Abstract
The increase in biodiesel production has been leading to an excess amount of crude glycerol and, consequently, serious environmental issues. For this reason, electrospun chitosan-based nanofibers (CB-EN), composed by chitosan and poly(ethylene oxide) (PEO), were synthesized to apply in the biosorption of impurities from industrial glycerol. To evaluate the biosorption efficiency, the chitosan-based nanofiber was compared to other chitosan-based biosorbents (chitosan biopolymeric film and chitosan powder). The equilibrium and thermodynamic studies were successfully performed to comprehend the interaction mechanisms through the biosorption of glycerol pigments onto electrospun chitosan-based nanofibers. The temperature effect was evaluated by experimental equilibrium curves. Freundlich and BET models were used to estimate isotherm parameters. Gibbs free energy change, enthalpy change, entropy change, and isosteric heat of biosorption were quantified. The equilibrium curves showed that the highest equilibrium relative adsorption (340.7 g−1) was reached at 60 °C. The BET model was the most suitable to represent the equilibrium behavior. The thermodynamic parameters indicated that the biosorption was spontaneous, exothermic, random, and energetic heterogeneous. Therefore, this work developed a green and efficient alternative to refine industrial glycerol.

Note: This data is mandatory. Please provide
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The demand for renewable fuels has been increasing due to environmental concerns. In fact, biodiesel is a promising renewable fuel, which is usually produced by the transesterification reaction of animal fats and vegetable oils. However, biodiesel production generates around 10% (w/w) of glycerol as the major by-product (Hoekman et al. 2012; Yang et al. 2012; Živković and Veljković 2018). The fast increase in biodiesel production has been leading to a large glut of crude glycerol, which has been caused a critical problem and an environmental liability for the biodiesel market (Hu et al. 2012). Therefore, it is extremely important to transform crude glycerol into an added-value product.
The glycerol with high purity has many applications in the pharmaceutical and food sectors. The glycerol purification generally includes three steps: neutralization, evaporation, and refinement. The last one is responsible for the achievement of glycerol with high purity (Ardi et al. 2015; Tan et al. 2013). Among the processes that can be used in the refinement step, adsorption with activated carbon is the most one employed to reduce the amount of pigments and other compounds (Dhabhai et al. 2016). However, chitosan could be a promising green alternative for this application, because this biopolymer is produced from renewable and natural resources (Kyzas et al. 2013).
Chitosan is a biopolymer constituted by residues of 2-acetamide-2-deoxy-β-D-glucopyranose and 2-amino-2-deoxy-β-D-glucopyranose. The presence of amino (–NH2) and hydroxyl (–OH) groups makes chitosan an interesting biosorbent to react with other species (Crini and Badot 2008; Dotto et al. 2017a; Frantz et al. 2017; Wan et al. 2018). Therefore, different types of chitosan-based biosorbents have already been extensively applied to remove pigments from dye wastewater (Hossini et al. 2017; León et al. 2018; Moura et al. 2016; Zhang et al. 2018). Furthermore, chitosan-based nanofibers are especially attractive due to the advantages of higher surface area and easy separation (Dotto et al. 2017b; Gore et al. 2018; Kwork et al. 2018; Li et al. 2017; Qin et al. 2017).
For better comprehension about the biosorption system design, the study of equilibrium and thermodynamic parameters are important. The equilibrium isotherms show the interaction type between biosorbent and adsorbate; in addition, they also provide information about the affinity among their molecules. Moreover, the adsorption thermodynamic parameters are required to elucidate the energy intensity of the operation, as well as the spontaneity and randomness of the system (Piccin et al. 2017; Pohndorf et al. 2016). Therefore, this work aimed to study the equilibrium and thermodynamic parameters in the biosorption of glycerol pigments onto electrospun chitosan/poly(ethylene oxide) nanofibers (CB-EN) in a batch system. The effectiveness of CB-EN was evaluated by comparing it to other chitosan-based biosorbents. Freundlich and Brunauer–Emmett–Teller (BET) models were applied to the equilibrium study. The thermodynamic study was performed by estimating the Gibbs free energy change (ΔG), enthalpy change (ΔH), entropy change (ΔS), and isosteric heat of biosorption (ΔHa). Scanning electron microscope (SEM), energy-dispersive spectroscopy (EDS), and infrared analysis with attenuated total reflectance (FTIR) were carried out for further information about the interaction between chitosan-based nanofibers and glycerol impurities.
