Abstract
The seasonal succession of phytoplankton functional groups (PFGs), their ecological preferences, relationships between environmental variables and PFGs, and ecological status were investigated in the Batman Dam Reservoir, a warm monomictic reservoir, located in the Tigris River basin of Turkey. Altogether 60 species, 19 functional groups, and 10 prevailing functional groups were identified, and prevailing functional groups showed strong seasonal changes. Centric diatoms Cyclotella ocellata (group B) and Aulacoseira granulata (group P) were dominant in the spring, with water mixing and low temperature. Groups F (Elakatothrix gelatinosa, Elakatothrix gelatinosa, and Sphaerocystis schroeteri), J (Pediastrum simplex and Coelastrum reticulatum), G (Eudorina elegans and Volvox aureus), LM (Ceratium and Microcystis), and H1 (Aphanizomenon flos-aquae and Anabaena spiroides) dominated the phytoplankton community from summer to mid-autumn, with thermal stratification. Groups H1 and P became dominant in the late autumn, with the breakdown of stratification. With the deepening of the mixing zone, groups P and T (Mougeotia sp.) were dominant in the winter. The reservoir was meso-eutrophic according to trophic state index values based on total phosphorus (TP), chlorophyll a, Secchi depth and total nitrogen, habitat preferences of PFGs, and diversity indices of phytoplankton. Redundancy analysis (RDA) revealed that NO3–N, SiO2, TP, pH, and water temperature (WT) were the most important environmental factors controlling PFGs in the BDR. Weighted averaging regression results indicated that among PFGs, groups F and T had a narrower tolerance range for WT, pH, and SiO2, while groups G and T had a narrower tolerance range for TP and NO3–N.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phytoplankton assemblages, which form the basis of aquatic food chains, are the principal primary producers in lakes and reservoirs. However, phytoplankton growth in aquatic ecosystems is limited by various factors such as water temperature, nutrient levels, pH, light availability, and grazing pressure. If one of these factors changes, it will affect the structure of phytoplankton community to a certain extent (Xiao et al. 2011; Jiang et al. 2014; Cao et al. 2018). It is not easy to illustrate relationships between phytoplankton community structure and environmental factors. Therefore, in recent years, multivariate statistical techniques have been widely utilized to examine these relationships in aquatic ecosystems (Wang et al. 2007; Çelekli and Öztürk 2014). Eutrophication is one of the most serious ecological problems of lakes and reservoirs and is responsible for water quality degradation and severe restriction in water uses (Codd 2000; Padedda et al. 2017). The use of phytoplankton species for assessment of water quality and trophic status of surface waters has a long history (Hutchinson 1944; Jarnefelt 1952; Padisak et al. 2006; Pasztaleniec and Poniewozik 2010).
Seasonal succession of phytoplankton composition in lakes and reservoirs has long fascinated aquatic ecologists. Sommer et al. (1986) developed a plankton ecology group (PEG) model describing seasonal succession of phytoplankton assemblages in lakes. According to this model, there is a succession within phytoplankton assemblages along four key temporal periods. Briefly, small cryptophytes and small centric diatoms develop under nutrient availability and increased light intensity towards the end of winter. In spring, these small algae are grazed by herbivorous zooplanktonic species. Thus, a clear-water phase occurs because phytoplankton abundance decreases rapidly. In summer, phytoplankton community starts to grow following the decline of herbivorous zooplanktonic populations due to limited food availability and fish predation. At first, small edible cryptophytes and large inedible colonial chlorophytes become predominant. Then, due to phosphate competition, chlorophytes are replaced by large diatoms. Silica depletion limits diatom growth. Thus, replacement of the large diatoms by large dinoflagellates and/or cyanobacteria occurs. However, nitrogen depletion favors a shift to nitrogen-fixing species of filamentous cyanobacteria. In autumn, as a result of physical changes which includes increased mixing depth resulting in nutrient replenishment, large unicellular or filamentous algal forms develop which is adapted to being mixed. In winter, phytoplankton biomass decreases to minimum levels because of low light energy and low temperature. These seasonal variations of phytoplankton in temperate lakes and reservoirs are related to temperature, light, nutrients, and grazing (Sommer et al. 1986; Grower and Chrzanowski 2006; Rolland et al. 2009; Maraslioglu and Soylu 2017).
Phytoplankton species have developed morphological and physiological adaptive strategies for surviving in different aquatic environments (Reynolds et al. 2002; Becker et al. 2010; Xiao et al. 2011; Salmaso et al. 2015). Reynolds (1997) defined several phytoplankton functional groups that may potentially dominate or co-dominate in a given environment. These groups are often polyphyletic and share adaptive features, based on the physiological, morphological, and ecological attributes of the species (Becker et al. 2010). The phytoplankton functional group (PFG) approach of Reynolds et al. (2002) used 31 phytoplankton associations, identified by alphanumeric labels (coda) according to their sensitivities and tolerances. However, a subsequent review by Padisak et al. (2009) recognized 40 coda, although not all of them yet sufficiently substantiated to be brought into the final classification. Because functional groups are well described in terms of habitat properties, environmental tolerance, and trophic state, they represent the classical and the widest used system of classifying the phytoplankton (Zutinic et al. 2014; Salmaso et al. 2015).
The Batman Dam Reservoir (BDR) is one of three major reservoirs in the Tigris River basin in Turkey. Some reports have been published on the water quality of this dam reservoir (Varol et al. 2012), but there are no studies reporting its phytoplankton community structure (Varol and Şen 2016). The aims of the present study were to assess seasonal variations in the phytoplankton community structure in the Batman Dam Reservoir, to explain these variations in relation to environmental factors using multivariate statistical approaches, to determine ecological preferences of phytoplankton using weighted averaging regression, and to assess ecological status of the reservoir using trophic state index, phytoplankton diversity indices, and habitat preferences of phytoplankton. In this study, the phytoplankton functional group approach was used to identify phytoplankton community structure and its succession.
Material and methods
Study area
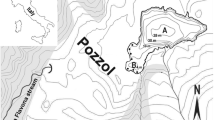
The BDR is located in the Tigris River basin in Turkey, which is a semi-arid region (Fig. 1). The BDR is a warm monomictic reservoir. The main features of this dam reservoir are given in Table S1. The continental climate of the basin is similar to that of the Mediterranean region. Between February 2008 and January 2009, the annual mean air temperature was 16.7 °C with the highest and the lowest temperature of 31.3 °C in July and August and 1.1 °C in January, respectively. Average annual total precipitation was 297.8 mm, of which 7.7% fell in the summer, 19.5% in the spring, 33.9% in the autumn, and 38.9% in the winter.
