °C, nitrogen absorption-desorption isotherms of the above bacteria-derived biochar samples were carried out. The surface area was calculated with the Brunauer-Ebammett-Teller (BET) method based on nitrogen sorption data in the relative pressure (P/P0) range of 0 to 1. Pore volume and pore diameter were determined using the Barret-Joyner-Halender (BJH) method based on N2 adsorption data. The total pore volume was obtained from the amount of nitrogen absorbed at a relative pressure (P/P0) of 0.98 approximately. The pore size distribution was analyzed by a nonlocal density functional theory method using the data of nitrogen adsorption, which was assumed a slit pore model (Yuan et al. 2018).
To evaluate influences of pyrolysis temperature on the bacteria-derived biochar structure ordering, Raman spectra measurements were carried out on a Renishaw InVia Raman microscope (inVia, Renishaw, UK) with the excitation laser wavelength of 532 nm at room temperature (Liu et al. 2014).
Morphology and microstructure characteristics of bacteria-derived biochars before and after the adsorption of Cd2+ were observed using field emission scanning electron microscopy (SEM, JSM-6610LV, JEOL, Japan) at an accelerating voltage of 30 kV and transmission electron microcopy (TEM, Tecnai G2F30, FEI, USA) coupled with an energy dispersive X-ray system (EDX) at an acceleration voltage of 300 kV. High-resolution transmission electron microscopy (HRTEM) equipped with the select area electron diffraction (SAED) and TEM mapping were further used to investigate the potential crystal structure and distribution of adsorbed Cd2+ on bacteria-derived biochar (Yoo et al. 2018).
The surface chemical compositions of bacteria-derived biochar before and after the adsorption of Cd2+ (here referring to the bonding energies of Cd, P, O, and C) were determined using X-ray photoelectron spectroscopy (XPS, Escalab 250xi, Thermo Scientific, USA) (Guo et al. 2017).
Sorption experiments
Cadmium stock solution (1000 mg/L) was prepared by dissolving 3CdSO4·8H2O in 0.01 mol/L NaNO3 solution that simulated soil water phase background electrolyte. All vessels and experimental apparatuses were strictly acid-cleaned before use so as to avert the residual of metal ions.
The adsorption kinetic experiments were performed as follows: 10 mg BBC250, BBC350, BBC450, and BBC550 samples were placed into 10-mL solution containing 25 mg/L Cd2+ in glass vials, respectively, and then, the above glass vials were placed in a shaker (150 rpm) at 25 °C. At certain sampling time interval (1, 2, 3, 5, 7, 9, 11, 24, and 48 h), 10-mL subsamples were taken and Cd concentrations after filtration with 0.22-μm filters (MCE, Green Union Science Instrument Co., Ltd., China) were measured by air-acetylene flame atomic absorption spectrometry (AAS, AA-6300C, Shimadzu Co. Itd., Suzhou, China) equipped with an air-acetylene flame atomizer and a hollow cathode lamp (AS-1, Beijing, China).
The adsorption isotherm experiments were carried out as the same conditions described above. In brief, 10-mg BBC250, BBC350, BBC450, and BBC550 samples were added into 10-mL NaNO3 solution with different Cd levels (10, 15, 20, 25, 50, 80, and 100 mg/L) in 20-mL glass vials with Teflon-lined screw caps, respectively. The above mixture was placed in a shaker under 150 rpm at 25 °C for 24 h. After equilibrium, BBC250, BBC350, BBC450, and BBC550 and liquid phases were separated by centrifugation (3000 rpm for 15 min) before filtering through 0.22-μm filters.
Adsorption model and kinetics analysis
The adsorption amount of Cd2+ on bacteria-derived biochar at the certain time t (qt, mg/g) was calculated using Eq. 1 (Wang et al. 2018),
where qt is the amounts of Cd2+ adsorbed onto the bacteria-derived biochar (mg/g), V is the volume of the solution (L), m is the amount of adsorbent (g), C0 and Ct represent the concentration of Cd2+ at initial time and at time t, respectively (mg/L), bacteria-derived biochar was referred to BBC250, BBC350, BBC450, and BBC550, respectively.
The pseudo-first-order and pseudo-second-order models (Luo et al. 2018) were used to investigate the effects of contact time on Cd adsorption on the bacteria-derived biochar, and the correlation coefficient (R2) was considered as an evaluation of the consistency between the experimental data and the models. The pseudo-first-order kinetic model (Eq. 2) is given as follows:
The pseudo-second-order kinetic model consists by all the steps of adsorption including adsorption, external film diffusion, and internal particle diffusion. The equation is defined as Eq. 3.