Material and methods
Materials
Chitosan powder (C-P) (molecular weight (MW) = 150 ± 4 kDa; deacetylation degree (DD) = 85.2 ± 1.1%; particle size (PS) = 100 ± 10 μm) was produced from shrimp waste (Penaeus brasiliensis), according to our previous works (Dotto et al. 2011; Moura et al. 2015; Weska et al. 2007). Poly(ethylene oxide) (PEO) (MW = 900 ± 5 kDa) was purchased from the Sigma-Aldrich (Sigma-Aldrich®, USA). The pretreated industrial glycerol (moisture content = 13.48%; pH = 4.6; Lovibond yellowness index = 15.0 and Lovibond redness index = 7.5; soap content = 0%; acidity content = 0%) was obtained from a local biodiesel production plant in Brazil. The pretreatment aimed to reduce salts, catalyst, free fatty acids, and methanol by neutralization and evaporation steps.
Development of chitosan biopolymeric films
Chitosan powder (1.5 g) was dissolved into 50 mL of acetic acid solution (0.1 mol L−1) at 25 ± 1 °C, under magnetic stirring (300 rpm) (Fisatom, 752, Brazil) for 12 h. The chitosan biopolymeric film (C-F) was obtained by solvent evaporation at 40 ± 2 °C for 24 h using an oven with air circulation (Dotto et al. 2013; Moura et al. 2015).
Development of electrospun nanofibers
Chitosan (5% w/v) was dissolved in acetic acid solution (90% v/v), and then, PEO (3% w/v) was added into the solution. The polymeric solution was performed at 25 ± 1 °C for 12 h under magnetic stirring (300 rpm) (Fisatom, 752, Brazil). The electrospun chitosan/poly(ethylene oxide) nanofibers (CB-EN) were synthesized by electrospinning technique under the following conditions, based on our previous work (Farias et al. 2018): capillary diameter of 0.55 mm; capillarity-collector distance of 150 mm; electric potential of 25 kV and feed rate of 600 μL h−1. The nanofiber development occurred at air conditions of 25 ± 1 °C and relative humidity of 65 ± 1%.
Characterization of chitosan-based biosorbents
The surface morphology of chitosan-based biosorbents (C-P, C-F, CB-EN) was evaluated by SEM (JEOL, JSM–6610, Akishima-shi, Tokyo, Japan). The analysis was operated at 10 kV, with a magnification of 250 and 2500 times. Prior to SEM analysis, the biosorbents were placed on stainless steel supports and for the coating was used 1 nm of gold layer (Goldstein et al. 2003). The average fiber diameter was calculated by randomly selected diameters of 50 nanofibers from each sample.
Biosorption equilibrium applied on glycerol purification
The equilibrium assays were performed under different CB-EN biosorbent concentrations (0.05, 0.15, 0.25, 0.35, and 2 mg g−1) and in different temperatures (60, 70, and 80 °C). All aliquots were measured after the equilibrium was reached. The removal of glycerol pigments was detected by UV-visible spectrophotometry (Shimadzu, UV–2550, Japan), with a wavelength of 265 nm. The amount of pigments adsorbed, qe (g mg−1), was described by the following equation (Eq. (1)):
where mo and mf (g) are the initial and final glycerol pigment weights, respectively, and mad (g) is the adsorbent weight.
Despite the fact that the nature of glycerol pigments was unknown, it was possible to quantify the pigments by employing a relative adsorption capacity (qr) (Eq. (2)), together with the Lambert–Beer law (Eq. (3)) (Swinehart 1962).
where A refers to glycerol pigment absorbance, εc (m3 g−1) is a constant related to analyte nature and cuvette size, C (g m−3) is the concentration, V (m3) is the solution volume, and f is the dilution factor (dimensionless).
The inclusion of Eq. (3) into Eq. (2) results in Eq. (4), considering that all volumes (V) and dilution factors (f) do not change for the whole samples.
where qre (g−1) refers to equilibrium relative adsorption capacity, and Ao and Ae refer to initial and equilibrium glycerol pigment absorbances (dimensionless), respectively.
In order to verify the potential of CB-EN biosorbent for pigment removal from glycerol, the equilibrium assays were also carried out using other chitosan-based biosorbents (chitosan powder (C-P) and chitosan film (C-F)) in batch system, using a thermostatic shaker at 150 rpm (Fanem, 315 SE, Brazil), in triplicate (n = 3). The comparisons were performed using 0.15 mg g−1 of each biosorbent at 60, 70, and 80 °C.