Sampling and analysis
Monthly phytoplankton and water samples were collected from at three sites on the BDR between February 2008 and January 2009. For the taxonomic analysis, qualitative phytoplankton samples from the surface water were taken with a plankton net (55 μm mesh size) and transferred to 100-mL polyethylene bottles. For the quantitative analysis of phytoplankton, surface water samples were taken and transferred to 500-mL polyethylene bottles. Both qualitative and quantitative phytoplankton samples were preserved with formaldehyde in the field (4% final concentration) (Varol et al. 2018). Phytoplankton species composition was determined under a light microscope (Olympus BX51) using × 20, × 40, and × 100 objectives. Phytoplankton identification was done according to Patrick and Reimer (1966, 1975), Krammer and Lange-Bertalot (1986), Hartley (1996), John et al. (2002), Krammer (2002), Wehr and Sheath (2003), Komarek and Komarkova (2002, 2006), and Komarek and Zapomelova (2007). Algal species were enumerated with an inverted microscope (Olympus CKX-41) using × 20 and × 40 objectives, according to the Utermöhl sedimentation method (Utermöhl 1958). The counting unit was the individual (unicell, coenobium, filament, or colony), and at least 100 individuals of the most abundant species were counted (Varol and Şen 2018).
Phytoplankton taxa were classified into PFGs according to Reynolds et al. (2002) and Padisak et al. (2009). PFGs contributing > 5% of total phytoplankton abundance were classified as prevailing groups (Reynolds et al. 2002).
Surface water samples were also taken simultaneously with the phytoplankton sampling, transferred to 2-L polyethylene bottles, and transported to the laboratory immediately for hydrochemical analyses. Electrical conductivity (EC), pH, dissolved oxygen (DO), and water temperature (WT) were measured in situ using a portable multimeter. Water transparency (Transp) was measured with a Secchi disk.
Water samples were filtered on the sampling day in the laboratory. Nitrate nitrogen (NO3–N), ammonium nitrogen (NH4–N), nitrite nitrogen (NO2–N), soluble reactive phosphorus (SRP), silica (SiO2), and total alkalinity (TA) were analyzed in the filtered water samples, while total nitrogen (TN), total phosphorus (TP), chemical oxygen demand (COD), and chlorophyll a (Chl-a) were analyzed in the unfiltered samples. All these parameters were analyzed on the day of sampling.
NO3–N (2,6-dimethylphenol method), NH4–N (phenate method), NO2–N (diazotization method), TN (persulfate digestion followed by the 2,6-dimethylphenol method), SiO2 (molybdosilicate method), SRP (ascorbic acid method), and TP (persulfate digestion followed by the ascorbic acid method) were analyzed by spectrophotometry (ISO 1986; APHA 1995). Chl-a was measured by spectrophotometry after acetone extraction (APHA 1995). TA was determined by the titration method and COD by the dichromate reflux method. Dissolved inorganic nitrogen (DIN) was calculated as the sum of NO3–N, NH4–N, and NO2–N (APHA 1995).
Data analysis
Direct gradient analysis was performed to determine the relationships between phytoplankton functional groups and environmental variables. Detrended correspondence analysis (DCA) of functional group abundance data was used to determine whether linear or unimodal ordination methods should be applied. DCA indicated that gradient lengths did not exceed 2 standard deviation units for all axes, which suggested a linear ordination model. Accordingly, redundancy analysis (RDA) was preferred for the direct gradient analysis. In RDA, the forward selection method with the Monte Carlo permutation test was performed to choose the environmental variables that represented the major gradients but did not correlate strongly with each other in explaining variability among functional groups. Therefore, environmental variables with a variance inflation factor (VIF) above 20 were removed to avoid multicollinearity among variables (Ter Braak and Smilauer 2002). From this process, DO, TN:TP, DIN:TP, DIN, EC, and T-Alk were found to be redundant and thus were removed from the analysis. The remaining variables, pH, WT, TN, NO3–N, NH4–N, TP, SRP, SiO2, COD, Transp, and Chl-a, had VIFs below 20, and these are final environmental variables considered in the final RDA. In addition, the conditional and marginal effects of environmental variables on functional groups were also assessed using the Monte Carlo permutation test. For data analysis, all phytoplankton data (10 prevailing functional groups) were Log10(x + a) transformed, where a corresponds to the minimum non-zero value of the variable in each case. In addition, all environmental variables except pH were Log10(x + 1) transformed. Both DCA and RDA were carried out using the CANOCO 4.5 package (Ter Braak and Smilauer 2002).
The Spearman correlation analysis was used to evaluate the relationships between abundance of phytoplankton functional groups and environmental factors using the SPSS 11.5 package. The differences in the spatial and temporal distribution of the environmental variables in the BDR were analyzed with one-way analysis of variance (ANOVA) using the SPSS 11.5 package. In addition, optima and tolerance ranges of functional groups for environmental variables were calculated through weighted averaging regression using the software package C2 (Juggins 2007).
The Shannon–Wiener diversity index (H′) and Margalef richness index (d) were used to calculate community metrics of phytoplankton. The indices were calculated by the following equations (Xu et al. 2017):
where Pi is the ratio of the individuals of the species i to the total individuals of all species (N), and S is the total number of species.
Carlson’s trophic state index (TSI) based on Chl-a, TP, TN, and SD values was used to assess the trophic status of the BDR (Carlson 1977; Kratzer and Brezonik 1981) (additional data are given in the supplemental material).
Results
Environmental variables
In this study, all environmental variables did not display significant spatial variations (p > 0.05, by ANOVA). However, they showed significant seasonal differences (p < 0.05, by ANOVA). As expected, WT values were lower in the winter (8.3 °C) and higher in the summer (25.8 °C). WT values ranged from 5.6 °C in February to 26.7 °C in August (Table 1). A thermal stratification was observed in the BDR between May and October. pH values averaged from 8.11 (December) to 8.92 (April). DO showed higher average in the spring (10.01 mg/L), but lower level in the autumn (7.38 mg/L). EC and TA showed a decreasing trend from February to July, but an increasing trend from August to January. TN and NO3–N showed similar seasonal variations. The average concentrations of TN, NO3–N, and DIN were higher in spring (0.923 mg/L, 0.584 mg/L, and 0.62 mg/L, respectively), while they were lower in the autumn (0.286 mg/L, 0.067 mg/L, and 0.12 mg/L, respectively). NO3–N comprised 63% of TN in the spring, but 47%, 25%, and 23% in the winter, summer, and autumn, respectively. NH4–N, contrary to TN and NO3–N, had a lower mean concentration in the spring (0.033 mg/L). NH4–N comprised 16% of TN in the autumn, but < 8% in the other seasons. NO2–N concentrations were lower than 0.01 mg/L in all seasons except winter (0.021 mg/L). COD values averaged from 2.33 mg/L in the winter to 5.66 mg/L in the autumn. Similar to TN and NO3–N, the average concentrations of TP and SRP were lower in the autumn (0.029 mg/L and 0.018 mg/L, respectively), while they were higher in the spring (0.11 mg/L and 0.043 mg/L, respectively). SRP comprised 74% of TP in the summer, but 62%, 59%, and 39% in the autumn, winter, and spring, respectively. TN:TP ranged from 6 in October to 26 in August, while DIN:TP ranged from 2.1 in September to 13.6 in January. SiO2 showed higher average in the winter (10 mg/L) and lower level in the spring (7.4 mg/L). Water transparency values were higher in the summer (3.94 m), but they were lower in the winter (2.14 m). Chl-a concentrations ranged from 1.54 μg/L in June to 11.43 μg/L in February (Table 1).