Elovich model is another common model to reveal the chemisorption process, which assumes that the adsorbate is adsorbed onto the solid surface without desorption, and the adsorption rate reduces with increasing contact time due to an increased surface (Chien and Clayton 1980). The equation is expressed as Eq. 4,
where qe and qt (mg/g) represent the content of adsorbed Cd at the equilibrium and at time t, respectively. k1 (h−1) and k2 (g/(mg/h)) represent adsorption rate constants. α (mg/(g·min)) is the initial adsorption rate and β (g/mg) is the constant concerned with the surface coverage and the chemisorption activation energy.
To compare the adsorption capacity and intensity of Cd2+ onto bacteria-derived biochar, Langmuir and Freundlich models were employed by fitting the experimental data of the adsorption isotherm. The equations of the two models are shown below (Gao et al. 2019):
where qe (mg/g) and Ce (mg/L) represent the adsorption amount and the equilibrium concentration of Cd, respectively. The KL (L/mg) and qmax (mg/g) express the Langmuir adsorption constant and the maximum adsorption amount, respectively. KF and n express the uptake capacity and adsorption intensity, respectively.
Sequestration capacity of Cd2+ by bacteria-derived biochar
To estimate the sequestration capacity of bacteria-derived biochar for Cd2+, BBC350 adsorbed Cd2+ was selected to carry out the leaching experiment. Briefly, Cd-loaded BBC350 (0.5 g) was placed in a 1000-mL flask containing 500-mL HNO3 solution (0.1 mol/L) in a shaker under 150 rpm at 25 °C for 480 min. After that, the supernatant was filtered by a 0.22-μm filter after centrifugation (3000 rpm for 15 min). The concentration of Cd2+ in filtrate was analyzed by AAS.
The sequestration ratio (SR) for Cd2+ by BBC350 was calculated as the following formulae, Eq. 7 and Eq. 8.
where C0 is the initial content of Cd2+ concentration (mg/L), Ca is the concentration of Cd2+ (mg/L) at the equilibrium time (24 h) during adsorption step in the section of 2.3, CL is the leaching concentration of Cd2+ (mg/L) at the equilibrium time (240 min) during leaching step. Ee (%) is the equilibrium elution efficiency of leaching step represented the proportion of the elution amount during the desorption step occupied in the amount of adsorption amount in previous adsorption step, which is calculated by the below Eq. 8.
Phosphorus release from bacteria-derived biochar
Phosphorus release from BBC250, BBC350, BBC450, and BBC550 was separately investigated through a batch leaching experiment (Zhao et al. 2016). Briefly, biochar was mixed with 0.01 mol/L NaNO3 solution at a solid/liquid ratio of 1:1 (w/v) and then agitated at 25 °C for 72 h. At certain sampling time intervals (1, 2, 3, 5, 7, 11, 24, 48, and 72 h), the leachate was collected and filtered using a 0.22-μm filter. After that, the phosphorus concentrations in the leachate were determined by colorimetry using the molybdate/ascorbic acid method (Cao and Harris 2010).
Quantitative analysis of sorption contribution ratio involved in different Cd2+ sorption mechanisms on bacteria-derived biochars
The sorption contributions of Cd2+ sorption on bacteria-derived biochars involving in the interaction and precipitation with minerals (Qmin), functional group complexation (Qco), and Cd2+-π coordination (Qcπ) were analyzed as the methods described by Pal and Maiti (2019). Briefly, the surface minerals on bacteria-derived biochars were removed by soaking in 1 mol/L HCl solution overnight and then washed using pure water until to the neutral pH value. The reduced amount of Cd2+ from Cd-loaded bacteria-derived biochars by acidic soaking was considered as the contribution of these removed minerals, and the yield of demineralized biochars from initial bacteria-derived biochars was calculated to offset the concentration effect. The sorption involved in the interaction with minerals (Qmin) was calculated by Eq. 9:
where Qmin (mg/g) is the amount of Cd2+ sorption attributed to the interaction with minerals, Qt (mg/g) is the total sorption of Cd2+ on bacteria-derived biochars, Qa (mg/g) is the amount of adsorbed Cd2+ on demineralized bacteria-derived biochars, Y is the yield of demineralized biochars from initial bacteria-derived biochars.
Additionally, in order to the coordination with the oxygen-containing organic groups on the surface of bacteria-derived biochars, a drop of 1 mol/L HCl solution was added to demineralized bacteria-derived biochars before and after Cd2+ sorption, where the chemical interaction below could exist:
Thus, the amount of H+ release could represent the contribution of functional group complexation (Qco) to the adsorption of Cd2+. It could be calculated by Eq. 10:
where Qco (mg/g) is the amount of Cd2+ sorption attributed to the functional groups complexation, QpH (mg/g) is the amount of adsorbed Cd2+ by functional groups complexation, Y is the yield of demineralized biochar from initial bacteria-derived biochars.
Cd2+ sorption on demineralized bacteria-derived biochars was the sum of Cd2+-π interaction and functional group complexation together, so the amount of Cd2+ sorption by Cd2+-π interaction (Qcπ) could be calculated by Eq. 11:
Finally, the contribution ratios of Cd2+ sorption mechanisms involved in the interaction and precipitation with minerals, functional group complexation, and Cd2+-π coordination were calculated using the Qmin/Qt, Qco/Qt, and Qcπ/Qt, respectively.
