Abstract
Metabolic processes of the submerged aquatic community (photosynthesis and respiration) play important roles in regulating diel cycles of dissolved inorganic carbon (DIC) and sequestering carbon in a karst stream. However, little is known of whether diel DIC cycling occurs during rainfall in a karst groundwater-fed stream, even though this question is critical for the accurate estimation of what may be a major terrestrial carbon sink. Here, we measured diel variations of water chemical composition in a small karst groundwater-fed stream in southwest China during a rainfall event to assess the influences of rainfall and rising discharge on DIC diel cycling and the potential carbon sink produced by in-stream metabolism. Our results show that water chemical composition at the source spring (CK site) is relatively stable due to chemostatic behavior during rising discharge after a rainfall period. This site lacked submerged aquatic vegetation and, thus, had no diel variations in water chemistry. However, diel cycles of all hydrochemical parameters occurred at a site 1.3 km downstream (LY site). Diel variations in pH, DO, and δ13CDIC were inversely related to diel changes in SpC, DIC, Ca2+, and pCO2. These results indicated that diel cycling of DIC due to in-stream metabolism of submerged aquatic community was still occurring during elevated discharge from rainfall. We estimate the carbon sink through the in-stream metabolism of the submerged aquatic community to be 5.6 kg C/day during the studied rainfall event. These results imply that submerged aquatic communities in a karst stream can significantly stabilize carbon originating from the carbonate rock weathering processes in karst areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The World Karst Aquifer Map (WOKAM) project has found that karstified rocks and aquifer systems cover approximately 14.7% of the Earth’s ice-free land (Chen et al. 2017). In karst areas, many surface streams are fed by karst groundwaters, which discharge from aquifer at springs. Karst groundwater is often rich in dissolved inorganic carbon (DIC) because of carbonate dissolution by carbonic acid generated during the hydration of CO2. The elevated DIC concentrations may lead karst groundwater-fed streams to play an important role in regulating regional and global carbon cycles through carbon transfer, CO2 degassing, carbon burial, and carbon assimilation (Atekwana and Krishnamurthy 1998; Covington 2016; Gombert 2002; Jiang and Yuan 1999; Kurz et al. 2013; Liu et al. 2010, 2015; Liu and Zhao 2000; Martin 2017; Martin et al. 2013; Pu et al. 2017; Yuan 1997). In particular, these streams may represent an important carbon sink, as the clear water allows light penetration to stream benthic environments and DIC is assimilated by submerged aquatic phototrophs (de Montety et al. 2011; Jiang and Yuan 1999; Liu and Dreybrodt 2015; Liu et al. 2010; Pu et al. 2017; Zhang et al. 2017a). The assimilated DIC is then buried and sequestered as organic carbon (OC). The net atmospheric CO2 uptake by interactions between water, carbonate minerals, dissolved CO2 (carbonic acid), and aquatic phototrophs on land has been estimated to be as large as 0.477 Pg C/a (Liu and Dreybrodt 2015), and thus a large fraction of the net terrestrial residual sink (i.e., 0.8~1.2 Pg C/year) (Ciais et al. 2013). Therefore, revealing DIC dynamics in karst streams is critical to understanding carbon cycling and its role in regional and global carbon budgets.
Karst aquifers are very sensitive to external environmental changes at a range of timescales, including diel, event, seasonal, annual, and multi-annual (Liu et al. 2015, 2007; Martin et al. 2016; Nimick et al. 2011; Shuster and White 1971; Vesper and White 2004; Zhang et al. 2012). Thus, DIC in karst groundwater-fed streams can display high temporal and spatial variability in coherence with aquifer hydrological variations (Zeng et al. 2016). Diel variations in DIC concentrations is a common phenomenon in a karst stream that results from alternating carbonate dissolution and aquatic metabolism (de Montety et al. 2011; Demars et al. 2015; Khadka et al. 2014; Lynch et al. 2010; Spiro and Pentecost 1991; Tobias and Boehlke 2011). These diel variations occur in all seasons, and they can be as large as seasonal variations (Nimick et al. 2011). Variations in stream flow may also play an important role in regulating karst stream DIC transport and transformation at diel, seasonal, and annual timescales (Atkins et al. 2017; Peter et al. 2014; Zeng et al. 2016). Dry seasons often slow water motion in channels and increase the time available for DIC transformations through mineral precipitation and plant assimilation (Mann et al. 2014; Shin et al. 2011). By contrast, rainfall events during wet season can export significant quantities of DIC from karst aquifer to surface streams, thereby altering the DIC cycling through changes to CO2 gas exchange, turbidity, water temperature, and flow velocity (Looman et al. 2016; Peter et al. 2014). The control of changing flow conditions was poorly constrained, however, because most earlier work occurred during lower-water-level or stable flow periods (de Montety et al. 2011; Jiang et al. 2013; Liu et al. 2015; Parker et al. 2014; Pu et al. 2017; Yang et al. 2015). Only a few studies have reported on DIC or the CO2 diel cycle under the impact of rainfall events in surface streams (Dinsmore et al. 2013; Johnson et al. 2009; Looman et al. 2016; Peter et al. 2014), but they mostly focused on peatland or silicate rock streams. Little is known of how flow variations may alter diel DIC cycling in a karst stream, even though this question is critical for the accurate estimation of what may be a major terrestrial carbon sink.