Phytoplankton community dynamics
In this study, a total of 60 species of phytoplankton belonging to eight phyla were identified in the BDR. The most diverse phylum was Chlorophyta (25 species) (41.67%), followed by Cyanophyta (17 species) (28.33%), Bacillariophyta (10 species) (16.67%), Pyrrophyta (3 species) (5%), Chrysophyta (2 species) (3.33%), Euglenophyta (1 species) (1.67%), Cryptophyta (1 species) (1.67%), and Prasinophyta (1 species) (1.67%) (Table 2). The number of phytoplankton species was higher in the autumn, followed by summer, winter, and spring.
Phytoplankton abundance did not display significant variations between sampling sites. The average phytoplankton abundance in the BDR was 289.1 × 103 indiv./L. However, there were significant differences in investigated months in terms of phytoplankton abundance. The highest phytoplankton abundance (809.7 × 103 indiv./L) was observed in December, while the lowest abundance (29.8 × 103 indiv./L) was recorded in March (Fig. S1). The average phytoplankton abundance was higher in the winter (477.9 × 103 indiv./L), followed by autumn (390.8 × 103 indiv./L), spring (166.3 × 103 indiv./L), and summer (121.6 × 103 indiv./L). Bacillariophyta (average 135.1 × 103 indiv./L) was the most abundant group during the study period, with an average contribution of 44% to the total abundance. Chlorophyta (average 83.8 × 103 indiv./L) was the second most abundant group, with an average contribution of 27.3% to the total abundance, while Cyanophyta (average 49.1 × 103 indiv./L) was the third most abundant group, accounting for 16% of the total abundance (Fig. 2a).
The phytoplankton community of the BDR was dominated by two centric diatoms (Aulacoseira granulata and Cyclotella ocellata) during the spring and winter (Table S2). The chlorophycean Mougeotia sp. occurred at a low frequency (December and January) but with a notable abundance. The filamentous cyanophyte Aphanizomenon flos-aquae was well represented in some months (February, March, October, and November) and contributed considerably to the abundance. Several chlorophycean (Pediastrum simplex and Sphaerocystis schroeteri) and dinophycean (Ceratium hirundinella and Peridinium cinctum) species were numerically abundant in certain seasons (late spring, summer, and early autumn), showing high contribution to the abundance. The colonial cyanophyte Microcystis aeruginosa and several chlorophyceans (Coelastrum reticulatum, Pediastrum duplex, Closteriopsis longissima, Eudorina elegans, and Volvox aureus), along with the pennate diatom Ulnaria acus, showed a relatively high frequency of occurrence, even though their contribution to abundance was low (Table S2).
The species richness and diversity indices were calculated for each month. The diversity index (H) ranged between 0.53 in March and 4.3 in September with a mean diversity value of 2.4, while the richness index (d) ranged between 0.68 in March and 4.0 in September with a mean value of 2.1. Both indices showed higher values in the autumn and summer and lower values in the spring and winter (Table 3).
Phytoplankton functional groups
In this study, 19 functional groups were recorded during the study period (Table 2). However, 10 functional groups (P, B, D, H1, J, F, G, T, LO, and LM) contributing more than 5% of total phytoplankton abundance per sample were classified as prevailing groups. Thus, these 10 prevailing functional groups including the 16 descriptive species were used for the analysis of phytoplankton community composition and its dynamics (Table 4).
At the beginning of the study period (February), functional groups P (Aulacoseira granulata) and B (Cyclotella ocellata) were dominant (36% and 28% of the total phytoplankton abundance, respectively), while group H1 (Aphanizomenon flos-aquae) was subdominant (Fig. 2b). In March, when the lowest phytoplankton abundance appeared due to a mixing event, groups P and H1 were co-dominant (45% and 27% of the total abundance, respectively), while group D (Ulnaria acus) was subdominant. In April, phytoplankton abundance increased in parallel to water column stability and group B (Cyclotella ocellata) dominated with 92% of the total phytoplankton abundance. Group B decreased in abundance in May (43%), while it remained dominant. In addition, groups F (Sphaerocystis schroeteri) and LO (Peridinium cinctum and Ceratium hirundinella) appeared in May but remained subdominant to group B. During the summer, phytoplankton abundance decreased, while species diversity increased with the stratification. In June, functional groups P, LO, (Peridinium cinctum and Ceratium hirundinella) and F shared dominance in the BDR. Group H1 (Anabaena spiroides and Aphanizomenon flos-aquae) appeared in July, and groups LM (Ceratium hirundinella and Microcystis aeruginosa) and J (Pediastrum simplex and Coelastrum reticulatum) increased together with the decreased abundance of F and LO. Dominant groups LM and H1 accounted for 29% and 20% of the total phytoplankton abundance in July, respectively. Group J dominated with 44% of the total phytoplankton abundance in August, while group LM decreased in abundance and remained subdominant together with groups H1 and G (Eudorina elegans and Volvox aureus). In autumn, both phytoplankton abundance and species diversity increased. Groups J (Pediastrum simplex and Coelastrum reticulatum) and F (Sphaerocystis schroeteri, Elakatothrix gelatinosa, and Kirchneriella lunaris) were co-dominant, while groups LM and H1 were subdominant in September. Group H1 established dominance together with group J in October (42% and 19% of the total abundance, respectively), while group LM was again subdominant. Phytoplankton abundance increased in November when the stratification breaks down, and it reached maximum in December, with the deepening of the mixing zone. Groups H1 and P were dominant in November (46% and 22% of the total abundance, respectively), while group J switched in subdominance. The dominant groups shifted to groups P and T (Mougeotia sp.) in December and January. Groups P and T accounted for 71% and 18% of the total abundance in December, respectively, while they accounted for 58% and 35% of the total abundance in January, respectively (Fig. 2b).
Correlation analysis
Most of phytoplankton functional groups were significantly correlated with many environmental variables (Table 5). Groups F, G, LO, and LM were significantly positively correlated with WT, while groups P and T were significantly negatively correlated with WT. Groups P and T were significantly positively correlated with EC, TN:TP, DIN:TP, NO2–N, SiO2, and TA, while they were significantly negatively correlated with pH. Groups H1, J, G, and LM were significantly positively correlated with COD. Groups H1, J, F, G, and LM were negatively correlated with TN, DIN, and NO3–N. Group B was significantly positively correlated with TP, while groups J, G, and LM were significantly negatively correlated with TP. Groups F and LO were significantly positively correlated with transparency. Among functional groups, group D was positively correlated with Chl-a, while group LO was significantly negatively correlated with Chl-a. Shannon–Wiener and Margalef indices of the BDR phytoplankton community were positively correlated with water temperature and COD but were negatively correlated with TN, TP, DIN, DIN:TP, and NO3–N (Table 5).