Soil experiment
To verify the adsorption and sequestration capacity for Cd2+ in soil by bacteria-derived biochars, BBC250, BBC350, BBC450, and BBC550 were added as a ratio of 1% (w/w) to the tested paddy soils in pots under 70% soil moisture content for 10 days. No biochar addition was used as the control check (CK). Each treatment was triplicate. Soil samples were collected after 10 days and extracted continuously by 1.0 mol/L MgCl2 at pH = 7 (exchangeable Cd) and 1.0 mol/L sodium acetate at pH = 5 (carbonate-bound Cd) (Tessier et al. 1979). The sum of exchangeable Cd and carbonate-bound Cd was considered to be the fractions easily absorbed by plants (FEA) for Cd in the tested soils, and FEA-Cd could be used to predict the bioavailabilities of heavy metals in soils and correlate with plant uptake (Walker et al. 2003).
Cadmium determination
Before measurement, samples were immediately acidified to pH < 2 with concentrated HNO3. Cd2+ concentrations were measured by AAS. The calibration curve of Cd2+ was calculated by serial gradient solutions of Cd2+ in the range from 0.025 to 1.0 mg/L. The detection limit of Cd2+ was 0.00337 mg/L, and the relative standard deviation (RSD) was 0.2% (n = 6). FEA-Cd contents in the tested paddy soils were determined with ICP-MS (iCAP Qc, Thermo Scientific, USA). The instrumental calibration, data acquisition, and data analysis were controlled by the instrument software with the detection limit of 0.0006 μg/L. The standard solutions of 0, 5.0, 10.0, 20.0, 40.0, and 50 μg/L were used for instrumental calibration. The correlation coefficient in standard curve was > 0.999. Each test was replicated three times.
Statistical analysis
One-way analysis of variance (ANOVA) was used to assess significant differences at p ≤ 0.05 among groups (Origin 8.5). All of the experiments were performed in triplicate and the data are presented as mean ± standard deviation (SD).
Results and discussion
Properties of bacteria-derived biochar
The surface area and total pore volume of biochar are significant parameters related to adsorption efficiency for heavy metal ions, which influences amounts of binding sites (O'Connor et al. 2018). The surface areas of BBC250, BBC350, BBC450, and BBC550 were 24.0 m2/g, 65.2 m2/g, 38.2 m2/g, and 4.7 m2/g, respectively. Nitrogen sorption analysis showed that BBC250, BBC350, BBC450, and BBC550 presented the type IV isotherm characteristics of mesoporous material, and their narrow pore width distribution centered at 53.61 nm, 53.34 nm, 62.66 nm, and 46.81 nm, respectively (Fig. 1a and Table S1).
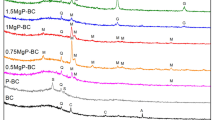
Raman spectra of BBC350, BBC450, and BBC550 showed that two important Raman peaks 1355 cm−1 (D-band) induced by sp3 hydridization and 1573 cm−1 (G-band) originated from crystalline graphitic/sp2 carbon atoms, respectively (Fig. 1b), suggesting the appearance of graphitic-like structure in biochar (Mendonca et al. 2017). The intensity ratio of the D and G bands (ID/IG) indicates the graphitization degree and density of defects for carbon materials (Jiang et al. 2016). Here, the ID/IG increased from 0.781 to 0.882 with an increased pyrolytic temperature from 350 to 550 °C, elucidating that newly formed sp2-hybridized domains were dominant with higher defects, and preserved six or more fused hexatomic rings (suggesting the presence of aromatic or graphitic structures). The G peaks of bacteria-derived biochars were broadened significantly with the increased pyrolytic temperature from 350 to 550 °C, for instance, the G peaks of BBC550 from 1466.8 to 1746.9 cm−1. This result could be related to the formation of graphitic and/or aromatic rings structures in biochar during the process of pyrolysis from 350 to 550 °C (Abdul et al. 2017).
The microstructures of BBC250, BBC350, BBC450, and BBC550 were presented by SEM and TEM images (Fig. 2 and Fig. S1). BBC250 and BBC350 showed typically rough and porous natures, while BBC450 and BBC550 clearly appeared polyptychial structures and visible pores, which convincingly supported the results of Raman spectra (Fig. 1b). Also, the TEM observations of BBC350 and BBC550 were similar with the membrane structure of graphene oxide (Chen et al. 2017). Overall, these results shown in Table S1, Figs. 1 and 2 show that these biochars had mesoporous, polyptychial, and graphene-like structure, which suggested a greater potential for bacteria-derived biochar to sequestrate Cd2+ compared to simple pore structure of other biochars.
