Abstract
This work examines the feasibility of fuelling biodiesel derived from Eichhornia crassipes in a compression ignition engine. This work also proposes water hyacinth biodiesel (WHB) as a potential alternative energy source since the above species is available extensively in freshwater, marine, and aquatic ecosystems throughout the world. WHB was blended with petroleum diesel fuel at various volume proportions of 10%, 20%, 30%, 40%, and 100% and their properties were analyzed as per ASTM standards for its application as biofuel. The prepared test fuels were analyzed experimentally in a single-cylinder diesel engine at constant speed (1500 rev/min) for its performance, combustion, and emission characteristics. Test results projected that the characteristics of 20% WHB + 80% diesel fuel blend were in par with neat diesel fuel in terms of thermal efficiency, HC, CO, and smoke emissions. However, WHB blends resulted in slightly higher levels of CO2 and NOx emissions. At full load, the attained cylinder pressure and heat release rate of WHB were comparatively lower than diesel fuel. Ignition delay is lowest for B100 blend and therefore the diffusion burning phase of biodiesel phase is found to be dominant in comparison with diesel fuel. For biodiesel blends, the combustion starts earlier due to higher cetane number, lessened delay period, and lowered calorific value followed by lowered HRR.

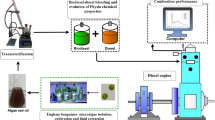
Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, energy has a key role over the cultural and economic fronts throughout the developing and developed countries. World’s energy needs are satisfied mainly via fossil fuels such as gas, coal, and mineral oils. Diesel engines are dominating on the transportation sector owing to its improved thermal efficiency. Faster depletion of fossil fuels and increasing exhaust emission norms have aggravated the researchers to find a suitable alternative feedstock for existing unmodified diesel engines (Konwar et al. 2014). Even though various alternatives for energy like fuel cells, hydrogen fuels, and biofuels, emergence of biofuel came as surprising and very promising for satisfying the fuel crisis (Demirbas 2009; Yusuf et al. 2011). It is noted that the very first engine invented by Rudolf diesel was run solely by peanut oil. Since vegetable oils have high viscosity and poor cold flow properties, its direct usage in diesel engine may pose serious consequence in terms of poor performance and emissions (Issariyakul et al. 2011). As emerging trends, various types of biodiesel such as waste cooking oil, waste pyrolysis oil, and waste plastic oil emerged as attractive alterations. Due to inherent advantages of biodiesel (transesterified vegetable oil) such as improved lubricity, improved cetane number, excess available O2, and safer transportation, biodiesel was found to be a suitable alternate for existing unmodified diesel engine.
The conventional sources of biodiesel were that of feedstocks of sunflower, olive, corn, palm, etc., which were known as first-generation biofuels. These were prone to certain drawbacks such as loss of food crops, excessive water consumption, and wasteful arable land (Bhuiya et al. 2014; Saladini et al. 2016). Some of the limitations while using edible vegetable oil as alternative feedstock includes the élan of global consumable food market, food security threat, and could even result in starvation on some developing countries (Atadashi et al. 2010). So, developing an appropriate alternative feedstock was favorable if the source was of non-edible/non-food feedstock so that it does not siphon off the existing vegetable oil cache (Umdu et al. 2009; Atabani et al. 2013; Huang et al. 2010).
Therefore, there arose a need for second-generation biodiesel which can be sourced from non-edible oils such as animal fats, jatropha oil, pongamia oil, karanja oil, and tamarind oil (Zhong et al. 2016; Raju et al. 2018). The latest trend of biodiesel production was from algae and sea weeds and hence it was considered as third-generation feedstocks (Srinophakun et al. 2017). Water hyacinth biomass was found to be suitable for production of bioethanol (Mishima et al. 2008; Abraham and Kurup 1996). Water hyacinth biomass contains hemicellulose (48%), cellulose (18%), and lignin (3.5%) (Nigam 2002). Water hyacinth is available abundantly throughout the world, especially at tropical regions where the backwater and marshy wetlands promote the growth of aquatic weed (Aswathy et al. 2010). Even though there were several reports of ethanol production from water hyacinth (Mishima et al. 2008; Abraham and Kurup 1996; Kahlon and Kumar 1987), so far there were very few researchers focused on usage of oil extracted from water hyacinth as an alternative feedstock for diesel engines. In recent times, Sanaa et al. (2018) examined the water hyacinth as potential source for biofuel production. They collected the water hyacinth seasonally from Zomor canal, Giza and Egypt. Later the hydrophytes were cleaned from debris by washing several times with distilled water, and then it was dried to remove moisture and ground into ultrapowder from. The fine ground water hyacinth sample mixed with methanol in 2:1 M ratio and the solution was stirred for 20 min with the micro ultrasonicator. Further, they were added mixture of 500 ml chloroform/water in 1:1 volume ratio and the solution was titrated for several times and it was settled at room temperature. Finally, they separated the residue and water hyacinth crude oil was collected. Later, they conducted transesterification for the raw oil to reduce the viscosity and improve the volatile nature of the biodiesel. The authors determined all the properties of the obtained biodiesel and concluded that all the properties are well within the standards of Egyptian fuel standards and they recommend the water hyacinth was promising source for the biodiesel production. Choudhary et al. (2015) studied the response surface methodology over water hyacinth biodiesel-diesel blends on diesel engine to optimize the combustion and performance characteristics. They were applied similar methodology for the extraction of the water hyacinth oil from the fresh matured water hyacinth leaves. The authors examined the water hyacinth biodiesel blends at various operating parameters such as compression ratio, injection pressure, and loads with diesel-bioethanol blends. From their test results, 10% bioethanol-diesel blends showed promising results in terms of performance and combustion parameters of diesel engine when compared to other biodiesel blends. In this research work also, similar methodology was implemented for the extraction of the biodiesel from the water hyacinth.