RDA
RDA was also performed to reveal the relationships between environmental variables and functional groups. The RDA ordination diagram including 11 environmental variables and 10 functional groups is presented in Fig. 3. The eigenvalues for the first two axes were 0.350 and 0.315, respectively, explaining 66.5% of total variance of the species data. The species–environment correlations for the first and second axes were high (0.955 and 0.978, respectively) (Table S3). The Monte Carlo permutation test indicated that the first two axes were statistically significant (p < 0.01). The position of each functional group with respect to the first two environmental axes is shown in Fig. 3. The first RDA species axis was positively related to NO3–N (r = 0.882), TP (r = 0.793), TN (r = 0.654), and SRP (r = 0.481) and negatively related to COD (r = − 0.580) and WT (r = − 0.440). The second RDA species axis was positively associated with SiO2 (r = 0.903), whereas it was negatively associated with pH (r = − 0.781) and WT (r = − 0.598). At the positive end of axis 1, functional group B was associated positively with TP and SRP. At the negative end of axis 1, functional groups F and LO were associated positively with WT and groups H1 and G with COD, while groups J, LM, and G were associated negatively with TN, NO3–N, and TP. At the positive end of axis 2, groups P and T were associated positively with SiO2 and negatively with pH and WT, while group D was associated negatively with WT and transparency (Fig. 3).
The marginal and conditional effects of 11 environmental variables are shown in Table 6. The marginal effect represents the independent effect of each environmental variable when the variable was treated separately. The conditional effect shows the effect that each environmental variable brings in addition to all the variables already selected and the most important variable included first. From the table of marginal effects, the most important factor for phytoplankton functional group composition was NO3–N, followed by SiO2, TP, pH, and WT. The conditional effects of TP, pH, WT, TN, COD, and SRP decreased dramatically after NO3–N was selected. However, all variables except TN and COD can be included in the final model when the 0.05 probability threshold level for the entry of a variable into the model is adopted. Among variables, NH4–N, Chl-a, transparency, and SRP have low marginal effects (Table 6).
Optima and tolerances of functional groups to environmental variables
Tolerance and optima statistics illustrate the variations in the ecology of phytoplankton functional groups in relation to five variables (WT, NO3–N, TP, SiO2, and pH) that have the highest marginal effects (lambda 1 > 0.2) in RDA (Fig. 4). Weighted averaging WT, NO3–N, TP, SiO2, and pH optima ranged between 9.78 and 24.04 °C, between 0.074 and 0.555 mg/L, between 0.028 and 0.1058 mg/L, between 7.34 and 70.77 mg/L, and between 8.18 and 8.83, respectively. As shown in Fig. 4, groups F, G, and LO were found in warmer water, while groups T and P preferred cooler water. Groups B, LO, and F were found in more alkaline water. Groups P and T preferred higher SiO2 levels, while groups B and D preferred higher TP and NO3–N levels. Among the FGs, groups F and T had a narrow tolerance range for WT, pH, and SiO2, while groups G and T had a narrow tolerance range for TP and NO3–N.
Discussion
Relationships between environmental variables and PFGs
In lentic ecosystems, temporal variations in the water column movements, associated with the water circulation patterns, are considered one of the main environmental forces that affect phytoplankton dynamics. The availability of light and nutrients, water temperature, and turbulence are the most variables in determining phytoplankton assemblages (Reynolds 2006; Becker et al. 2010; Crossetti et al. 2013). The climate of the study region is similar to that of the Mediterranean region. Therefore, air temperature is high and the duration of daylight is longer between late spring and mid-autumn in the study area. The BDR was thermally stratified between May and October, while mixing occurred between November and April. Similar water circulation patterns (mixing vs. stratification) were also observed in some reservoirs located in the Mediterranean region (Moreno-Ostos et al. 2008; Becker et al. 2010; Çelekli and Öztürk 2014; Sevindik et al. 2017). In this study, relationships between phytoplankton functional groups and environmental variables were examined across a horizontal gradient.
The RDA results indicated that phytoplankton functional groups in the BDR were regulated by NO3–N, SiO2, TP, pH, and WT. Species of group P tend to be present in the more eutrophic waters with mild light and are commonly found in lower latitudes (Padisak et al. 2009; Cao et al. 2018). Group P (Aulacoseira granulata) was found in the BDR in all months, except May, when the lowest concentration of SiO2 was recorded. Reynolds et al. (2002) reported that functional group P is sensitive to Si depletion. Results of RDA and weighted averaging (WA) confirmed that this species preferred high SiO2 concentrations (10.45 ± 0.91 mg/L) (Figs. 3 and 4). This species reached the highest densities in November, December, and January, when maximum SiO2 concentrations were observed. Although group P is also sensitive to stratification, it can be found in the epilimnia of stratified lakes when the mixing criterion is satisfied (Reynolds et al. 2002). In this study, A. granulata was found at low densities in the BDR during the summer due to its sensitivity to stratification. In addition, group P in the BDR had a negative correlation with water temperature and a positive correlation with DIN:TP and EC. In November, December, and January months in the BDR, water temperature was low, and SiO2 concentrations, EC levels, and DIN:TP ratios were high, promoting the growth of group P. The presence of group P in high density was observed in other temperate reservoirs and lakes such as Ömerli Reservoir (Albay and Akçaalan 2003), Lake Garda (Salmaso 2002), and Valparaiso Reservoir (Negro et al. 2000).
Group T (Mougeotia sp.) can grow in more persistently mixed lakes where light is increasingly the limiting factor (Reynolds et al. 2002; Padisak et al. 2009; Cao et al. 2018). However, this species was found to be in high densities in December and January, when water mixing was homogeneous. Group T had a positive correlation with SiO2, TA, and TN:TP and a negative correlation with WT and pH. After November, the decreased water temperature, daylight time, and pH, and increased NO3–N, TA, and SiO2 concentrations could stimulate the growth of group T. Indeed, WA results indicated that group T preferred cooler water (9.78 ± 2.97 °C), lower pH level (8.18 ± 0.08), and higher SiO2 concentration (10.77 ± 0.77 mg/L) than those of other functional groups. In addition, this group had narrower tolerance ranges for pH, SiO2, TP, and NO3–N compared to other groups (Fig. 4). The presence of Mougeotia sp. was also reported from Çaygören Reservoir (Çelik and Sevindik 2015) and Lake Garda (Salmaso 2002).