Adsorption isotherms and kinetics of Cd2+ on bacteria-derived biochar
The adsorption kinetics of Cd2+ on BBC250, BBC350, BBC450, and BBC550 including the pseudo-first-order, pseudo-second-order, and Elovich models are shown in Fig. 2 while the kinetics fitting parameters for the adsorption of Cd2+ onto BBC250, BBC350, BBC450, and BBC550 are listed in Table S2. As shown in Fig. 2a and Table S2, the pseudo-second-order model better fitted the adsorption of Cd2+ on bacteria-derived biochar as suggested by their higher correlation coefficients (R2) than that of pseudo-first-order model. This indicated that the adsorption of Cd2+ on bacteria-derived biochar was dominated by chemisorption mechanism (Guo et al. 2017). Furthermore, the correlation coefficient of Elovich model was higher than 0.90 except for that of BBC250, which also suggested that chemical interactions played an important role during the adsorption process of Cd2+ onto bacteria-derived biochar (Deng et al. 2019). Additionally, hexagon graphitic sheets occurred within BBC350, BBC450, and BBC550 (Fig. 1b), which could induce cation-π interaction, and thereby increase the adsorption of Cd2+ on bacteria-derived biochars. This result was consistent with the findings of Harvey et al. (2011)), where they confirmed that cation-π bonding mechanisms play a crucial role in the sorption of HM ions on biochars.
As shown in SEM images (Figs. 2 and S1), bacteria-derived biochars have more homogeneous surface and more obvious multilayered structure with the increase of pyrolytic temperature, which was against the initial cadmium sorption and had much longer diffusive interaction time. For instance, the adsorption rates (k2) of BBC250, BBC350, and BBC450 decreased from 0.063 to 0.008 g/(mg/h) with temperature increasing from 250 to 450 °C. The higher k2 values signified the higher initial sorption rate in the pseudo-second-order model. Also, the initial sorption rate is related to the diffusive interaction time affected by biochar structure. The structure with more active adsorption sites would have higher k2 values. So, the rough and monolayer structure of BBC250 with more active adsorption sites contribute to the high initial sorption rate. These results implied that porosities and structural differences of bacteria-derived biochar could cause different diffusive interaction time so as to affect the initial cadmium sorption, which is similar with the conclusion of Abdul et al. (2017). The parameters of Elovich model (α and β) (Table S2) showed that the values of α (mg/(g/h)) were remarkably bigger than β (g/mg) for the tested bacteria-derived biochars (p < 0.05), which suggested that adsorption was larger than desorption during the adsorption process of Cd2+ onto bacteria-derived biochar (Liang et al. 2014).
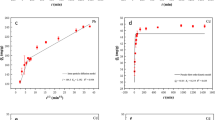
The adsorption isotherms (including Langmuir and Freundlich) and their fitting parameters of Cd on biochar are shown in Fig. 3b and Table S3, respectively. The R2 of Langmuir model (the R2 value of BBC250, BBC350, BBC450, and BBC550 is 0.9516, 0.9597, 0.9985, and 0.9925, respectively) was higher than Freundlich model (the R2 value of BBC250, BBC350, BBC450, and BBC550 is 0.9145, 0.6417, 0.8942, and 0.6981, respectively), suggesting that Langmuir model could better describe the adsorption process of Cd2+ onto bacteria-derived biochar and the monolayer coverage sorption of Cd2+ and homogeneous distribution of adsorption sites on the surface of biochar (Li et al. 2016). The maximum adsorption capacities of BBC250, BBC350, BBC450, and BBC550 for Cd2+ calculated by Langmuir fitting were 51.52 mg/g, 38.49 mg/g, 22.21 mg/g, and 13.02 mg/g, respectively (Table S3), whereas the experimental adsorption capacities qe of BBC250, BBC350, BBC450, and BBC550 were 34.0 mg/g, 34.6 mg/g, 20.7 mg/g, and 12.0 mg/g, respectively (Fig. 3b). As shown in Table S1, BBC350 obviously possessed the largest surface area and total pore volume, which may be one of the reasons for its higher adsorption capacity. X-ray diffraction analysis (XRD, Fig. S6) exhibits that the (002) peak of BBC250 shifts to lower angle as compared with BBC450, meaning larger interlayer distance of BBC250 attributed to its layered structure, which lead to higher specific surface area (SSA) for BBC250 (Long et al. 2015). The graphene-like layers may stack up and the differences of interlayer distance may be the reason for biochar prepared in low temperature has bigger SSA. Notably, the adsorption capacity of bacteria-derived biochar for Cd2+ decreased with an increased pyrolytic temperature from 250 to 550 °C, which might be because that the functional group C=C (1582 cm−1) became stronger whereas C=O (1647 cm−1) became weaker when temperature increased from 250 to 550 °C, resulting in a weaker cation-π interaction for BBC450 and BBC550 (as the results of FTIR shown in Fig. S3) (Deng et al. 2019).