The Freundlich (Eq. (5)) and BET (Eq. (6)) models were fitted to the experimental data obtained by CB-EN biosorbent for further comprehension about the biosorption equilibrium behavior (Ebadi et al. 2009; Freundlich 1906).
where KF (g−1/n) is the Freundlich constant, 1/n is the heterogeneity factor (dimensionless), qm (g−1) is the monolayer adsorption capacity, and KS and KL are the BET equilibrium constants for the monolayer (dimensionless) and consecutive layers (dimensionless), respectively.
Thermodynamic study
The Gibbs free energy change (ΔG0) (kJ mol−1), entropy change (ΔS0) (J mol−1 K−1), and enthalpy change (ΔH0) (kJ mol−1) parameters were estimated by Eq. (7) and Van’t Hoff plot (Eq. (8)) (Liu 2009; Milonjić 2007).
where R (8.314 J mol−1 K−1) is the gas constant, T (K) is the absolute temperature, ρ is the glycerol density (g L−1), and KD (L−1 g) is the thermodynamic equilibrium constant.
The isosteric heat of biosorption (ΔHa) (kJ mol−1) was determined by the following equation derived from the Clausius–Clapeyron (Bayrak 2003) associated with the Lambert–Beer Law (Swinehart 1962) (Eq. (9)):
where L' is an integration constant (dimensionless).
Statistical regression analysis
The equilibrium relative adsorption capacity values (qre) of biosorbents were compared by mean differences, utilizing the software Statistica 7.0 (StatSoft, USA). The values were considered significantly different at a 95% level (p < 0.05).
The equilibrium models were fitted to the experimental data by nonlinear Quasi–Newton estimation method (Statistic 7.0, Statsoft, USA). The graphics were plotted using OriginPro 8 (OriginLab, USA).
Interaction analysis between electrospun chitosan-based nanofibers and the glycerol pigments
The possible interactions between CB-EN biosorbent and glycerol pigments were evaluated by scanning electron microscope (SEM), energy-dispersive spectroscopy (EDS) (Jeol, JSM–6610LV, Japan), and infrared analysis with attenuated total reflectance (FTIR) (Shimadzu, Prestige 21, Japan) (Goldstein et al. 2003; Silverstein et al. 2005).
Results and discussion
Characterization of chitosan-based biosorbents
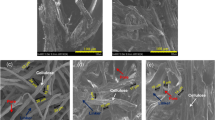
The SEM images (Fig. 1a–d) revealed that the CB-EN were successfully developed, with an average diameter of 526 ± 101 nm. Furthermore, the SEM images (Fig. 1b–e) demonstrated that C-P showed heterogeneous surface, with protuberances and cavities for all over the irregular particles. The SEM images also (Fig. 1c–f) showed that the C-F had a relatively homogenous flat surface, with some roughness.
Biosorption equilibrium applied on glycerol purification
In order to evaluate the effectiveness of CB-EN in the biosorption process, the equilibrium relative adsorption capacity values (qre) were determined, and they were compared with the qre values of the C-P and C-F. The equilibrium relative adsorption capacities are shown in Table 1. The higher values of the relative adsorption capacity of CB-EN, through the temperature range studied (from 60 to 80 °C), could be associated with the increased surface area of the biosorbent, which was resulted by chitosan on nanoscale. In fact, the nanoscale may improve the accessibility of glycerol pigments onto chitosan adsorptive sites.
Figure 2 shows the biosorption equilibrium curves for glycerol pigments onto CB-EN. The equilibrium curves indicated that the highest equilibrium relative adsorption was of 340.7 g−1 at 60 °C. Indeed, the temperature increase from 60 to 80 °C led to a reduction in biosorption capacity throughout the whole biosorbent concentration range (from 0.05 to 2 mg g−1). This result can be attributed to the increase in the pigment solubility, which can lead to a stronger interaction between the glycerol and pigments than the biosorbent and pigments (Dotto et al. 2015; Wisniewska 2012).