The centric diatom Cyclotella ocellata, representative of group B, occupied a large proportion of total abundance in April, when the maximum concentration of TN was recorded in the BDR. Group B can grow in well-mixed lakes (Reynolds et al. 2002). In this study, during the mixing period, C. ocellata was present in the BDR but its densities were low. Tilman et al. (1986) stated that diatoms are poor competitors for nitrogen, but good competitors for phosphorus, whereas their better competitive ability is gained at low temperatures. In this study, group B had a positive correlation with TP and a negative correlation with WT. According to the WA results, group B preferred a higher pH level (8.83 ± 0.23) and higher TP (0.1058 ± 0.026 mg/L) and NO3–N (0.555 ± 0.158 mg/L) concentrations than those of other functional groups (Fig. 4). However, it is acknowledged that the occurrence of planktonic diatoms is often related to low water temperature, irradiance, and high turbulence and Si concentrations (Schlegel and Scheffler 1999). Hoyer et al. (2009) reported that non-buoyant and non-motile phytoplankton species such as Cyclotella are characterized by rapid sinking during thermal stratification. This case was also observed in the BDR because C. ocellata was absent during the summer due to high water temperature and thermal stratification. Members of group B were also reported from Lake Kozjak (Zutinic et al. 2014), Liman Lake (Soylu and Gönülol 2010), Lake Dagow (Schlegel and Scheffler 1999), Lake Vela (Abrantes et al. 2006), and Spanish reservoirs (Negro and De Hoyos 2005).
Functional group H1 including nitrogen-fixing species (Aphanizomenon flos-aquae and Anabaena spiroides) was one of the important groups in the BDR between July and November. Especially, Aphanizomenon flos-aquae contributed a high proportion to the total abundance in October and November. Group H1 is tolerant to low N concentrations, but sensitive to mixing, low light intensity, and low P concentrations (Reynolds et al. 2002; Padisak et al. 2009). Correlation analysis and RDA demonstrated that TN, NO3–N, and DIN were significantly negative environmental variables influencing functional group H1. Indeed, in this study, DIN concentration showed a decreasing trend from June (0.33 mg/L) to October (0.10 mg/L). Thus, the decreased DIN availability in July (0.16 mg/L) stimulated the growth of nitrogen-fixing species until November. With the rapid decrease in the water temperature from 17.9 to 11.7 °C and daylight duration and the increase in the TN concentration from 0.38 to 0.83 mg/L, the growth of group H1 was suppressed in the December. Smith (1983) predicted that TN:TP ratios strongly affected the dominance of cyanophytes in the lakes; when TN:TP < 29 (by weight), cyanophytes tended to dominate, and when TN:TP > 29 (by weight), cyanophytes tended to be rare. In the BDR, TN:TP ratios were below 29 during the study period, indicating that the BDR has a favorable environment for the growth of cyanophytes. WA results indicated that group H1 preferred warm water (19.54 ± 3.58 °C) and low NO3–N (0.133 ± 0.127 mg/L) and TP (0.028 ± 0.0226 mg/L) concentrations. Group H1 was also reported from El Gergal Reservoir (Moreno-Ostos et al. 2008), Reservoir Marne (Rolland et al. 2009), Lake Vela (Abrantes et al. 2006), Yedikır Dam Lake (Maraşlıoğlu and Gönülol 2014), and Devegeçidi Dam Lake (Baykal et al. 2004).
Ceratium hirundinella and Microcystis aeruginosa, representatives of functional group LM, often co-exist in the summer epilimnia of eutrophic temperate lakes (Reynolds et al. 2002; Padisak et al. 2009; Varol 2016). Both species have a good adaptability to thermal stratification conditions (Cao et al. 2018). In the BDR, group LM was present from June to December. This group had a positive correlation with WT, COD, and water transparency and a negative correlation with DO, TN, NO3–N, DIN, and TP. Combined with high water temperature, COD, and water transparency, and low DIN concentrations, group LM was promoted in summer and autumn. The low DO concentrations in summer and autumn were also caused by high water temperature and COD. Similar to group H1, group LM preferred warm water (22.10 ± 4.71 °C) and low NO3–N (0.114 ± 0.099 mg/L) and TP (0.0321 ± 0.0093 mg/L) concentrations. The presence of group LM was also reported from Lake Arancio (Naselli-Flores 2013), Lake Charzykowskie (Wisniewska and Luscinska 2012), and Lake Glebokie (Pasztaleniec and Poniewozik 2010).
The motile dinoflagellates Peridinium cinctum and Ceratium hirundinella belonging to the functional group LO were found in the BDR in all months, except February, March, and December. However, group LO occurred at higher densities in June and July, when water column thermal stratification was present. This group is tolerant to nutrient deficiency and sensitive to prolonged or deep mixing (Reynolds et al. 2002; Padisak et al. 2009). Species of group LO prefer stable conditions, usually in the summer epilimnia of temperate lakes (Zutinic et al. 2014). As given in Fig. 3, this group preferred warm water (22.49 ± 5.03 °C) and alkaline pH (8.71 ± 0.21), which was also confirmed by RDA and correlation analysis. Therefore, a steadily formed thermal stratification, together with their motility capabilities, and high water temperature were responsible for high densities of group LO in June and July. Group LO was also reported from Liman Lake (Soylu and Gönülol 2010), Lake Vransko (Udovic et al. 2015), Skalenski Lakes (Teneva et al. 2014), and Alleben Reservoir (Çelekli and Öztürk 2014).
Species (Sphaerocystis schroeteri, Elakatothrix gelatinosa, and Kirchneriella lunaris) of group F made a high contribution to the total phytoplankton abundance during the stratification period. The members of this group are able to grow under thermal stratification and at a relatively deep optical depth (Happey-Wood 1988). In addition, group F can develop better in clear waters, because colonial green algae generally have a substantially higher light requirement than most planktonic blue-green algae or diatoms (Huszar et al. 2003). Indeed, correlation analysis indicated that there was a positive correlation between group F and water transparency. Reynolds et al. (2002) reported that this group is sensitive to low nutrient concentrations and high turbidity. In addition, group F in the BDR had a negative correlation with TN, DIN, and NO3–N and a positive correlation with water temperature. Therefore, low nutrient concentrations, high water temperature, and increased transparency were important factors leading to the growth of functional group F. WA results indicated that group F preferred warmer water (24.04 ± 2.17 °C) than that of other functional groups (Fig. 3). The presence of group F was also reported from Lake Mogan (Demir et al. 2014), Yedikır Dam Lake (Maraşlıoğlu and Gönülol 2014), and Lake Arancio (Naselli-Flores 2013).
Functional groups J (Pediastrum simplex and Coelastrum reticulatum) and G (Eudorina elegans and Volvox aureus) contributed considerably to the total abundance between August and November, when daylight is long. Reynolds et al. (2002) and Wang et al. (2011) reported that group J is mostly found in shallow, highly enriched lakes, while group G is found in nutrient-rich lakes and tolerant to high light. Both group J and group G in the BDR had a positive correlation with WT and COD and a negative correlation with pH, DO, TN, NO3–N, DIN, and TP. WA indicated that groups J and G preferred warm water (22.0 ± 5.02 °C and 22.5 ± 4.68 °C, respectively). Although both of groups are sensitive to nutrient deficiency, groups J and G preferred low TP (0.031 ± 0.0092 mg/L and 0.0329 ± 0.0085 mg/L, respectively) and NO3–N (0.091 ± 0.093 mg/L and 0.074 ± 0.082 mg/L, respectively) concentrations. Consequently, these groups adapted to high water temperature, and the increased COD concentrations could stimulate the growth of these groups. The presence of group J in high density was observed in Lake Arancio (Naselli-Flores 2013), Lake Vela (Abrantes et al. 2006), and Ömerli Reservoir (Albay and Akçaalan 2003), while group G was reported from Lake Gölköy (Çelekli et al. 2007), Borovitsa Reservoir (Teneva et al. 2010), and Lake Arancio (Naselli-Flores 2013).