The high temperature was favored for sorption of Cd2+ on straw biochar because of their richer pore structure with the increase of pyrolysis temperature (Liu and Fan 2018; Gao et al. 2019); however, straw biochar can only grab Cd2+ on the surface. The sorption capacity of bacterial biochar for Cd2+ decreased as the pyrolysis temperature increased, which might be due to larger interlayer distance and rougher surface in low temperature biochar such as BBC250. However, the polyptychial mesoporous structure of bacterial biochar could help the precipitate contained Cd entering into the layer space and subsequently be sequestrated, which was verified by SEM image (Fig. 2d) and the lattice line and diffraction ring in HRTEM image (Fig. 4g, h). More importantly, the differences presented between bacterial biochar and straw biochar are derived from their natural structure differences. Straw are mainly consisted of hollow tubular structure while bacteria are mainly composed by peptidoglycan layer. After pyrolysis, straw biochar or bacteria-derived biochar remained corresponding natural structures. Thus, the properties and structures of bacteria-derived biochar are different with that of straw biochar, resulting in many differences described above.
Sequestration of Cd2+ by bacteria-derived biochar
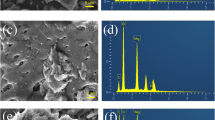
As expected, the changes of the structural morphology and properties for BBC250, BBC350, BBC450, and BBC550 were favorable to adsorption and sequestration of Cd2+. As shown in SEM images Fig. 2c, d, the surface of BBC550 was relatively uniform and smooth before adsorption of Cd2+. While some visible particles (possibly precipitates and/or minerals) appeared on the surface and inside layers of BBC550, suggesting Cd2+ reacted with the functional groups and/or components of biochar to form the Cd-related compounds (Zhang et al. 2015).
Compared to pre-sorption bacteria-derived biochars, many black depositional dots with distinct shape were remarkably observed on HRTEM image of Cd-loaded BBC450 (Fig. 4a). The corresponding EDS spectra of the depositional dots showed high peaks of Cd, P, and O elements (Fig. 4i), which demonstrated the sequestration of Cd2+ was strongly associated with P and O (Chen et al. 2018). The results were further supported by the results of TEM-mapping analysis (Fig. 4b–f). The phosphate-bridged ternary complexes, such as P–Cd and P–O–Cd, might be the key compounds responsible for effective sequestration of Cd2+ (Ma et al. 2015; Liu et al. 2018b; Lei et al. 2019). To obtain an insight into the detailed characteristics of flocculent sedimentary particles, HRTEM equipped with electron diffraction was carried out. BBC450 after adsorption of Cd2+ displayed obvious crystal and amorphous natures, where the lattice fringe spacing of observed crystal particles was approximately 0.225 nm (Fig. 4g), and the diffraction ring features were remarkable (Fig. 4h). In contrast, no lattice fringe (Fig. S3b) and corresponding diffraction ring (Fig. S3c) were observed in the control biochar (without Cd2+ sorption). These results suggested that Cd2+ adsorbed onto bacteria-derived biochar formed polycrystalline cadmium salt (Li et al. 2018), and highlighted the critical role of phosphate-bridged ternary complexes formation in the sequestration of Cd2+ and thereby reduced bioavailability of Cd2+.
To reveal chemical components of the formed mineral particles on bacteria-derived biochar after adsorption of Cd2+, the Cd-loaded BBC450 were further examined using XPS analysis. As shown in Fig. 5a, two new peaks appeared at binding energy of 405.8 eV and 412.8 eV, indicating the precipitation of Cd mineral(s) onto the bacteria-derived biochar surface. As shown in Fig. 5c, the highest peak energy of “P 2p” for the BBC450 before Cd adsorption was 133.13 eV, whereas that of “P 2p” for BBC450 after Cd adsorption increased to 133.83 eV. This observation indicated that element P might bond with Cd and O atoms during the adsorption process of Cd2+, which was also supported by the higher binding energy of Cd 3d and O 1s (Fig. 5b, d). The results in this study indicated the formation of phosphate-bridged ternary complexes (Zhu et al. 2014; Liu et al. 2018b) probably the cadmium orthophosphate, despite previous studies also reported the other three types of Cd minerals, cadmium carbonate, and cadmium chloride (Zhang et al. 2015) were identified in the post-sorption biochar.
Notably, after Cd2+ adsorption, the peaks at 292.9 eV and 295.7 eV disappeared in C 1s spectrum, referring to K 2p3/2 and K 2p1/2, respectively (Fig. S4), which suggested that K+ was released from BBC450 surface (Chen et al. 2018), implying that the ion exchanges occurred between Cd and surface ions of biochar.