The equilibrium curves were also described according to the initial slope. The isotherms showed a small slope, followed by a plot more vertically oriented. According to Giles et al. (1960), this behavior is classified as S type isotherm. This isotherm type suggests that as the adsorbate concentration enhances, the solute molecules may change their orientation, allowing that more adsorptive sites become available for biosorption. Freundlich and BET models were fitted to the isotherm experimental curves to provide more information about the biosorption of glycerol pigments onto CB-EN. The isotherm parameter results are presented in Table 2. The lowest values of average relative error (ARE) and the high values of the coefficient of determination (R2) showed that BET model was the most suitable to represent the biosorption of glycerol pigments onto CB-EN. The BET isotherm indicated that the pigment biosorption occurred in multilayer. Two different types of adsorptive sites led to the formation of the multilayer. The first ones were the chitosan functional groups, where the monolayer biosorption took place. Then, the second one was the adsorbate layer constituted by the glycerol pigments, which acted as new adsorptive sites.
Thermodynamic results
The Van’t Hoff plot and the thermodynamic parameters are shown in Table 3. The negative values of ΔG0 in Table 3 revealed the spontaneity of the reaction, which suggested that biosorption of glycerol pigments was energetically favorable. The positive values of ΔS0 evidenced the randomness in the biosorption process, which can indicate the structural change over the multilayer complex composed by the glycerol pigments adsorbed onto CB-EN. The negative values of ΔH0 showed that the biosorption of glycerol pigments was an exothermic process. Furthermore, the magnitude of ΔH (< 20 kJ mol−1) pointed out the van der Waals interactions between glycerol pigments and CB-EN, which confirmed the physisorption (Crini and Badot 2008; Dotto et al. 2015; Piccin et al. 2017).
Figure 3 presents the isosteric heat of biosorption (ΔHa) for the different biosorbent concentrations. The lowest values of ΔHa corresponded to the lowest biosorbent concentration, and the ΔHa varied as the biosorbent surface was covered. Indeed, this fact can be related to the different energetic adsorptive sites over CB-EN, whereby the glycerol pigments were adsorbed preferentially at the adsorptive sites with the highest energy. However, when the biosorbent concentration was reduced, the pigments were adsorbed by the lowest energetic adsorptive sites (Pohndorf et al. 2016; Silva et al. 2013). Moreover, Fig. 3 also shows a smooth deflection in the isosteric heat of biosorption curve, which can be associated with a change in the interaction of the biosorbent with the glycerol pigments due to the beginning of the multilayer.
Interaction analysis between electrospun chitosan-based nanofibers and glycerol pigments
Figure 4 shows the morphology of the CB-EN after the biosorption process. The SEM images (Fig. 4a, b) evidenced a great morphological change through the nanofiber structure. This modification can be due to the swelling behavior of the nanofibers and, probably, the flow of glycerol into the nanofibers by capillary forces facilitated the interaction between glycerol pigments and chitosan functional groups.
The EDS analysis quantified the atomic percentage of each element found in CB-EN prior and after the biosorption process. Prior biosorption process, the C and O atomic percentage values were 39.68% and 60.32%, respectively. After biosorption process, the C, O, Na, and Cl atomic percentage values were 30.65, 58.07, 5.99, and 5.29%, respectively. The C and O elements refer to the chitosan and poly(ethylene oxide); however, the Na and Cl elements can be attributed to sodium chloride due to the fact that the atomic percentage represents approximately 1:1 ratio. These ions may have remained from the first step of glycerol purification (neutralization) (Ardi et al. 2015).
The FTIR spectra of CB-EN before and after the biosorption of glycerol pigments were also evaluated in order to identify the functional groups that could act as adsorptive sites through the binding of glycerol pigments onto CB-EN. The FTIR spectrum of CB-EN before biosorption process pointed out the typical bands of chitosan. The bands at 3612 and 3312 cm−1 are associated to the O–H and N–H stretching vibration, respectively. The stretching vibration of C=O from the amides and C–O–H deformation was found at 1660 cm−1 and 1413 cm−1, respectively. The FTIR spectrum of CB-EN also identified some characteristics of PEO bands. The band at 2884 cm−1 is assigned to –CH2 stretching. The C–O–C stretching vibration were observed at 1083 and 941 cm−1. Furthermore, the FTIR spectrum of CB-EN after the biosorption process revealed the majority of the chitosan and PEO functional groups. Nevertheless, the biosorption process significantly reduced the –NH2 and –OH groups at 3297 and 3612 cm−1, respectively. Therefore, it is reasonable to suggest that the monolayer biosorption occurred through the interaction of the hydroxyl and amine groups with glycerol pigments.