In order to overcome the above addressed benefits of using water hyacinth as alternative fuel in diesel engines, it is proposed to synthesize the biodiesel from water hyacinth by conventional transesterification and experimenting it with a single-cylinder diesel engine for analyzing its performance, combustion, and emission characteristics of test fuels namely, B10 (10% WHB + 90% diesel), B20 (20% WHB + 80% diesel), B30 (30% WHB + 70% diesel), B40 (40% WHB + 60% diesel), and B100 (100% WHB) and diesel fuel, respectively.
Biodiesel preparation
Biomass and oil extraction
Initially, water hyacinth plants were collected from backwaters in Pondicherry district, Tamil Nadu, India. The stalks and leaves of water hyacinth were partially dried in sun and stored in laboratory. For removing the residual moisture, the stalk and leaves were subjected to 70 °C air owen. Before initiating the pretreatment, milling was done for dried biomass to lower the grain size of about 1–2 μm. In general, alkali/dilute acids were ideal for pretreatment of oil. Biomass samples at 10% loading were treated with 2% sodium hydroxide and 0.88% sulfuric acid for a reaction time of 1 h and at reaction temperature of 95 °C. After it attains room temperature, the biomass samples were washed 3–4 times in running water in order to attain pH neutralization after which it is rinsed with distilled water and spread on paper after drying.
Water hyacinth oil is extracted from the above mixture by mixing through ether and hexane solution through Soxhlet apparatus. The resulting mixture is allowed for deposit for about 24 h. Water hyacinth oil is extracted from the collected biomass (after filtration and weighing) through vacuum evaporation, thereby reducing the absorbed hexane and ether solution through rotary evaporator. The preparation of water hyacinth oil from water hyacinth plant is illustrated via flow diagram in Fig. 1.
Transesterification-water hyacinth biodiesel preparation
Water hyacinth biodiesel is produced using a 2-L batch reactor, a magnetic stirrer, a condenser, a sampling outlet unit, and a thermometer. Biodiesel preparation was carried out using acid-base catalyst procedure. Initially before starting esterification, the raw oil is heated up to 65 °C using a rotary evaporator in vacuum for moisture removal. For esterification, a mixture of 6:1 M ratio of methanol to raw oil and 1.5% (v/v) sulfuric acid (H2SO4) are treated with above preheated raw oil and stirred for 1 h at 65 °C at 600 rpm. Then, using a funnel separator, the esterified oil is separated from alcohol, H2SO4, and impurities. Separated esterified oil was now heated at 65 °C for 1 h in a rotary evaporator for removing water and methanol. After esterification, transesterification is carried out to further reduce the viscosity as the raw oil is very viscous. For transesterification, a mixture of 6:1 M ratio of methanol to oil ratio and 0.9 (wt%) of KOH (potassium hydroxide) were added with the esterified oil and stirred for 1 h at 65 °C at 600 rpm.
Then, the obtained solution is transferred to a funnel separator and allowed to settle for 24 h. The final product (water hyacinth biodiesel) is obtained at the top layer and glycerol is settled over the bottom which is drained. The obtained biodiesel has several impurities which are removed by treating with distilled water and again allowing for settling in the funnel separator for 24 h. Now the pure biodiesel without impurities is obtained. The fatty acid composition of water hyacinth biodiesel (WHB) is illustrated in Table 1.The transesterification reaction for conversion of triglycerides to biodiesel was illustrated in Eq. 1.
Materials and method
Water hyacinth—an outline
Water hyacinth (Eichhornia crassipes), a perennial aquatic weed native to Brazil, is recently considered to be the most successful colonizers of the plant kingdom. It is an ornamental plant as well as parasitic plant capable of clogging the waterways and chocking the local ponds where it is introduced. The common water hyacinth (Eichhornia crassipes) is capable of growing and doubling itself in size in less than 2 weeks. The plant requires minimum growth temperature of 12 °C and a maximum growth temperature of 35 °C and its pH tolerance lies between 5 and 7.5. Since this weed consumes excessive oxygen content available where it grows, it is named as invasive species and in Asian subcontinent, it is named as “Terror of Bengal.” Water hyacinth has excellent source of biomass. Each hectare cultivation (2.5 acres) of standing crop of water hyacinth is capable of producing 70,000 m3/ha of biogas (Mitchell 1976; National Academy of Sciences 1976) and 1 kg of dry matter of water hyacinth is capable of yielding a heating value of 22,000 kJ/m3 (Curtis and Duke 1982).
Test fuel preparation
The test fuel blends used were B10, B20, B30, B40, B100, and diesel fuel which were prepared in volumetric basis. B10 is composed of 10% WHB + 90% diesel fuel, B20 is composed of 20% WHB + 80% diesel fuel, B30 comprises 30% WHB + 70% diesel fuel, B40 comprises 40% WHB + 60% diesel fuel, and B100 denotes 100% WHB, respectively. All the WHB-diesel blends were stirred magnetically for 30 min followed by ultrasonication of 10 min. The test fuel blends were investigated for settling and till 96 h, there were no reported settling. Only after prior investigation of settling, the test blends were subjected to characterization, fuel property investigation, and experimentation purposes. The various thermophysical properties of the prepared fuel samples were determined by standard testing procedure with respect to ASTM standards in fuel testing laboratory. The ASTM standards used for the determination of the biodiesel properties were as follows. The standards like ASTM-D270 for calorific value; ASTM-D976 for cetane number; ASTM-D93 for flash point; ASTM-D 4052 for density and ASTM-D445 for kinematic viscosity; ASTM-D 4530 for carbon residue; ASTM-D 664 for acid value; ASTM-D 2709 for water content; and ASTM-D 874 for ash content used in this investigation and measured values are listed out in Table 2.