Phosphorus release from biochar favoring sequestration of Cd2+
The aforementioned results illuminated that phosphorus played an important role in Cd2+ sequestration, the phosphorus (here referring to PO43−) release from biochar was thus investigated. As shown in Fig. 6a, BBC250 and BBC350 released more phosphorus than that of BBC450 and BBC550 (i.e., 8.0 and 2.3 mg/g for BBC250 and BBC350 vs. 0.085 and 0.021 mg/g for BBC450 and BBC550, respectively). This result might be ascribed to the decrease of the soluble phosphorus in the biochar produced at more higher pyrolysis temperatures (> 450 °C) because amorphous P-Ca-Mg could crystallize to form less soluble phases at higher temperatures (Cao and Harris 2010). When Cd2+ was added, the concentration of PO43− in supernatant decreased, suggesting that the released phosphorus (e.g., PO43−) favored the binding with Cd2+ and even finally formed polycrystalline particles (Xu et al. 2013; Zhao et al. 2016). In a word, biochar could be considered as a kind of soil additive that could sequestration cadmium ions coupling with slow phosphorus release in term of fertilizers.
The evaluation for sequestration of Cd2+ by bacteria-derived biochar
In order to evaluate the sequestration efficiency of Cd2+ by bacteria-derived biochar, the leaching experiment of Cd2+ from Cd-loaded BBC350 was carried out. As shown in Fig. 7, the adsorption amount of Cd2+ by BBC350 achieved 32.0 mg/g in the sorption stage; however, when the BBC350 adsorbed Cd2+ was added into HNO3 solution (0.1 mol/L), absorbed Cd2+ was released approximately 90% of the adsorption amount of Cd2+ on BBC350 during whole 240 min. The residual Cd amount on BBC350 was 3.2 mg/g, and the residual Cd was sequestrated in the pores of BBC350 in form of insoluble polycrystalline and/or amorphous phosphate-bridged ternary complex.
Contribution of Cd2+ sorption mechanisms
The contribution of the interaction with minerals (Qmin), functional groups complexation (Qco), and Cd2+-π coordination (Qcπ) to the total Cd2+ sorption (Qt) on biochars is shown in Fig. 8. The contribution of Cd2+-π coordination increased with increasing pyrolysis temperature from 350 to 550 °C, while the contribution of interaction with minerals presented the reverse trend. For example, the Qcπ/Qt value of BBC350 was 1.3%; however, the Qcπ/Qt value of BBC550 increased to 13.1%. Conversely, the Qmin/Qt value of BBC350 was 98.4%, but the Qmin/Qt value of BBC550 was only 84.8%. This phenomenon might be mainly because the pyrolysis temperature affected the reserved amount of oxygen containing functional groups and aromaticity difference of biochars (Cui et al. 2016). These results were also consistent with the analysis of the FIRT spectrum (Fig. S2). The Qmin values of bacteria-derived biochars accounted for 70.2–98.4% of their Qt values, indicating that the interaction between Cd2+ and minerals is a dominant mechanism of Cd2+ sorption on bacteria-derived biochars.
FEA-Cd in paddy soil
As shown in Fig. 9, after 10 days, almost all amendments of BBC250, BBC350, BBC450, and BBC550 decreased significantly the content of FEA-Cd in the tested soils (p < 0.05). For instance, the lowest FEA-Cd (49.96%) was achieved by BBC250 amendment compared to the other bacteria-derived biochars. This might be because BBC250 had higher sorption capacity (Fig. 3b). Nevertheless, the addition of bacteria-derived biochars could reduce Cd bioavailability in the tested paddy soils, which would be potentially applied to immobilize Cd in the Cd-contaminated soil.
Conclusions
The results of this study showed that the polyptychial mesoporous graphite-like properties of BBC350 promoted the adsorption and sequestration of Cd2+ indicated by the images of lattice line and diffraction ring. The interaction with minerals (including surface precipitation with phosphorus and ion exchange) played a dominant role by forming insoluble cadmium salt composed by polycrystalline and/or amorphous phosphate-bridged ternary complex during the process of Cd2+ sorption and sequestration. The saturated sorption capacities of bacteria-derived biochars for Cd2+ were 12~34 mg/g in simulated soil water phase. Moreover, bacteria-derived biochar amendments (1%, w/w) reduced the fractions easily absorbed by plants for Cd in the test paddy soils by 1.9–26% during 10 days. Therefore, bacteria-derived biochar would be promisingly applied in remediation of soils contaminated by heavy metals.