Conclusion
Electrospun chitosan/poly(ethylene oxide) nanofibers (CB-EN) were successfully developed for pigment biosorption from pretreated industrial glycerol. The biosorption of glycerol pigments onto CB-EN was investigated by equilibrium and thermodynamic studies. The CB-EN showed higher relative adsorption capacity when compared to other chitosan-based biosorbents. Moreover, the BET model revealed the multilayer biosorption. In fact, the first layer of biosorption occurred through the interaction between the chitosan adsorptive sites and glycerol pigments, followed by successive layers composed by the interaction between glycerol pigments. The isosteric heat of biosorption confirmed the heterogeneity of the adsorptive sites. The thermodynamic parameters proposed that the biosorption of glycerol pigments onto CB-EN was spontaneous, random, and exothermic. Therefore, this study elucidated the biosorption mechanisms and the interactions in the glycerol pigment biosorption onto CB-EN, which are fundamental parameters for the industrial scale-up. Nevertheless, future research should be devoted to apply the amounts of CB-EN needed to obtain high-purity glycerol (> 99%) in order to evaluate its physicochemical properties. Moreover, this study showed a sustainable and efficient alternative to transform industrial glycerol into an added-value product.
Abbreviations
- 1/n :
-
Heterogeneity factor (dimensionless)
- A :
-
Glycerol pigment absorbance (dimensionless)
- A o, A e :
-
Initial and equilibrium glycerol pigment absorbances (dimensionless)
- f :
-
Dilution factor (dimensionless)
- K D :
-
Thermodynamic equilibrium constant (L−1 g)
- K F :
-
Freundlich constant (g−1/n)
- K S, K L :
-
BET equilibrium constants (dimensionless)
- L' :
-
Integration constant (dimensionless)
- m ad :
-
Adsorbent weight (g)
- m o, m f :
-
Initial and final glycerol pigment weights (g)
- q e :
-
Equilibrium adsorption capacity (g mg−1)
- q m :
-
Monolayer adsorption capacity (g−1)
- q r :
-
Relative adsorption capacity (g−1)
- q re :
-
Equilibrium relative adsorption capacity (g−1)
- R :
-
Gas constant (kJ mol−1 K−1)
- R 2, ARE :
-
Statistical coefficients (%)
- T :
-
Absolute temperature (K)
- V :
-
Solution volume (mL)
- ΔG 0 :
-
Gibbs free energy change (kJ mol−1)
- ΔH 0 :
-
Enthalpy change (kJ mol−1)
- ΔH a :
-
Isosteric heat of biosorption (kJ mol−1)
- ΔS 0 :
-
Entropy change (J mol−1 K−1)
- ρ :
-
Glycerol density (g L−1)
- εc :
-
Constant related to analyte nature and cuvette size (m3 g−1)
References
Ardi MS, Aroua MK, Hashim NA (2015) Progress, prospect and challenges in glycerol purification process: a review. Renew Sust Energ Rev 42:1164–1173. https://doi.org/10.1016/j.rser.2014.10.091
Bayrak Y (2003) Adsorption isotherms in bleaching hazelnut oil. J Am Oil Chem Soc 80:1143–1146. https://doi.org/10.1007/s11746-003-0833-7
Crini G, Badot PM (2008) Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption process using batch studies: a review of recent literature. Prog Polym Sci 33(4):399–447. https://doi.org/10.1016/j.progpolymsci.2007.11.001
Dhabhai R, Ahmadifeijani E, Dalai AK, Reaney M (2016) Purification of crude glycerol using a sequential physico–chemical treatment, membrane filtration, and activated charcoal adsorption. Sep Purif Technol 168:101–106. https://doi.org/10.1016/j.seppur.2016.05.030
Dotto GL, Souza VC, Moura JM, Moura CM, Pinto LAA (2011) Influence of drying techniques on the characteristics of chitosan and the quality of biopolymer films. Dry Technol 29(15):1784–1791. https://doi.org/10.1080/07373937.2011.602812
Dotto GL, Moura JM, Cadaval TRS Jr, Pinto LAA (2013) Application of chitosan films for the removal of food dyes from aqueous solutions by adsorption. Chem Eng J 214:8–16. https://doi.org/10.1016/j.cej.2012.10.027