Engine test procedure
A stationary diesel-powered Kirloskar engine (Model: TAF-1 Make: Kirloskar) was employed for experimentation. The specifications of test engine are given in Table 3. The engine was coupled to eddy current dynamometer with electrical resistance followed by a dynamometer controller. Data acquisition system (DAS) collects the cylinder pressure and the collected data were stored in computer for analysis. Using the combustion analyzer kit, heat release rate (HRR), cumulative heat release rate (CHRR), ignition delay (ID), start of combustion (SOC), combustion duration (CD), and mass fraction burnt in the cylinder were evaluated. A non-contact type optical sensor was mounted near flywheel to ensure the constant speed of engine (1500 rpm). K-type thermocouples were installed at various locations for measuring exhaust gas temperatures (EGT). The exhaust concentrations such as HC, CO, NOx, CO2, and O2 were measured by using the 5 gas analyzer (AVL444). During the steady-state engine operation at every stage of experiment, exhaust gas was permitted to surge by the probe followed by the condensation trap filter for evolving the moisture free gas. The filtered gas was now allowed to pass to NDIR sensor for the measurement of HC, CO, and CO2 emissions. With electrochemical sensor, NOx was measured. With smoke meter (AVL 437 C), smoke opacity was measured. The schematic layout of test installation is shown in Fig. 2. Tests were performed at five load intervals of 0%, 25%, 50%, 75%, and 100% which are equivalent to their respective brake power ranges. Initially, the engine was run with diesel for 20 min to warm it up before the testing of other blends. Similarly, between one test fuel and other, engine was allowed to run with diesel fuel for 10 min to ensure complete combustion of the residual fuel and in-cylinder homogeneity. The experimental tests were carried out using six fuel blends namely, B10 (10% WHB–90% diesel), B20 (20% WHB–80% diesel), B30 (30% WHB–70% diesel), B40 (40% WHB–60% diesel), and B100 (100% WHB) and diesel fuel, respectively.
Performance analysis
The instrument used to measure the output power is dynamometer, which has a stationary rotor bounded by electromagnets and coupled with crankshaft by flexible coupling. The rotor’s rotary motion acts against the magnetic field generated by the electromagnet when supplied with sufficient electric current. This in turn creates resistance to rotor and thereby happens to apply load to engine. Hence, varying the current supplied to electromagnet can alter the load. The opposing force created on dynamometer is measured by the change in strain gauge, based on which the “Torque” is measured by multiplying the R-distance between the center of pivotal point (strain gauge) with the varied opposing force.
Based on the obtained torque, the brake power (BP) output can be evaluated for the test engine which operates at a constant speed of 1500 rpm as follows:
Where, “BP” denotes brake power, “N” denotes constant engine speed (here 1500 rpm), “T” denotes the torque generated, and “S” denotes dynamometer constant. From the above equation, the respective brake powers generated by the engine at engine loads of 0%, 25%, 50%, 75%, and 100% at speed of 1500 rpm can be evaluated. The total fuel consumed by the engine is evaluated by the consumption of certain quantity of fuel (10 cc) at varying time period with the help of stopwatch. With increasing load, more fuel is injected to maintain the engine speed constant (at 1500 rpm).
“TFC” can be calculated by the formula below:
Where “ρ” denotes fuel density, “v” denotes the volume of fixed quantity (here it is 10 cc), and “t” denotes the time taken by stopwatch during 10 cc fuel consumed. From BP and TFC, the brake-specific fuel consumption (BSFC) can be evaluated by the amount of fuel consumed for getting 1 kW power output. In general, BSFC is preferred for using experimental fuels which has similar values of density and calorific values. However, BSEC (brake-specific energy consumption) is more specific than BSFC as it correlates the varying density and calorific value of experimental fuels. “BSEC” is nothing but amount of energy supplied (MJ) to engine operated for 1 h and for getting 1 kW output power. It can be calculated by the formula represented below:
Where, “BSEC” denotes the brake-specific energy consumption, “BP” denotes brake power developed by the engine, and “CV” denotes the calorific value of the test fuel subjected to experimentation. Finally, the brake thermal efficiency is determined for each fuel as a tendency to find how the fuel is utilized inside the combustion zone efficiently to convert it into useful work output.
Where, “BTE” denotes brake thermal efficiency, “TFC” denotes the total fuel consumption, and “CV” denotes the calorific value of the test fuel subjected to experimentation. Overall, it is concluded that the performance characteristics for the experimentation. Actual air intake measurements were done using “U” tube manometer with 20 mm orifice diameter. By using the equation below, the difference in manometer water column is noted and the volume of air flow is calculated through the coefficient of discharge.
Where, “VA” denotes volume of air through orifice, “Cd” denotes coefficient of discharge (0.62), “A” denotes area of orifice cross section (m2), “Ha” water column’s height (m3), and “g” denotes acceleration due to gravity (9.81 m/s2).
Combustion analysis
The combustion parameters involved for analyzing the combustion characteristics were cylinder pressure, heat release rate (HRR), and cumulative heat release rate (CHRR) in reference to crank angle position. The in-cylinder pressure is recorded by piezoelectric pressure sensor fixed on cylinder head. The sensor operates at sensitivity range of 55 pC/bar with maximum operating range of 250 bar. To cool the sensor, a cooling adapter is provided adjacent to the sensor. Based on the signals received, there will be an optimum charge output from sensor which is directly proportional to the in-cylinder pressure. Charge amplifier amplifies the output to equivalent voltage while the signal conditioner converts the signal from analog to digital signal. The DAS (data acquisition system) records the pressure data and gets stored in high-speed computer at every 100 consecutive cycles.