This study assesses DIC concentration variations during changing flow conditions and how the variations may alter the carbon cycle in a karst system. This assessment is based on data collected from a subtropical karst groundwater-fed stream (Guancun stream (GS)) located in southwest China. The study is based on a 3-day record of high-resolution measurements of diel variations within a stream of water for DIC concentrations, δ13CDIC values, and physicochemical parameters. These results will help to improve evaluations of the amount of carbon that could be sequestered in karst streams and improve the understanding of carbon transportation and transfer with varying hydrological processes in inland water bodies.
Study area
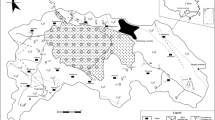
The Guancun surface stream (GSS) is a tributary of the Shimen River and a part of the Rongjiang River drainage system, which is located in Daliang town in Rong’an county of Guangxi Zhuang Autonomous Region, China (Fig. 1). GSS is a typical subtropical headwater stream and almost entirely fed by karst groundwater from an upper Devonian (D3r) limestone aquifer (Pu et al. 2017). The outlet of the Guancun underground stream (GUS) is the head of the GSS with no surface tributaries flowing into it (Fig. 1). The GSS channel is underlain by Lower Carboniferous (C1y) limestone interbedded with dolomite of the Yingtang Formation. The length and average width of the GSS are 1.32 km and 3.5 m, respectively. At base level, the water depth is shallow (0.2~1.2 m). The study area is characterized by a cold–dry winter from late November through March and a hot–rainy summer from April through October and has an annual average temperature of 19.7 °C. The area is dominated by the East Asian Monsoon with an annual average precipitation of 1726 mm, 72% of which occurs in the wet season from late April to early September. As a typical monsoon region, air temperature and precipitation in the GSS catchment co-vary, both of which being high in the wet season and low in the dry season.
Maps of Guancun surface stream and sampling sites. a Photograph is the location of Guancun stream in SW China. b Photographs are the scenes in Guancun stream from groundwater outlet to stream mouth. c Map shows the surface stream flow route and sampling sites in study area (modified from Google Earth 2015) (Pu et al. 2017)
This study focuses on monitoring sites for detailed diel monitoring and sampling (Fig. 1). The upstream CK (24° 52′ 10″, 109° 20′ 07″) site is located at a GUS outlet, which receives typical karst groundwater. The downstream LY (24° 51′ 32″, 109° 20′ 01″) site is approximately 1.30 km downstream from CK near the stream mouth and is the site of a gauging station.
Methods
Data for this study was acquired August 17–19, 2013. Two multi-parameter meters (WTW 3430, WTW GmbH, Weilheim, Germany) were set at both the CK and LY sites. Hydrochemical variables including water temperature, pH, dissolved oxygen (DO), and specific conductivity (SpC) were continuously measured in situ at 15-min time intervals at both sites. Resolutions for water temperature, pH, dissolved oxygen (DO), and specific conductivity (SpC) are 0.1 °C, 0.004 pH units, 0.01 mg/L, and 1 μS/cm, respectively. Specific conductivity (SpC) refers to the electrical conductivity corrected from ambient temperature to 25 °C by a nonlinear correction function. Turbidity was measured in situ at 15-min time intervals at the LY site using a YSI 6600 datasonde (Yellow Springs, OH, USA). All monitoring probes for the instruments were calibrated according to manufacturer’s specifications prior to deployment. Weir water stages were also continuously monitored at 15-min time intervals at the LY site using the same YSI 6600 datasonde. These water stage measurements were converted to discharge units using the rectangular-weir-discharge formula (Pu et al. 2017).