Dotto GL, Sharma SK, Pinto LAA (2015) Biosorption of organic dyes: research opportunities and challenges. Scrivener Publishing, Massachusetts
Dotto GL, Campana-Filho SP, Pinto LAA (2017a) Chitosan based materials and its applications. Bentham Science Publishers, Sharjah
Dotto GL, Santos JMN, Tanabe EH, Bertuol DA, Foletto EL, Lima EC, Pavan FA (2017b) Chitosan/polyamide nanofibers prepared by Forcespinning® technology: a new adsorbent to remove anionic dyes from aqueous solutions. J Clean Prod 144:120–129. https://doi.org/10.1016/j.jclepro.2017.01.004
Ebadi A, Mohammadzadeh JSS, Khudiev A (2009) What is the correct form of BET isotherm for modeling liquid phase adsorption? Adsorption (Boston) 15(1):65–73. https://doi.org/10.1007/s10450-009-9151-3
Farias BS, Vidal ÉM, Ribeiro NT, Silveira N Jr, Vaz BS, Kuntzler SG, Morais MG, Cadaval TRS Jr, Pinto LAA (2018) Electrospun chitosan/poly(ethylene oxide) nanofibers applied for the removal of glycerol impurities from biodiesel production by biosorption. J Mol Liq 268:365–370. https://doi.org/10.1016/j.molliq.2018.07.081
Frantz TS, Silveira N Jr, Quadro MS, Andreazza R, Barcelos AA, Cadaval TRS Jr, Pinto LAA (2017) Cu(II) adsorption from copper mine water by chitosan films and the matrix effects. Environ Sci Pollut Res 24:5908–5917. https://doi.org/10.1007/s11356-016-8344-z
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–471
Giles CH, MacEwan TH, Nakhwa SN, Smith D (1960) Studies in adsorption. Part XI.* A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurements of specific surface areas of solids. J Chem Soc:3973–3993. https://doi.org/10.1039/JR9600003973
Goldstein JI, Newbury DE, Echlin P, Joy DC, Romig AD Jr, Lyman CE, Fiori C, Lifshin E (2003) Scanning electron microscopy and X–ray microanalysis. Springer US, New York
Gore PM, Khurana L, Siddique S, Panicker A, Kandasubramanian B (2018) Ion–imprinted electrospun nanofibers of chitosan/1–butyl–3–methylimidazolium tetrafluoroborate for the dynamic expulsion of thorium (IV) ions from mimicked effluents. Environ Sci Pollut Res 25(4):3320–3334. https://doi.org/10.1007/s11356-017-0618-6
Hoekman SK, Broch A, Robbins C, Ceniceros E, Natarajan M (2012) Review of biodiesel composition, properties, and specifications. Renew Sust Energ Rev 16(1):143–169. https://doi.org/10.1016/j.rser.2011.07.143
Hossini H, Soltani RD, Safari M, Maleki A, Rezaee R, Ghanbari R (2017) The application of a natural chitosan/bone char composite in adsorbing textile dyes from water. Chem Eng Commun 204(9):1082–1093. https://doi.org/10.1080/00986445.2017.1340274
Hu S, Luo X, Wan C, Li Y (2012) Characterization of crude glycerol from biodiesel plants. J Agric Food Chem 60:5915–5921. https://doi.org/10.1021/jf3008629
Kwork KCM, Koong LF, Ansari TA, McKay G (2018) Adsorption/desorption or arsenite and arsenate on chitosan and nanochitosan. Environ Sci Pollut Res 25(15):14734–14742. https://doi.org/10.1007/s11356-018-1501-9
Kyzas GZ, Fu J, Matis KA (2013) The change from past to future for adsorbent materials in treatment of dyeing wastewaters. Materials (Basel) 6(11):5131–5158. https://doi.org/10.3390/ma6115131
León O, Muñoz-Bonilla A, Soto D, Perez D, Rangel M, Colina M, Fernandez-García M (2018) Removal of anionic and cationic dyes with bioadsorbent oxidized chitosans. Carbohydr Polym 194:375–383. https://doi.org/10.1016/j.carbpol.2018.04.072
Li L, Zhang J, Yanxiang L, Yang C (2017) Removal of CR (VI) with spiral wound chitosan nanofiber membrane module via dead–end filtration. J Membr Sci Technol 544:333–341. https://doi.org/10.1016/j.memsci.2017.09.045
Liu Y (2009) Is the free energy change of adsorption correctly calculated? J Chem Eng Data 54(7):1981–1985. https://doi.org/10.1021/je800661q
Milonjić SK (2007) A consideration of the correct calculation of thermodynamic parameters of adsorption. J Serb Chem Soc 72(12):1363–1367. https://doi.org/10.2298/JSC0712363M