The engine cylinder is coupled with data acquisition system (DAS) wich records the cylinder pressure data relevant to crank angle and computes combustion parameters like heat release rate, combustion duration, and ignition delay. DAS processes the real-time data through a series of operation like signal generation (through transducer), signal conditioning (through filters and circuitry), analog to digital signal conversion (through ADC converter), data storage and display (through PC-based arrangement), and processing of data (through software “Engine soft 4.0”).
For identifying the position of crank angle during the engine operation, the crank angle encoder (Make: Encoders India Pvt. Ltd., resolution 1°) is mounted on the flywheel which instantly sends the signal about the position with respect to TDC (Top Dead Centre). For measuring the engine speed, a proximity sensor (Make: Instron Engg Pvt. Ltd., range 0–9999 rpm) is placed below the engine coupling. Resistance temperature detector (RTD) (range 0–200 °C) is mounted to measure air temperature at inlet, inlet and outlet temperature of cooling water. A K-type thermocouple is placed near the exhaust valve for sensing the temperature of exhaust gases (range 0–1500 °C).
Emission analysis
The exhaust gas analyzer employed for the current experiment is QRO-402 (Make: QROTECH Ltd., Korea). It measures HC, CO, and CO2 at operating ranges of 0–1500 ppm, 0–9.99%, and 0–20% through NDIR (non-dispersive infrared) method. NOx measurement is in the range of 0–500 ppm and is measured by electrochemical method. Smoke measurements were done using AVL 437 C equipment (Type: IP 52) operating at 190–240 V AC; 50 Hz, 2.5 A. Smoke measurements were read at 0–100% smoke opacity and 0–99.99 m−1 smoke absorbance.
Uncertainty analysis
Errors and uncertainties can emerge from various factors like selection and calibration of instruments, changing environment conditions, tests and observations, etc. In general, uncertainty can be grouped into two major factors, namely fixed errors and random errors. The former scenario deals with repeatability while the latter deals with the analytical measurements. In this current work, the uncertainty of measured variable (ΔX) is evaluated by Gaussian distribution as shown in Eq. 7 within the confidence limits of ± 2σ. 2σ is the mean limit in which the 95% of measured values rely upon.
Where, Xi is the number of readings, \( \overline{X_i} \) denotes the experimental reading, and σi represents standard deviation. The uncertainties of calculated parameters were assessed using the expression given below:
Where, R in Eq. 8 represents the function of X1, X2,……..Xn and X1, X2,…….Xn represent number of readings taken. Hence ΔR is computed by RMS (root mean square) of errors associated with measured parameters. The uncertainties of various measuring instruments were illustrated in Table 4. By using Eq. 9, the uncertainties in various measured parameters were evaluated and tabulated in Table 5.
Results and discussion
Performance and combustion analysis
Brake-specific energy consumption
The variation of brake-specific energy consumption (BSEC) for different blends of WHB-diesel blends was illustrated in Fig. 3. BSEC indicates the measure of efficiency of the fuel whose energy is utilized in test engine for developing a unit power output. It is observed that BSEC of diesel is lower than the test fuel samples at all load conditions. Also, B10 is lower BSEC values at part load and full load conditions when compared to the other tested water hyacinth biodiesel blends. This is because of the fuel properties of B10, especially that of kinematic viscosity and calorific value which are closer to diesel fuel. These are in line with previous findings using various biodiesel sources (Balasubramanian et al. 2018; Wagner et al. 1984; Venu and Madhavan 2017; Raheman and Phadatare 2004; Ramadhas et al. 2005).
Brake thermal efficiency
Brake thermal efficiency (BTE) indicates how the fuel’s heat energy is converted to mechanical output. Various fuel properties responsible for BTE are kinematic viscosity, oxygen output, calorific value, and cetane index. Figure 4 clearly depicts the variation of BTE for all the test fuels throughout the engine load condition. BTE of B10 lies closer to diesel fuel throughout the engine load which could be attributed to improved heating value of the biodiesel blends due to in-built oxygen content in the blend. With increasing biodiesel concentration, there is a drop in BTE owing to higher fuel viscosity and poor energy content. Lower blend ratios of water hyacinth biodiesel till B30 result in higher BTE due to improved fuel atomization, vaporization, and air-fuel interaction process. Diesel exhibits highest BTE (32.6%), followed by B10 (29.6%), B20 (28.3%), B30 (27.9%), B40 (26.85%), and B100 (24.8%), where all the biodiesel-diesel blends have lowered BTE in comparison with neat diesel. These are in good agreement with the findings of Wagner et al. (1984), Raheman and Phadatare (2004), Dhana Raju et al. (2016), and Ramadhas et al. (2005).
Ignition delay
Ignition delay is the actual time period between start of injection and start of combustion. ID is an important parameter used to define the ignition quality (in terms of cetane index). Lower the ID, higher the cetane number, owing to well-improved diffusion combustion period. Biodiesel usually has higher cetane number than diesel fuel and therefore contributes to lowered ignition delay period in comparison with diesel fuel. Figure 5 illustrates the variation of ignition delay period for WHB-diesel blends. It is observed that, with increasing biodiesel concentration, the ignition delay is higher owing to sharp regress in atomization, improved cetane number, lowered cone angle, and excess oxygen content available in the blend. B100 exhibits lowered delay (5.8 °CA) in comparison with diesel (9.2 °CA). As the engine load increases, ignition delay decreases as a result of faster fuel-air mixing rate, higher in-cylinder temperature, exhaust gas dilution, and retainment of excess heat during the previous cycle of combustion. At 100% engine load, the ignition delay of B10, B20, B30, B40, B100, and diesel is 8.5 °CA, 7.6 °CA, 6.9 °CA, 6.6 °CA, 5.8 °CA, and 9.3 °CA, respectively. This is because at higher temperatures, vaporization rate is enhanced; chemical delay is lowered (Murugan et al. 2008).