Functional group D (Ulnaria acus) occupied a small proportion of the total abundance. It was only found in the BDR in February, March, September, and December months. The growth of this group was favorable in shallow, nutrient-enriched, and turbid waters (Reynolds et al. 2002). In addition, U. acus can grow in both colder and warmer waters (Zutinic et al. 2014). In this study, among the PFGs, group D had the widest tolerance range for water temperature (± 7.24 °C) and NO3–N (± 0.278 mg/L) and TP (± 0.0412 mg/L) concentrations. This group was also reported from Lake Kozjak (Zutinic et al. 2014) and Çaygören Reservoir (Sevindik 2010).
Phytoplankton succession pattern in the BDR
A typical phytoplankton succession pattern consistent with the PEG model was observed in the BDR. As described in the PEG model (Sommer et al. 1986), centric diatoms (groups B and P) were dominant in the spring in the BDR due to water mixing and low temperature, while colonial chlorophytes (groups F, J, and G), dinoflagellates (group LO), group LM (C. hirundinella and M. aeruginosa), and filamentous cyanobacteria (group H1) dominated the phytoplankton community in the summer due to thermal stratification. Filamentous cyanobacteria and colonial chlorophytes remained dominant in the autumn, with the breakdown of stratification. Diatoms (group P) and filamentous chlorophytes (group T) dominated the phytoplankton community in the winter. Physical factors, concentrations of nutrients, and grazing pressure were considered to be the driving factors of phytoplankton seasonal succession in the BDR (Grower and Chrzanowski 2006; Rolland et al. 2009; Zutinic et al. 2014).
Trophic status of the BDR
According to Carlson’s TSI, lakes or reservoirs with TSI values between 40 and 50 are classified as mesotrophic, TSI values greater than 50 are classified as eutrophic, and values less than 40 are classified as oligotrophic (Carlson 1977; Kratzer and Brezonik 1981). TSI values derived from TN (49), SD (44), and Chl-a (43) indicated mesotrophic conditions in the BDR, while the TSI value for TP (64) indicated a eutrophic status. An advantage of functional groups is that ecological features are linked with the trophic state. Therefore, PFGs can be used for the trophic evaluation of reservoirs (Reynolds 2006; Padisak et al. 2009). In this study, 10 prevailing PFGs (P, B, D, H1, J, F, G, T, LO, and LM) were used for the assessment of trophic status of the BDR. Salmaso et al. (2015) reported that functional groups B, F, T, and LO are found in mesotrophic lakes and reservoirs, while groups P, D, H1, J, G, and LM are found in eutrophic lakes and reservoirs (Table 4). Also, phytoplankton diversity indices have been used to assess the trophic status of lakes and reservoirs (Soylu et al. 2007; Zhang and Zang 2015; Xu et al. 2017). The Shannon–Wiener index values usually range between 0 and 1 in eutrophic lakes, while they usually range between 2 and 3 in mesotrophic lakes. The Margalef index values of eutrophic lakes usually range between 0 and 3, while they usually range between 4 and 5 in mesotrophic lakes. In the study, the average value of the Shannon–Wiener index was 2.4, indicating mesotrophic conditions, while that of the Margalef index was 2.1, indicating eutrophic conditions (Zhang and Zang 2015) (Table 3). Thus, the BDR could be classified as a meso-eutrophic reservoir according to TSI values, functional groups, and diversity indices.
Limiting nutrient in the BDR
The TN:TP ratio is commonly used to determine limiting nutrient for phytoplankton growth (Guildford and Hecky 2000; Becker et al. 2010). However, Morris and Lewis (1988)) and Bergström (2010) found that the DIN:TP ratio is the most effective indicator of nutrient limitation for phytoplankton growth. Bergström (2010) reported that N limitation occurs at the DIN:TP ratio less than 1.5 (by mass), P limitation occurs at the DIN:TP ratio greater than 3.4 (by mass), while the DIN:TP ratio between 1.5 and 3.4 is considered to be under both N and P limitations. In the BDR, the DIN:TP ratio was between 1.5 and 3.4 in September and October, while it was greater than 3.4 in other months (Table 1). The DIN:TP ratio results indicated that phytoplankton growth in the BDR was largely limited by phosphorus.
Conclusion
-
1.
This study confirmed that the concept of phytoplankton functional groups can be successfully used for understanding phytoplankton dynamics, adaptations, tolerances, and sensitivities to environmental conditions and for assessing the trophic state of monomictic reservoirs.
-
2.
Water circulation patterns (stratification vs. mixing) affected water temperature, light availability, and nutrient concentrations, and consequently, phytoplankton succession.
-
3.
RDA and correlation analysis indicated that NO3–N, SiO2, TP, pH, and WT were the most important environmental variables governing the temporal dynamics of the phytoplankton functional groups in the BDR.
-
4.
According to the trophic state index values based on TP, Chl-a, Secchi disk depth and TN, habitat preferences of functional groups, and phytoplankton diversity indices, the BDR was classified as meso-eutrophic.
-
5.
According to weighted averaging regression, among the PFGs, groups F, G, and LO preferred warmer water, while groups T and P preferred cooler water. Groups P and T preferred higher SiO2 levels, while groups B and D preferred higher TP and NO3–N levels.