From August 17 to 19, 2013, water samples were collected every 2 h at both sites from the mid-channel of the stream using the ISCO 6712 autosampler (Teledyne ISCO, Inc., USA). Water was pumped from 0.1 m below the water surface. Every water sample was temporarily stored in 2-L pre-rinsed high-density polyethylene (HDPE) bottles in the autosampler base after samplings. To keep water samples chilled, the autosampler base was filled with ice for entire sampling time. Unfiltered water samplers were titrated for alkalinity immediately in the field with an accuracy of 0.05 mmol/L using a portable testing kit by Merck KGaA Co. (Germany). Because HCO3− constitutes ~ 90% of DIC over the pH range of 7.3–8.5 (Rice et al. 2012), we used alkalinity measurements as an approximation of DIC concentration for this study. Water from the autosampler bottles was immediately filtered (0.45-μm cellulose acetate membrane) into other small clean bottles for later analysis of major cation (Ca2+, Mg2+, K+, and Na+), major anions (Cl−, SO42−), and δ13CDIC. Samples used to detect cations were acidified with trace metal grade nitric acid (7 M HNO3) to a pH of < 2.0. Samples for δ13CDIC were collected in 25-mL acid-washed dry HDPE bottles, and three drops of HgCl2 were added in order to prevent microbial activity. A portable cooler was used to store all samples in the field. Samples were delivered to a laboratory where they were stored and chilled in a refrigerator at 4 °C until analysis.
Meteorological parameters, including rainfall, air temperature, wind speed, barometric pressure, and solar radiation, were measured using an on-site Vantage Pro 2 weather station (Davis Instruments Corp., USA) from August 16 to 20, 2013. Resolutions of rainfall, air temperature, wind speed, barometric pressure, and solar radiation were 0.2 mm, 0.1 °C, 5%, 0.1 hPa, and 1 W/m2, respectively.
Major anions and cations were measured by an automated Dionex ICS-900 ion chromatograph and an ICP-OES (IRIS Intrepid II XSP, Thermo Fisher Scientific, USA), respectively, using procedures based on APHA 2012 methods (Rice et al. 2012). The calculated errors of charge balance were within ± 5%. The δ13CDIC values of water samples were analyzed using a MAT-253 mass spectrometer coupled with a Gas Bench II automated device. The results are expressed as δ13CDIC (‰) with respect to the Vienna Pee Dee Belemnite (V-PDB) standard with an analytical precision of ± 0.15‰. All lab analyses were carried at the Environmental and Geochemical Analysis Laboratory of the Institute of Karst Geology, Chinese Academy of Geological Science (Pu et al. 2017; Zhang et al. 2017a).
The partial pressure of CO2 (pCO2) and saturation index of calcite (SIc) in the stream waters were calculated through the program WATSPEC (Wigley 1977) according to the hydrochemical data sets, including pH, water temperature, and concentrations of K+, Na+, Ca2+, Mg2+, Cl−, SO42−, and HCO3−.
CO2 fluxes across water–air interface were calculated using a molecular diffusion model (Raymond et al. 2012):
where F is the CO2 evasion flux (mg/m2/h) between water and atmosphere, k is the gas transfer velocity (cm/h), and [CO2]water − [CO2]air is the CO2 concentration gradient between the water and air. Previous studies measured atmospheric CO2 concentrations 1.5 m above the water surface that is to be 445 ppmv (Mo 2015). k is a key parameter for accurately calculating CO2 evasion flux. We calculated k using the temperature-dependent Schmidt number (ScT) for freshwater:
with
where k600 is the k for CO2 at 20 °C in freshwater, that is, k at a Schmidt number of 600 (Raymond et al. 2012), and T is the in situ water temperature (°C). k600 was derived using an equation described by Raymond et al. (2012):
where v is the velocity (m/s), S is the channel slope (m/km), Q is the stream discharge (m3/s), and D is the water depth (m).
Results
Hydrological and meteorological variations
There were some sporadic rain events with a total rainfall of 0.8 mm on the afternoon of August 17, 2013, which did not raise the GSS water level. During the study period, a concentrated rainfall of ~ 22.8 mm fell from 0700 to 1345 h on August 18, 2013 at an average intensity of 3.38 mm/h and increased the discharge from the base flow value of 20.34 to 83.91 L/s (Figs. 2 and 3). Discharge in GSS is usually controlled by groundwater discharge at GUS outlet (Pu et al. 2017) and had a mean discharge of 46.13 L/s and median of 55.28 L/s during this study (Figs. 2 and 3). Discharge rose quickly in response to the rain event approximately 2.0 h after the rain started. The discharge of GSS was elevated following the peak discharge for the remainder of the study. Turbidity increased after rainfall coincident with the increased discharge, showing two peaks, but a 2-h lagging discharge (Figs. 2 and 3).