Cylinder pressure
In CI engines, the cylinder pressure depends on the amount of fuel burnt during the premixed combustion phase. Factors such as viscosity, air-fuel mixing rate, and cetane index are the main factors responsible for the variation of cylinder pressure. Figure 6 shows the variation of cylinder pressure for all the test fuels. It is observed that all the WHB-diesel blends exhibits lowered cylinder pressure in comparison with diesel, with higher biodiesel concentration giving rise to lowered cylinder pressure due to high viscous and low calorific value of biodiesel blends which affects the atomization, vaporization, and air-fuel mixing rate invariably affecting the cylinder pressure. In addition, the lowest ignition delay of B100 results in lower amount of accumulation of fuel quantity at time of combustion, resulting in lowered cylinder pressure.
Heat release rate
The heat release rate (HRR) at each crank angle for various test fuels was calculated using the correlation derived from first law of thermodynamics by Eq. 10:
Where, \( \frac{d{Q}_n}{d\theta} \) is the net heat release rate (J/°CA), P is the instantaneous cylinder pressure (N/m2), V is the instantaneous cylinder volume (m3), θ is crank angle (degree), and γ is the ratio of specific heats Cp/Cv (kJ/kgK). γ depends on temperature and influences the intensity of \( \frac{d{Q}_n}{d\theta} \) and \( \overset{\bullet }{Q_{lw}} \). \( \overset{\bullet }{Q_{lw}} \) is the blow by losses which are evaluated by Rakopoulos 2012. The gross heat release rate can be calculated by Eq. 11:
Where, \( \frac{{d Q}_{\mathrm{lw}}}{d\theta} \) is the amount of heat transfer rate to the combustion chamber walls (Rakopoulos 2012). Figures 7 and 8 show the variation of heat release rate (HRR) and cumulative heat release rate (CHRR) for all the test fuels with respect to crank angle at 100% engine load condition. It is observed that the combustion starts earlier for WHB-diesel blends due to higher cetane number, lowered delay period, and calorific value which lowers the HRR significantly (Mazumdar and Agarwal 2008). Diffusion combustion phase of biodiesel blends were in par with diesel fuel owing to higher O2 content in biodiesel, which are left over at previous cycle and continues to combust even at later stages of combustion. Initially, the CHRR graph is negative due to vaporization of test fuel; however, on initiation of combustion, the value becomes positive. The diffusion combustion phase of WHB-diesel blends was dominant in comparison with diesel fuel owing to the twin effect of complete combustion due to the presence of O2 molecules in its structure and higher heating value of diesel fuel than WCB-diesel blends (Serrano et al. 2012). With increasing WCB concentration, the CHRR lowers at premixed combustion duration due to high O2 content of biodiesel promoting later stages of combustion.
Exhaust gas temperature
Exhaust gas temperature (EGT) indicates the amount of heat released during combustion and has certain influence over the pollutant formation. EGT gives a clear insight of O2 availability, air-fuel ratio, engine performance, and heat liberated during the diffusion combustion phase. From Fig. 9, it is observed that WCB blends have higher EGT in comparison to diesel fuel due to higher O2 content in biodiesel. EGT increases with increasing engine load due to more fuel injected into the cylinder. With increasing concentration of biodiesel, the fuel properties are altered especially higher viscosity, improved bulk modulus, and advancement in start of combustion (Tan et al. 2012), which altogether makes EGT to surge. B40 exhibits highest EGT of about 530 °C at full load condition. At part loads, the EGT of B20 and B100 were in par, while these relative differences were higher at higher loads due to more fuel takes part in combustion at higher loads. These are in good agreement with findings of Nwafor (2004), Kumar et al. (2003), Ramadhas et al. (2005), and Balasubramanian et al. (2018).
Emission analysis
Hydrocarbon
Hydrocarbon (HC) formations in CI engines are very complex and dependent on various sources like fuel trapped in nozzle, crevice areas, and cylinder piston interface. Biodiesel combustion in diesel engine causes HC to be more predominant due to incomplete evaporation of fuel-air mixture, local over-rich/lean mixture, and excess spray impingement (Heywood 1988). From Fig. 10, it is observed that WHB-diesel blends exhibit lowered HC emission throughout the engine load due to the presence of O2 in biodiesel, improving the combustion efficiency. With increasing engine load, HC emission tends to increase due to excess fuel burnt in the combustion chamber. At 100% engine load, HC is highest for diesel fuel (67 ppm), followed by B10 (63 ppm), B100 (69 ppm), B40 (58 ppm), and B20 (56 ppm). Lowered HC emissions for WHB-diesel blends can be attributed to improved mixing rate, higher vaporization rate, and more O2 availability in blends. These are in good agreement with previous findings where there is higher viscosity of biodiesel improving the spray penetration and mixing rate (Xue et al. 2011). Contrasting results were also observed where increased viscosity of biodiesel leading to increasing HC emissions as a result of poor atomization and combustion.