References
Abdul G, Zhu X, Chen B (2017) Structural characteristics of biochar-graphene nanosheet composites and their adsorption performance for phthalic acid esters. Chem Eng J 319:9–20
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS (2014) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99:19–33
Cao X, Harris W (2010) Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour Technol 101:5222–5228
Chen L, Shi G, Shen J, Peng B, Zhang B, Wang Y, Bian F, Wang J, Li D, Qian Z, Xu G, Liu G, Zeng J, Zhang L, Yang Y, Zhou G, Wu M, Jin W, Li J, Fang H (2017) Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 550:380–383
Chen Z, Liu T, Tang J, Zheng Z, Wang H, Shao Q, Chen G, Li Z, Chen Y, Zhu J, Feng T (2018) Characteristics and mechanisms of cadmium adsorption from aqueous solution using lotus seedpod-derived biochar at two pyrolytic temperatures. Environ Sci Pollut Res 25:11854–11866
Chien SH, Clayton WR (1980) Application of Elovich equation to the kinetics of phosphate release and sorption in soils 1. Soil Sci Soc Am J 44:265–268
Cui X, Fang S, Yao Y, Li T, Ni Q, Yang X, He Z (2016) Potential mechanisms of cadmium removal from aqueous solution by Canna indica derived biochar. Sci Total Environ 562:517–525
Deng Y, Huang S, Laird DA, Wang X, Meng Z (2019) Adsorption behaviour and mechanisms of cadmium and nickel on rice straw biochars in single- and binary-metal systems. Chemosphere 218:308–318
Gao LY, Deng JH, Huang GF, Li K, Cai KZ, Liu Y, Huang F (2019) Relative distribution of Cd2+ adsorption mechanisms on biochars derived from rice straw and sewage sludge. Bioresour Technol 272:114–122
Guo Z, Zhang X, Kang Y, Zhang J (2017) Biomass-derived carbon sorbents for cd(II) removal: activation and adsorption mechanism. ACS Sustain Chem Eng 5:4103–4109
Harvey OR, Herbert BE, Rhue RD, Kuo LJ (2011) Metal interactions at the biochar-water interface: energetics and structure-sorption relationships elucidated by flow adsorption microcalorimetry. Environ Sci Technol 45:5550–5556
Jiang Y, Yan J, Wu X, Shan D, Zhou Q, Jiang L, Yang D, Fan Z (2016) Facile synthesis of carbon nanofibers-bridged porous carbon nanosheets for high-performance supercapacitors. J Power Sources 307:190–198
Klupfel L, Keiluweit M, Kleber M, Sander M (2014) Redox properties of plant biomass-derived black carbon (biochar). Environ Sci Technol 48:5601–5611
Lei S, Shi Y, Qiu Y, Che L, Xue C (2019) Performance and mechanisms of emerging animal-derived biochars for immobilization of heavy metals. Sci Total Environ 646:1281–1289
Li F, Li D, Li X, Liao J, Li S, Yang J, Yang Y, Tang J, Liu N (2016) Microorganism-derived carbon microspheres for uranium removal from aqueous solution. Chem Eng J 284:630–639
Li F, Wang W, Li C, Zhu R, Ge F, Zheng Y, Tang Y (2018) Self-mediated pH changes in culture medium affecting biosorption and biomineralization of Cd2+ by Bacillus cereus Cd01. J Hazard Mater 358:178–186
Liang Y, Cao X, Zhao L, Xu X, Harris W (2014) Phosphorus release from dairy manure, the manure-derived biochar, and their amended soil: effects of phosphorus nature and soil property. J Environ Qual 43:1504–1509
Liu J, Liu W, Ji S, Wan Y, Yin H, Zhou Y (2014) Facile Synthesis of Carbon-Encapsulated Li4Ti5O12@C Hollow Microspheres as Superior Anode Materials for Li-Ion Batteries. Eur J Inorg Chem 12:2073–2079
Liu L, Fan S (2018) Removal of cadmium in aqueous solution using wheat straw biochar: effect of minerals and mechanism. Environ Sci Pollut Res 25:8688–8700
Liu L, Li W, Song W, Guo M (2018a) Remediation techniques for heavy metal-contaminated soils: principles and applicability. Sci Total Environ 633:206–219
Liu J, Zhu R, Liang X, Ma L, Lin X, Zhu J, He H, Parker SC, Molinari M (2018b) Synergistic adsorption of cd(II) with sulfate/phosphate on ferrihydrite: an in situ ATR-FTIR/2D-COS study. Chem Geol 477:12–21
Long C, Chen X, Jiang L, Zhi L, Fan Z (2015) Porous layer-stacking carbon derived from in-built template in biomass for high volumetric performance supercapacitors. Nano Energy 12:141–151
Luo M, Lin H, Li B, Dong Y, He Y, Wang L (2018) A novel modification of lignin on corncob-based biochar to enhance removal of cadmium from water. Bioresour Technol 259:312–318
Ma L, Zhu J, Xi Y, Zhu R, He H, Liang X, Ayoko GA (2015) Simultaneous adsorption of cd (II) and phosphate on Al 13 pillared montmorillonite. RSC Adv 5:77227–77234
Matias VRF, Beveridge TJ (2005) Cryo-electron microscopy reveals native polymeric cell wall structure in Bacillus subtilis 168 and the existence of a periplasmic space. Mol Microbiol 56:240–251