Hydrochemical variations
At the CK site, the coefficient of variation (CV) for all measured parameters was very small during the study period. The CV of pH, water temperature, and DO are 0.23%, 0.28%, and 0.16%, respectively, indicating a minor variation during the study period. However, SpC exhibited relatively larger variations, although still small, with a range of 435.0 to 470 μs/cm with a CV of 2.4% (Fig. 2, Table S1). DIC and Ca2+ varied from 278.8 to 287.2 mg/L and 81.0 to 86.1 mg/L with CV values of 0.87% and of 1.7%, respectively (Table S1). Minor variations also occurred in pCO2 with a CV of 3.9%. Water at CK was supersaturated with respect to calcite, with SIc values ranging from 0.08 to 0.14, with an average value of 0.10. The observed small CV for all the measured parameters at CK suggests no distinct diel variations during the study period (Fig. 2). Although the discharge of GSS quickly rose in response to rainfall, hydrochemical parameters at CK (GUS outlet) show a relative steady status with little storm effect, piston effect, or dilution as can commonly occur in some karst springs (Hess and White 1988; Liu et al. 2004, 2007; Pu et al. 2014; Vesper and White 2003a, b). The small variations appear to reflect chemostatic behavior in the GUS during the rain event (Clow and Mast 2010; Godsey et al. 2009; Karis et al. 2016).
At the LY site, water temperature, SpC, pH, DO, Ca2+, DIC, pCO2, and SIc values showed pronounced temporal variations during the study period (Fig. 3). The mean stream water temperature was 23.1 °C, with a range from 22.3 °C just prior to dawn to 24.0 °C in the afternoon, consistent with, but slightly lagging with, solar radiation and air temperature variations (Fig. 3). Changes in pH were temporally coincident with changes in DO, with peaks occurring in the afternoon (14:00–16:00) (Fig. 3). pH ranged from a high of 8.11 during the day to a low of 7.99 at night with a mean of 8.05. DO varied from 7.51 mg/L during the day to 6.56 mg/L at night with a mean of 7.18 mg/L. Notably, the pH and DO maxima roughly coincided with the peak of discharge but had an approximate 2-h lag. The temporal pattern of SpC showed an inverse correlation with pH and DO with minima occurring in the afternoon and maxima at night.
There was a significant decrease in the concentrations of DIC and Ca2+ between site CK and LY (Table S1, t test, p < 0.001). DIC and Ca2+ concentrations have diel patterns that are 180° out of phase with those of pH and DO at LY (Fig. 3). DIC and Ca2+ concentrations increased to maximum values of 287.9 mg/L and 84.3 mg/L, respectively, at 0800 h on August 18. The peaks were followed by fast decline to afternoon lows of 221.6 mg/L and 69.9 mg/L, respectively at 1400 h (Fig. 3). The peak of discharge was consistent with the troughs of DIC and the Ca2+ time series. The water SIc values at LY were more than 0.7, reflecting greater supersaturation than at CK. The changing diel pattern of SIc at LY was similar with the curve of Ca2+ and DIC concentrations, with low values in the afternoon and high values occurring at night. The derived pCO2 also showed a pronounced temporal change and was in phase with Ca2+, DIC, and SIc (Fig. 3). The pCO2 values at LY were significantly lower than at CK (Table S1, t test, p < 0.01). These results indicated that the diel cycles of hydrochemical parameters at LY still occurred during rain and increasing discharge events.
Variation in carbon isotope and CO2 evasion flux
The range of measured δ13CDIC values in CK was − 14.03 to − 13.24‰, with a mean of − 13.74‰; although they a large range, the values show no regular diel pattern. The δ13CDIC values at LY were significantly higher than at CK (Table S1, p < 0.003) and vary from − 12.93 to − 11.80‰. In contrast to CK, LY showed diel variations of δ13CDIC values with lower values at night and higher values in the afternoon. The δ13CDIC maxima at LY corresponded to DIC, Ca2+, SpC, SIc, and pCO2 minima and pH, DO, and water temperature maxima (Fig. 3). The δ13CDIC peaks also coincide roughly with the discharge and turbidity peaks but lagged about 2 h after discharge.
The difference of CO2 evasion flux between CK and LY is also significant (Table S1, t test, p = 0.000). CO2 evasion at CK varied from 1138.8 to 1505.3 mg/m2/h with a mean value of 1302.8 mg/m2/h, which was about eight times higher than at LY, which ranged from 103.5 to 201.9 mg/m2/h with a mean value of 153.1 mg/m2/h (Table S1, Fig. 3).