Carbon monoxide
Generally, diesel engines liberate lower carbon monoxide (CO) due to its operation well over the lean mixture one and hence it is very less significant in emissions. However, biodiesel usage alters the O2 content, oxidation rate, fuel spray characteristics, in-cylinder temperature, and ignition centers which plays a vital role in CO formation (Balaji and Cheralathan 2015). From Fig. 11, it is found that CO of diesel fuel is higher than WHB-diesel blends due to O2 deficiency in diesel fuel, overriding the WHB-diesel blends which have in-built fuel O2 content. O2 presence enhances the combustion rate and promotes faster conversion of CO to CO2. At 100% load, B40 blend exhibits lowest CO formation in comparison with diesel fuel (0.144%). Increasing engine loads causes increasing CO emissions due to more fuel taking part in combustion and formation of more fuel-rich zones with O2 deficiency. At full load, CO emissions emitted by B100 are lower than B20, B30, and B40 by 3.7%, 6.9%, and 8.2%, respectively.
Carbon dioxide
Diesel engine combustion consists of air, fuel, and some quantum of heat to ignite the heterogeneous fuel-air mixture attained by compression. Combustion process generates products of complete combustion and products of incomplete combustion. While carbon dioxide (CO2) and H2O were the products of complete combustion, CO, HC, NOx, and smoke were considered as products of incomplete combustion. More the CO2 emissions, better the fuel oxidation as there is a trade-off between CO and CO2 emissions. From Fig. 12, it is observed that WHB-diesel blends have higher CO2 emissions throughout the engine load indicating the presence of oxygen content promoting complete combustion. In addition, it is observed that B100 has a noticeable drop in CO2 emission in comparison with other test blends such as B30 and B40, which can be attributed to lowered calorific value and higher density of fuel mixtures which affects the complete combustion process thereby leading to less CO2 emissions.
Nitrogen oxides
Nitrogen oxide (NOx) emission in general occurs only at gas temperatures greater than 1500 °C at the end of combustion, where O2 and N2 in air react to form by products. The presence of O2 content in biodiesel plays a vital role in NOx formation. NOx emissions surge at lean mixture ranges (ƛ = 1.1–1.2) where the O2 molecule profusion occurs, resulting in higher combustion temperatures (Zhao 2007; Koç et al. 2009; Kim and Lee 2007; Park et al. 2010; Uyumaz 2015). Figure 13 shows the variation of NOx emissions for diesel and WHB-diesel blends. It is observed that, with increasing engine loads for biodiesel blends, NOx emissions increase owing to improved combustion rate, O2 availability, and net heat released in the previous combustion cycles. Higher NOx emissions for WHB-diesel blends can be attributed to higher fuel density in relation to higher injection pressures consuming excess fuel delivery (at same injector settings). Moreover, WHB-diesel blend’s combustion lasts only for shorter time, which in turn affects the cooling time via heat transfer and dilution which altogether results in higher NOx emissions. Increasing biodiesel concentration tends to increase the NOx emissions linearly. While for B100, there is a drop in NOx emissions due to lowered peak in-cylinder pressure and calorific value which affects the combustion rate and in-cylinder temperature resulting in lowered NOx emissions.
Smoke opacity
Smoke emissions in CI engines are predominant at diffusion combustion phase where the entire atomized droplets are transformed to elemental “C” particles which are further oxidized in reaction zones (i.e., soot precursor oxidation). Additional reasons for smoke emission occurrence are air deficiency in combustion-rich zones, higher fuel viscosity, excess C/H ratio, and over accumulation of fuel in combustion chamber fuel-rich zones (Murugan et al. 2008). Figure 14 shows the variation of smoke emissions of WHB-diesel blends with respect to higher loads. It is observed that biodiesel blends resulted in lowered smoke emissions in comparison to diesel fuel due to lowered C/H ratio, higher O2 content, and absence of aromatic chain in biodiesel thereby resulting in improved combustion and oxidation of fuel-rich zones. It is observed that till 40% addition of biodiesel concentration, there is a noticeable drop in smoke generation which can be intrigued with more O2 content, less “C” content, and less C-C bonds in the biodiesel structure which promotes oxidation of soot precursors followed by lessened smoke (Utlu and Koçak 2008). Highest smoke is observed for B100 in comparison with other test blends. This is because of animosity in the oxidation of high viscous fuel as a result of huge-sized droplets, lessened evaporation rate, and poor air-fuel mixing rate therefore resulting in higher possibility of carbonaceous soot particles which occurs as smoke emissions.
Conclusion
The present experimental work focuses on the investigation of performance, combustion, and emission characteristics of a diesel engine powered by water hyacinth biodiesel (WHB) and its blends with diesel fuel. Based on the experimental results, the following conclusions were drawn:
-
The various blends prepared from the water hyacinth-diesel fuels were successfully operated on diesel engine without any hardware modifications.
-
Test results revealed lower BTE for the WHB blends when compared to the diesel fuel. Among the several of blends of WHB, 20% water hyacinth biodiesel blend shown promising results in terms of enhanced performance and reduced tailpipe emissions.
-
WHB and its blends resulted in lowered ignition delay, peak cylinder pressure, and instantaneous heat release when compared to the diesel fuel at all load conditions.
-
WHB and its blends possessed lower level of HC, CO, and smoke emissions due to the presence of inherent fuel bound O2 atoms. However, the CO2 and NOx levels were considerably higher than neat diesel fuel, especially at part load conditions.
Overall, it can be concluded that B20 (20% WHB + 80% diesel fuel) blend can be used as a potential alternative feedstock for future diesel engine applications towards green and sustainable energy resources.