Moura JM, Farias BS, Rodrigues DAS, Moura CM, Dotto GL, Pinto LAA (2015) Preparation of chitosan with different characteristics and its application for biofilms production. J Polym Environ 23(4):470–477. https://doi.org/10.1007/s10924-015-0730-y
Moura JM, Gründmann DDR, Cadaval TRS Jr, Dotto GL, Pinto LAA (2016) Comparison of chitosan with different physical forms to remove reactive black 5 from aqueous solutions. J Environ Chem Eng 4(2):2259–2267. https://doi.org/10.1016/j.jece.2016.04.003
Piccin JS, Cadaval TRS Jr, Pinto LAA, Dotto GL (2017) Adsorption isotherms in liquid phase: experimental, modeling, and interpretations. Springer International Publishing, Switzerland
Pohndorf RS, Cadaval TRS Jr, Pinto LAA (2016) Kinetics and thermodynamics adsorption of carotenoids and chlorophylls in rice bran oil bleaching. J Food Eng 185:9–16. https://doi.org/10.1016/j.jfoodeng.2016.03.028
Qin W, Li J, Tu J, Yang H, Chen Q, Liu H (2017) Fabrication of porous chitosan membranes composed of nanofibers by low temperature thermally induced phase separation, and their adsorption behavior for Cu2+. Carbohydr Polym 178:338–346. https://doi.org/10.1016/j.carbpol.2017.09.051
Silva SM, Sampaio KA, Ceriani R, Verhé R, Stevens C, De Greyt W, Meirelles AJA (2013) Adsorption of carotenes and phosphorus from palm oil onto acid activated bleaching earth: equilibrium, kinetics and thermodynamics. J Food Eng 118(4):341–349. https://doi.org/10.1016/j.jfoodeng.2013.04.026
Silverstein RM, Webster FX, Kiemle DJ (2005) Spectrometric identification of organic compounds. Wiley, New Jersey
Swinehart DF (1962) The Beer–Lambert law. J Chem Educ 39(7):333. https://doi.org/10.1021/ed039p333
Tan HW, Aziz ARA, Aroua MK (2013) Glycerol production and its application as a raw material: a review. Renew Sust Energ Rev 27:118–127. https://doi.org/10.1016/j.rser.2013.06.035
Wan Z, Li M, Zhang Q, Fan Z, Verpoort F (2018) Concurrent reduction–adsorption of chromium using m–phenylediamine–modified magnetic chitosan: kinetics, isotherm, and mechanism. Environ Sci Pollut Res 25(18):17830–17841. https://doi.org/10.1007/s11356-018-1941-2
Weska RF, Moura JM, Batista LM, Rizzi J, Pinto LAA (2007) Optimization of deacetylation in the production of chitosan from shrimp wastes: use of response surface methodology. J Food Eng 80:749–753. https://doi.org/10.1016/j.jfoodeng.2006.02.006
Wisniewska M (2012) The temperature effect on the adsorption mechanism of polyacrylamide on the silica surface and its stability. Appl Surf Sci 258(7):3094–3101. https://doi.org/10.1016/j.apsusc.2011.11.044
Yang F, Hanna MA, Sun R (2012) Value–added uses for crude glycerol—a byproduct of biodiesel production. Biotechnol Biofuels 5(1):13. https://doi.org/10.1186/1754-6834-5-13
Zhang C, Chen Z, Guo W, Zhu C, Zou Y (2018) Simple fabrication of chitosan/graphene nanoplates composite spheres for efficient adsorption of acid dyes from aqueous solution. Int J Biol Macromol 112:1048–1054. https://doi.org/10.1016/j.ijbiomac.2018.02.074
Živković S, Veljković M (2018) Environmental impacts the of production and use of biodiesel. Environ Sci Pollut Res 25(1):191–199. https://doi.org/10.1007/s11356-017-0649-z
Funding
The authors would like to thank CAPES (Brazilian Agency for Improvement of Graduate Personnel) and CNPQ (National Council of Science and Technological Development) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Farias, B.S., Gründmann, D.D.R., Strieder, M.M. et al. Biosorption of glycerol impurities from biodiesel production onto electrospun chitosan-based nanofibers: equilibrium and thermodynamic evaluations. Environ Sci Pollut Res 26, 28436–28443 (2019). https://doi.org/10.1007/s11356-019-04525-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04525-3