References
Abrantes N, Antunes SC, Pereira MJ, Gonçalves F (2006) Seasonal succession of cladocerans and phytoplankton and their interactions in a shallow eutrophic lake (Lake Vela, Portugal). Acta Oecol 29:54–64
Albay M, Akçaalan R (2003) Factors influencing the phytoplankton steady state assemblages in a drinking-water reservoir (Ömerli Reservoir, Istanbul). Hydrobiologia 502:85–95
APHA (1995) Standard methods for examination of water and wastewater. American Public Health Association, Washington
Baykal T, Açıkgöz Ü, Yıldız K, Bekleyen A (2004) A study on algae in Devegeçidi Dam Lake. Turk J Bot 28:457–472
Becker V, Caputo L, Ordonez J, Marce R, Armengol J, Crossetti LO, Huszar VLM (2010) Driving factors of the phytoplankton functional groups in a deep Mediterranean reservoir. Water Res 44:3345–3354
Bergström AK (2010) The use of TN:TP and DIN:TP ratios as indicators for phytoplankton nutrient limitation in oligotrophic lakes affected by N deposition. Aquat Sci 72:277–281
Cao J, Hou Z, Li Z, Chu Z, Yang P, Zheng B (2018) Succession of phytoplankton functional groups and their driving factors in a subtropical plateau lake. Sci Total Environ 631–632:1127–1137
Carlson RE (1977) A trophic state index for lakes. Limnol Oceanogr 22:361–369
Çelekli A, Öztürk B (2014) Determination of ecological status and ecological preferences of phytoplankton using multivariate approach in a Mediterranean reservoir. Hydrobiologia 740:115–135
Çelekli A, Albay M, Dügel M (2007) Phytoplankton (except Bacillariophyceae) flora of Lake Gölköy (Bolu). Turk J Bot 31:49–65
Çelik K, Sevindik TO (2015) The phytoplankton functional group concept provides a reliable basis for ecological status estimation in the Çaygören Reservoir (Turkey). Turk J Bot 39:588–598
Codd GA (2000) Cyanobacterial toxins, the perception of water quality, and the prioritisation of eutrophication control. Ecol Eng 16:51–60
Crossetti LO, Becker V, Cardoso LS, Rodrigues LR, Costa LS, Motta-Marques D (2013) Is phytoplankton functional classification a suitable tool to investigate spatial heterogeneity in a subtropical shallow lake? Limnologica 43:157–163
Demir AN, Fakıoğlu Ö, Dural B (2014) Phytoplankton functional groups provide a quality assessment method by the Q assemblage index in Lake Mogan (Turkey). Turk J Bot 38:169–179
Grower JP, Chrzanowski TH (2006) Seasonal dynamics of phytoplankton in two warm temperate reservoirs: association of taxonomic composition with temperature. J Plankton Res 28:1–17
Guildford SJ, Hecky RE (2000) Total nitrogen, total phosphorus, and nutrient limitation in lakes and oceans: is there a common relationship? Limnol Oceanogr 45:1213–1223
Happey-Wood CM (1988) Ecology of freshwater planktonic green algae. In: Sandgren CD (ed) Growth and reproductive strategies of freshwater phytoplankton. Cambridge University Press, Cambridge, pp 175–226
Hartley B (1996) An atlas of British diatoms. Biopress Limited, Bristol
Hoyer AB, Moreno-Ostos E, Vidal J, Blanco JM, Palomino-Torres RL, Basanta A, Escot C, Rueda FJ (2009) The influence of external perturbations on the functional composition of phytoplankton in a Mediterranean reservoir. Hydrobiologia 636:49–64
Huszar V, Kruk C, Caraco N (2003) Steady-state assemblages of phytoplankton in four temperate lakes (NE USA). Hydrobiologia 502:97–109
Hutchinson GE (1944) Limnological studies in Connecticut. VII. A critical examination of the supposed relationship between phytoplankton periodicity and chemical changes in lake waters. Ecology 25:3–26
ISO (1986) Water quality determination of nitrate. In: Part 1: 2,6-dimethylphenol spectrometric method. International Organization for Standardization, Geneva
Jarnefelt H (1952) Plankton als Indikator der Trophiegruppen der Seen. Ann Acad Sci Fenn A IV Biol 18:1–29
Jiang YJ, He W, Liu WX, Qin N, Ouyang HL, Wang QM, Kong XZ, He QS, Yang C, Yang B, Xu FL (2014) The seasonal and spatial variations of phytoplankton community and their correlation with environmental factors in a large eutrophic Chinese lake (Lake Chaohu). Ecol Indic 40:58–67
John DM, Whitton BA, Brook AJ (2002) The freshwater algal flora of the British Isles: an identification guide to freshwater and terrestrial algae. Cambridge University Press, Cambridge
Juggins S (2007) C2 version 15 user guide software for ecological and palaeoecological data analysis and visualisation. Newcastle University, Newcastle upon Tyne, UK
Komarek J, Komarkova J (2002) Review of the European Microcystis morphospecies (cyanoprokaryotes) from nature. Czech Phycol 2:1–24
Komarek J, Komarkova J (2006) Diversity of Aphanizomenon-like cyanobacteria. Czech Phycol 6:1–32
Komarek J, Zapomelova E (2007) Planktic morphospecies of the cyanobacterial genus Anabaena = subg. Dolichospermum – 1. part: coiled types. Fottea 7:1–31
Krammer K (2002) Diatoms of Europe. Cymbella. A.R.G. Gantner Verlag K.G., Ruggell
Krammer K, Lange-Bertalot H (1986) Süßwasserflora von Mitteleuropa. Band 2. Bacillariophyceae, Teil 1. Naviculaceae. Gustav Fischer Verlag, Stuttgart
Kratzer CR, Brezonik PL (1981) A Carlson-type trophic state index for nitrogen in Florida lakes. Water Resour Bull 17:713–715
Maraşlıoğlu F, Gönülol A (2014) Phytoplankton community, functional classification and trophic state ındices of Yedikır Dam Lake (Amasya). J Biol Environ Sci 8:133–141
Maraslioglu F, Soylu EN (2017) Relationship of epilithic diatom communities to environmental variables in Yedikır Dam Lake (Amasya, Turkey). Turk J Fish Aquat Sci 17:1347–1356
Moreno-Ostos E, Cruz-Pizarro L, Basanta A, George DG (2008) The spatial distribution of different phytoplankton functional groups in a Mediterranean reservoir. Aquat Ecol 42:115–128
Morris DP, Lewis WM (1988) Phytoplankton nutrient limitation in Colorado Mountain lakes. Freshw Biol 20:315–327
Naselli-Flores L (2013) Morphological analysis of phytoplankton as a tool to assess ecological state of aquatic ecosystems: the case of Lake Arancio, Sicily, Italy. Inland Waters 4:15–26
Negro AI, De Hoyos C (2005) Relationships between diatoms and the environment in Spanish reservoirs. Limnetica 24:133–144
Negro AI, De Hoyos C, Vega JC (2000) Phytoplankton structure and dynamics in Lake Sanabria and Valparaiso reservoir (NW Spain). Hydrobiologia 424:25–37
Padedda BM, Sechi N, Lai GG, Mariani MA, Pulina S, Sarria M, Satta CT, Virdis T, Buscarinu P, Luglie A (2017) Consequences of eutrophication in the management of water resources in Mediterranean reservoirs: a case study of Lake Cedrino (Sardinia, Italy). Glob Ecol Conserv 12:21–35
Padisak J, Borics G, Grigorszky I, Soroczki-Pinter E (2006) Use of phytoplankton assemblages for monitoring ecological status of lakes within the Water Framework Directive: the assemblage index. Hydrobiologia 553:1–14