Abbreviations
- ASTM:
-
American Society for Testing and Materials
- BSEC:
-
Brake-specific fuel consumption
- BTE:
-
Brake thermal efficiency
- WHB:
-
Water hyacinth biodiesel
- CHRR:
-
Cumulative heat release rate
- CO:
-
Carbon monoxide
- CO2 :
-
Carbon dioxide
- EGT:
-
Exhaust gas temperature
- H2O:
-
Water
- HRR:
-
Heat release rate
- ID:
-
Ignition delay
- MR:
-
Methanol to oil ratio
- NOx:
-
Oxides of nitrogen
- O2 :
-
Oxygen
- HC:
-
Hydrocarbon
- P :
-
Instantaneous heat release rate, N/m2
- V :
-
Instantaneous cylinder volume, m3
- ΔX :
-
Uncertainty of measured variables
- X i :
-
Number of readings
- \( \overline{X_i} \) :
-
Experimental readings
- θ :
-
Crank angle, degree
- γ :
-
Ratio of specific heats (Cp/Cv), kJ/kgK
- \( \overset{\bullet }{Q_{lw}} \) :
-
Blow by losses, J/°CA
- \( \frac{d Qlw}{d\theta} \) :
-
Heat transfer to combustion chamber walls, J/°CA
- \( \frac{d{Q}_n}{d\theta} \) :
-
Net heat release rate, J/°CA
- \( \frac{d{Q}_g}{d\theta} \) :
-
Gross heat release rate, J/°CA
References
Abraham M, Kurup GM (1996) Bioconversion of tapioca (Manihotesculenta) waste and water hyacinth (Eichhorniacrassipes)—influence of various physico-chemical factors. J Ferment Bioeng 82(3):259–263
Aswathy US, Sukumaran RK, Devi GL, Rajasree KP, Singhania RR, Pandey A (2010) Bio-ethanol from water hyacinth biomass: an evaluation of enzymatic saccharification strategy. Bioresour Technol 101(3):925–930
Atabani AE, Mahlia TMI, Badruddin IA, Masjuki HH, Chong WT, Lee KT (2013) Investigation of physical and chemical properties of potential edible and non-edible feedstocks for biodiesel production, a comparative analysis. Renew Sust Energ Rev 21:749–755
Atadashi IM, Aroua MK, Aziz AA (2010) High quality biodiesel and its diesel engine application: a review. Renew Sust Energ Rev 14(7):1999–2008
Balaji G, Cheralathan M (2015) Experimental investigation of antioxidant effect on oxidation stability and emissions in a methyl ester of neem oil fueled DI diesel engine. Renew Energy 74:910–916
Balasubramanian D, Arumugam SRS, Subramani L, Chellakumar IJLJS, Mani A (2018) A numerical study on the effect of various combustion bowl parameters on the performance, combustion, and emission behavior on a single cylinder diesel engine. Environ Sci Pollut Res 25(3):2273–2284
Bhuiya MMK, Rasul MG, Khan MMK, Ashwath N, Azad AK, Hazrat MA (2014) Second generation biodiesel: potential alternative to-edible oil-derived biodiesel. Energy Procedia 61:1969–1972
Choudhary AK, Chelladurai H, Kannan C (2015) Optimization of combustion performance of bio ethanol (water hyacinth) diesel blends on diesel engine using response surface methodology. Arab J Sci Eng 40:3675–3695
Curtis CR, Duke JA (1982) An assessment of land biomass and energy potential for the Republic of Panama. Institute of Energy Conversion. Univ, Delaware, p 3
Demirbas A (2009) Progress and recent trends in biodiesel fuels. Energy Convers Manag 50(1):14–34
Dhana Raju V, Kumar KK, Kishore PS (2016) Engine performance and emission characteristics of a direct injection diesel engine fuelled with 1-hexanol as a fuel additive in Mahua seed oil biodiesel blends. Int J Therm Environ Eng 13(2):121–127
Heywood J (1988) Internal combustion engine fundamentals, 2nd edn. McGraw-Hill, New York, pp 284–286
Huang G, Chen F, Wei D, Zhang X, Chen G (2010) Biodiesel production by microalgal biotechnology. Appl Energy 87(1):38–46
Issariyakul T, Dalai AK, Desai P (2011) Evaluating esters derived from mustard oil (Sinapisalba) as potential diesel additives. J Am Oil Chem Soc 88(3):391–402
Kahlon SS, Kumar P (1987) Simulation of fermentation conditions for ethanol production from water-hyacinth. Indian J Ecol 14(2):213–217
Kim MY, Lee CS (2007) Effect of a narrow fuel spray angle and a dual injection configuration on the improvement of exhaust emissions in a HCCI diesel engine. Fuel 86(17–18):2871–2880
Koç M, Sekmen Y, Topgül T, Yücesu HS (2009) The effects of ethanol–unleaded gasoline blends on engine performance and exhaust emissions in a spark-ignition engine. Renew Energy 34(10):2101–2106
Konwar LJ, Boro J, Deka D (2014) Review on latest developments in biodiesel production using carbon-based catalysts. Renew Sust Energ Rev 29:546–564
Kumar MS, Ramesh A, Nagalingam B (2003) An experimental comparison of methods to use methanol and Jatropha oil in a compression ignition engine. Biomass Bioenergy 25(3):309–318
Mazumdar B, Agarwal AK (2008) Performance, emission and combustion characteristics of biodiesel (waste cooking oil methyl ester) fueled IDI diesel engine (No. 2008-01-1384). SAE Technical Paper
Mishima D, Kuniki M, Sei K, Soda S,. Ike M, M. Fujita (2008) Ethanol production from candidate energy crops: water hyacinth (Eichhornia crassipes) and water lettuce (Pistia stratiotes L.). Bioresource technology 99(7):2495–2500
Mitchell DS (1976) The growth and management of Eichhornia crassipes and Salvinia spp. in their native environment and in alien situations. In: Varshnay CK, Rz6ska J (eds) Aquatic weeds in South East Asia. Dr. W. Junk, The Hague, pp 167–176
Murugan S, Ramaswamy MC, Nagarajan G (2008) Performance, emission and combustion studies of a DI diesel engine using distilled Tyre pyrolysis oil-diesel blends. Fuel Process Technol 89(2):152–159
National Academy of Sciences (1976) Making aquatic weeds useful: some perspectives for developing countries. Report of an Ad Hoc Panel of the advisory Committee on Technology Innovation Board on Science and Technology for International Development. Commission on International Relations, Washington, D.C.