Mendonca FG, Cunha ITD, Soares RR, Tristao JC, Lago RM (2017) Tuning the surface properties of biochar by thermal treatment. Bioresour Technol 246:28–33
MEP (2016) The Ministry of Land and resources Report on the national soil contamination survey. http://www.gov.cn/foot/2014-2004/2017/content2661768.htm (in Chinese). Accessed 29 Sept 2016
O'Connor D, Peng T, Zhang J, Tsang DCW, Alessi DS, Shen Z, Bolan NS, Hou D (2018) Biochar application for the remediation of heavy metal polluted land: a review of in situ field trials. Sci Total Environ 619-620:815–826
Pal D, Maiti SK (2019) Abatement of cadmium (cd) contamination in sediment using tea waste biochar through meso-microcosm study. J Clean Prod 212:986–996
Peng JF, Song YH, Yuan P, Cui XY, Qiu GL (2009) The remediation of heavy metals contaminated sediment. J Hazard Mater 161:633–640
Qiao JT, Liu TX, Wang XQ, Li FB, Lv YH, Cui JH, Zeng XD, Yuan YZ, Liu CP (2018) Simultaneous alleviation of cadmium and arsenic accumulation in rice by applying zero-valent iron and biochar to contaminated paddy soils. Chemosphere 195:260–271
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem:544e551
Walker DJ, Clemente R, Roig A, Bernal MP (2003) The effects of soil amendments on heavy metal bioavailability in two contaminated Mediterranean soils. Environ Pollut 122:303–312
Wang RZ, Huang DL, Liu YG, Zhang C, Lai C, Zeng GM, Cheng M, Gong XM, Wan J, Luo H (2018) Investigating the adsorption behavior and the relative distribution of Cd2+ sorption mechanisms on biochars by different feedstock. Bioresour Technol 261:265–271
Xiao X, Chen B, Chen Z, Zhu L, Schnoor JL (2018) Insight into multiple and multilevel structures of biochars and their potential environmental applications: a critical review. Environ Sci Technol 52:5027–5047
Xu X, Cao X, Zhao L, Wang H, Yu H, Gao B (2013) Removal of cu, Zn, and cd from aqueous solutions by the dairy manure-derived biochar. Environ Sci Pollut Res 20:358–368
Yang H, Yan R, Chen H, Lee DH, Zheng C (2007) Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86:1781–1788
Yoo S, Chung CC, Kelley SS, Park S (2018) Graphitization behavior of loblolly pine wood investigated by in situ high temperature X-ray diffraction. ACS Sustain Chem Eng 6:9113–9119
Yu WS (2016) Agricultural and Agri-environment policy and sustainable agricultural development in China. IFRO report 247, University of Copenhagen, pp 6–15. https://www.researchgate.net/publication/301635451_Agricultural_and_agrienvironment_policy_and_sustainable_agricultural_development_in_China
Yu H, Zou W, Chen J, Chen H, Yu Z, Huang J, Tang H, Wei X, Gao B (2018) Biochar amendment improves crop production in problem soils: a review. J Environ Manag 232:8–21
Yuan J, Xu S, Zeng HY, Cao X, Dan Pan A, Xiao GF, Ding PX (2018) Hydrogen peroxide biosensor based on chitosan/2D layered double hydroxide composite for the determination of H2O2. Bioelectrochemistry 123:94–102
Zhang F, Wang X, Yin D, Peng B, Tan C, Liu Y, Tan X, Wu S (2015) Efficiency and mechanisms of cd removal from aqueous solution by biochar derived from water hyacinth (Eichornia crassipes). J Environ Manag 153:68–73
Zhao L, Cao X, Zheng W, Scott JW, Sharma BK, Chen X (2016) Copyrolysis of biomass with phosphate fertilizers to improve biochar carbon retention, slow nutrient release, and stabilize heavy metals in soil. ACS Sustain Chem Eng 4:1630–1636
Zhu R, Li M, Ge F, Xu Y, Zhu J, He H (2014) Co-sorption of cd and phosphate on the surface of a synthetic hydroxyiron-montmorillonite complex. Clay Clay Miner 62:79–88
Funding
This work was supported by the National Natural Science Foundation of China (21577118 and 41701580), National Key Research and Development Plan of China (2016YFD0800700), China Postdoctoral Science Foundation Funded Project (2016M590749, 2017T100605, and 2018M642992), the Natural Science Foundation of Hunan Province (2016JJ1024), Key Research and Development Plan of Hunan Province (2018SK2045 and 2018SK2042) and Collaborative Innovation Center of New Chemical Technologies for Environmental Benignity and Efficient Resource Utilization as well as Key Laboratory of Soil Environment and Pollution Remediation, Institute of Soil Science, Chinese Academy of Sciences (SER2017-05).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2897 kb)
Rights and permissions
About this article
Cite this article
Li, F., Tang, Y., Li, C. et al. Adsorption and sequestration of cadmium ions by polyptychial mesoporous biochar derived from Bacillus sp. biomass. Environ Sci Pollut Res 26, 23505–23523 (2019). https://doi.org/10.1007/s11356-019-05610-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05610-3