Padisak J, Crossetti LO, Naselli-Flores L (2009) Use and misuse in the application of the phytoplankton functional classification: a critical review with updates. Hydrobiologia 621:1–19
Pasztaleniec A, Poniewozik M (2010) Phytoplankton based assessment of the ecological status of four shallow lakes (Eastern Poland) according to Water Framework Directive—a comparison of approaches. Limnologica 40:251–259
Patrick R, Reimer CW (1966) The diatoms of the United States, exclusive of Alaska and Hawaii, vol volume I. Monographs of the Academy of National Sciences, Philadelphia
Patrick R, Reimer CW (1975) The diatoms of the United States, exclusive of Alaska and Hawaii, volume II. Part I. Monographs of the Academy of National Sciences, Philadelphia
Reynolds CS (1997) In: Kinne O (ed) Vegetation process in the pelagic: a model for ecosystem theory. Excellence in ecology. ECI, Oldendorf
Reynolds CS (2006) Ecology of phytoplankton. Cambridge University Press, Cambridge
Reynolds CS, Huszar VLM, Kruk C, Nasseli-Flores L, Melo S (2002) Towards a functional classification of the freshwater phytoplankton. J Plankton Res 24:417–428
Rolland A, Bertrand F, Maumy M, Jacquet S (2009) Assessing phytoplankton structure and spatio-temporal dynamics in a freshwater ecosystem using a powerful multiway statistical analysis. Water Res 43:3155–3168
Salmaso N (2002) Ecological patterns of phytoplankton assemblages in Lake Garda: seasonal spatial and historical features. J Limnol 61:95–115
Salmaso N, Naselli-Flores L, Padisak J (2015) Functional classifications and their application in phytoplankton ecology. Freshw Biol 60:603–619
Schlegel I, Scheffler W (1999) Seasonal development and morphological variability of Cyclotella ocellata (Bacillariophyceae) in the eutrophic Lake Dagow (Germany). Int Rev Hydrobiol 84:469–478
Sevindik TO (2010) Phytoplankton composition of Çaygören Reservoir, Balikesir-Turkey. Turk J Fish Aquat Sci 10:295–304
Sevindik TO, Çelik K, Naselli-Flores L (2017) Spatial heterogeneity and seasonal succession of phytoplankton functional groups along the vertical gradient in a mesotrophic reservoir. Ann Limnol Int J Limnol 53:129–141
Smith VH (1983) Low nitrogen to phosphorus ratios favor dominance by blue- green algae in lake phytoplankton. Science 221:669–671
Sommer U, Gliwicz ZM, Lampert W, Duncan A (1986) The PEG-model of seasonal succession of planktonic events in fresh waters. Arch Hydrobiol 106:433–471
Soylu EN, Gönülol A (2010) Functional classification and composition of phytoplankton in Liman Lake. Turk J Fish Aquat Sci 10:53–60
Soylu EN, Maraşlıoğlu F, Gönülol A (2007) Phytoplankton seasonality of a shallow turbid lake. Algol Stud 123:95–110
Teneva I, Gecheva G, Cheshmedjiev S, Stoyanov P, Mladenov R, Belkinova D (2014) Ecological status assessment of Skalenski Lakes (Bulgaria). Biotechnol Biotechnol Equip 28:82–95
Teneva I, Mladenov R, Belkinova D, Dimitrova-Dyulgerova I, Dzhambazov B (2010) Phytoplankton community of the drinking water supply reservoir Borovitsa (South Bulgaria) with an emphasis on cyanotoxins and water quality. Cent Eur J Biol 5:231–239
Ter Braak CJF, Smilauer P (2002) CANOCO reference manual and CanoDraw for windows user’s guide: software for canonical community ordination (version 45). Microcomputer Power, Ithaca
Tilman D, Kiesling R, Sterner R, Kilham SS, Johnson FA (1986) Green, blue-green and diatom algae: taxonomic differences in competitive ability for phosphorus, silicon and nitrogen. Arch Hydrobiol 106:473–485
Udovic MG, Zutinic P, Borojevic KK, Plenkovic-Moraj A (2015) Co-occurrence of functional groups in phytoplankton assemblages dominated by diatoms, chrysophytes and dinoflagellates. Fundam Appl Limnol 187:101–111
Utermöhl H (1958) Zur vervollkommnung der quantitativen phytoplankton methodik. Mitt Int Ver Theor Angew Limnol 9:1–38
Varol M (2016) External morphological variations and temporal distribution of the dinoflagellate Ceratium hirundinella in two dam reservoirs in the Tigris River basin (Turkey). Turk J Bot 40:112–119
Varol M, Şen B (2016) New records of Euglenophyceae for Turkish freshwater algae. Turk J Fish Aquat Sci 16:219–225
Varol M, Şen B (2018) Abiotic factors controlling the seasonal and spatial patterns of phytoplankton community in the Tigris River, Turkey. River Res Appl 34:13–23
Varol M, Gokot B, Bekleyen A, Şen B (2012) Spatial and temporal variations in surface water quality of the dam reservoirs in the Tigris River basin, Turkey. Catena 92:11–21
Varol M, Blanco S, Alpaslan K, Karakaya G (2018) New records and rare taxa for the freshwater algae of Turkey from the Tatar Dam Reservoir (Elazığ). Turk J Bot 42:533–542
Wang XL, Lu YL, He GZ, Han JY, Wang TY (2007) Exploration of relationships between phytoplankton biomass and related environmental variables using multivariate statistic analysis in a eutrophic shallow lake: a 5-year study. J Environ Sci 19:920–927
Wang L, Cai Q, Tan L, Kong L (2011) Phytoplankton development and ecological status during a cyanobacterial bloom in a tributary bay of the Three Gorges Reservoir, China. Sci Total Environ 409:3820–3828
Wehr JD, Sheath RG (2003) Freshwater algae of North America. Academic, Boston
Wisniewska M, Luscinska M (2012) Long-term changes in the phytoplankton of Lake Charzykowskie. Oceanol Hydrobiol Stud 41:90–98
Xiao LJ, Wang T, Hu R, Han BP, Wang S, Qian X, Padisak J (2011) Succession of phytoplankton functional groups regulated by monsoonal hydrology in a large canyon-shaped reservoir. Water Res 45:5099–5109
Xu Y, Li AJ, Qin J, Li Q, Ho JG, Li H (2017) Seasonal patterns of water quality and phytoplankton dynamics in surface waters in Guangzhou and Foshan, China. Sci Total Environ 590–591:361–369
Zhang NN, Zang SY (2015) Characteristics of phytoplankton distribution for assessment of water quality in the Zhalong Wetland, China. Int J Environ Sci Technol 12:3657–3664
Zutinic P, Udovic MG, Borojevic KK, Plenkovic-Moraj A, Padisak J (2014) Morpho-functional classifications of phytoplankton assemblages of two deep karstic lakes. Hydrobiologia 740:147–166
Acknowledgements
Special thanks are given to the editor Prof. Philippe Garrigues and the three anonymous reviewers for their constructive comments and suggestions for improving this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 29 kb)
Rights and permissions
About this article
Cite this article
Varol, M. Phytoplankton functional groups in a monomictic reservoir: seasonal succession, ecological preferences, and relationships with environmental variables. Environ Sci Pollut Res 26, 20439–20453 (2019). https://doi.org/10.1007/s11356-019-05354-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05354-0