Nigam JN (2002) Bioconversion of water-hyacinth (Eichhorniacrassipes) hemicellulose acid hydrolysate to motor fuel ethanol by xylose–fermenting yeast. J Biotechnol 97(2):107–116
Nwafor OMI (2004) Emission characteristics of diesel engine operating on rapeseed methyl ester. Renew Energy 29(1):119–129
Park C, Choi Y, Kim C, Oh S, Lim G, Moriyoshi Y (2010) Performance and exhaust emission characteristics of a spark ignition engine using ethanol and ethanol-reformed gas. Fuel 89(8):2118–2125
Raheman H, Phadatare AG (2004) Diesel engine emissions and performance from blends of karanja methyl ester and diesel. Biomass Bioenergy 27(4):393–397
Raju VD, Kumar MH, Kishore PS, Venu H (2018) Combined impact of EGR and injection pressure in performance improvement and NOx control of a DI diesel engine powered with tamarind seed biodiesel blend. Environ Sci Pollut Res 25:36381–36393
Rakopoulos DC (2012) Heat release analysis of combustion in heavy-duty turbocharged diesel engine operating on blends of diesel fuel with cottonseed or sunflower oils and their bio-diesel. Fuel 96:524–534
Ramadhas AS, Muraleedharan C, Jayaraj S (2005) Performance and emission evaluation of a diesel engine fueled with methyl esters of rubber seed oil. Renew Energy 30(12):1789–1800
Saladini F, Patrizi N, Pulselli FM, Marchettini N, Bastianoni S (2016) Guidelines for emergy evaluation of first, second and third generation biofuels. Renew Sust Energ Rev 66:221–227
Sanaa MMS, Eman AH, Emad AS (2018) Water hyacinth as non-edible source for biofuel production. Waste Biomass Valor 9:255–264
Serrano LM, Câmara RM, Carreira VJ, Da Silva MG (2012) Performance study about biodiesel impact on buses engines using dynamometer tests and fleet consumption data. Energy Convers Manag 60:2–9
Srinophakun P, Thanapimmetha A, Rattanaphanyapan K, Sahaya T, Saisriyoot M (2017) Feedstock production for third generation biofuels through cultivation of Arthrobacter AK19 under stress conditions. J Clean Prod 142:1259–1266
Tan PQ, Hu ZY, Lou DM, Li ZJ (2012) Exhaust emissions from a light-duty diesel engine with Jatropha biodiesel fuel. Energy 39(1):356–362
Umdu ES, Tuncer M, Seker E (2009) Transesterification of nannochloropsisoculata microalga’s lipid to biodiesel on Al2O3 supported CaO and MgO catalysts. Bioresour Technol 100(11):2828–2831
Utlu Z, Koçak MS (2008) The effect of biodiesel fuel obtained from waste frying oil on direct injection diesel engine performance and exhaust emissions. Renew Energy 33(8):1936–1941
Uyumaz A (2015) An experimental investigation into combustion and performance characteristics of an HCCI gasoline engine fueled with n-heptane, isopropanol and n-butanol fuel blends at different inlet air temperatures. Energy Convers Manag 98:199–207
Venu H, Madhavan V (2017) Effect of diethyl ether and Al2 O3 nano additives in diesel-biodiesel-ethanol blends: performance, combustion and emission characteristics. J Mech Sci Technol 31(1):409–420
Wagner LE, Clark SJ, Schrock MD (1984) Effects of soybean oil esters on the performance, lubricating oil, and water of diesel engines (No. 841385). SAE Technical Paper, pp 57–72
Xue J, Grift TE, Hansen AC (2011) Effect of biodiesel on engine performances and emissions. Renew Sust Energ Rev 15(2):1098–1116
Yusuf NNAN, Kamarudin SK, Yaakub Z (2011) Overview on the current trends in biodiesel production. Energy Convers Manag 52(7):2741–2751
Zhao H (2007) HCCI and CAI engines for the automotive industry, 1st edn. Woodhead, Cambridge, pp 68–74
Zhong W, Xuan T, He Z, Wang Q, Li D, Zhang X, Huang YY (2016) Experimental study of combustion and emission characteristics of diesel engine with diesel/second-generation biodiesel blending fuels. Energy Convers Manag 121:241–250
Acknowledgements
The authors thank Centre for biotechnology, Anna University for its assistance in aquatic growth, culture, and oil extraction. The author also thanks the Chemical engineering department, Anna University for fuel property characterization.
Funding
The author also discloses that there are no external grants received for this research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Eichhornia crassipes (water hyacinth) were grown in aquatic ecosystems, harvested, and oil is extracted
• Water hyacinth biodiesel (WHB) were synthesized from raw oil using transesterification
• WHB and its blends with diesel fuel were investigated for performance, combustion, and emissions
• B20 blend has lowest smoke, CO, and HC emission, but with highest NOx and CO2 formation
Rights and permissions
About this article
Cite this article
Venu, H., Venkataraman, D., Purushothaman, P. et al. Eichhornia crassipes biodiesel as a renewable green fuel for diesel engine applications: performance, combustion, and emission characteristics. Environ Sci Pollut Res 26, 18084–18097 (2019). https://doi.org/10.1007/s11356-019-04939-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04939-z