CO2 evasion did not show diel variation at the CK site over the study period, but it gradually decreased after the discharge and turbidity peaks (Y = − 259.58X + 107, R2 = 0.70, p < 0.01). The curve of CO2 evasion at LY was smoother, and a diel pattern occurred with maxima at night that were about 1.3 times higher than during the daytime minima (Fig. 3). After the peaks of discharge and turbidity, CO2 evasion decreased linearly at LY (Y = − 28.562X + 106, R2 = 0.19, p < 0.01). CO2 evasion at LY was positively correlated with DIC, Ca2+, and pCO2 and inversely correlated with pH, DO, water temperature, and δ13CDIC (Fig. 3). These results also suggest that the diel cycles of carbon isotope and CO2 evasion flux at LY still occur in the periods of rain and rising discharge.
Discussions
As diel frequency is an important timescale in this study, we focus on the data from 0600 h on August 18 to 0600 h on August 19 to evaluate factors controlling DIC and to estimate the carbon budget relative to increased discharge related to a rain event.
Controls on carbon cycling
Rainwater can directly fall into the GSS channel and contribute to increased streamflow. The mean width of the water surface of the GSS was about 4.5 m during the study period. Although such a measurement has a large degree of uncertainty due to the limited information on channel width between two sites, it offered first-hand data for discussing the influence of rainwater. Regardless of the uncertainty in width, the measured regional rainfall into the GSS open channel contributed only about 1.9% of total discharge during the study period. Stream evapotranspiration will decrease water discharge (de Montety et al. 2011; Nimick et al. 2011). However, GUS discharge continuously increased after rain events during the study period, indicating that evapotranspiration was limited for influencing discharge variation. Therefore, in the following calculations and discussions, we neglect the influence of precipitation directly recharging into the GSS open channel and evapotranspiration on the chemical compositions of stream water (Nimick et al. 2011).
Because CK is located at the outlet of the GUS, its water source is groundwater and its hydrochemistry is controlled by the GUS karst system. The composition of this water was chemostatic throughout the study, yielding low CV values (Clow and Mast 2010; Karis et al. 2016). The lack of compositional variation resulted in weak correlations of DIC vs. δ13CDIC, DIC vs. pCO2, DIC vs. DO, DIC vs. CO2 evasion flux, and DIC vs. discharge at CK at diel timescale (24 h) (Fig. 4a–i). Karst groundwater typically shows that the DIC and δ13CDIC originate from soil CO2 produced by the degradation of organic matter and plant root respiration and the dissolution of carbonate minerals (Marx et al. 2017; Nimick et al. 2011; Tobias and Böhlke 2010). The chemostatic characteristics of DIC and δ13CDIC suggest that inorganic carbon at CK also originates from multiple invariant processes (carbonate rock dissolution and soil CO2 hydration) in the subsurface as suggested during a seasonal (dry versus wet) study at this location (Zhang et al. 2017b).
Cross-plots between a δ13CDIC and DIC, bpCO2 and DIC, c DO and DIC, d CO2 evasion flux and DIC, e DO and δ13CDIC, f DO and pCO2, g DO and CO2 evasion flux, h δ13CDIC and CO2 evasion flux, i discharge and DIC, and j DO and discharge at extractive 24-h timescale from 0600 h on August 18 to 0600 h on August 19
In contrast to CK, LY showed significant correlations of DIC with δ13CDIC, pCO2, DO, CO2 evasion flux, and discharge over the study period (Fig. 4a–i). These correlations suggest that other processes, in addition to soil-CO2 production and carbonate rock dissolution, could affect water chemistry in the GSS. Commonly, varying temperature could decrease pH by increasing CO2 evasion as water warms during the day (de Montety et al. 2011; Nimick et al. 2011; Spiro and Pentecost 1991). In Fig. 3, pCO2 and CO2 evasion were lower and DO concentration was higher during the day than at night at the LY site. Water temperature at LY showed weak positive correlations with DO concentration (R2 = 0.15, p < 0.01; Fig. S1) and a slightly stronger negative correlation with CO2 evasion (R2 = 0.34, p < 0.01; Fig. S1). Moreover, DO concentrations showed a significant and strong negative correlation with both pCO2 (R2 = 0.77, p < 0.01; Fig. 4f) and CO2 evasion (R2 = 0.86, p < 0.01; Fig. 4g). Consequently, temperature does not appear to be the primary controlling factor on pCO2 in the GSS. CO2 evasion at LY should decrease DIC concentration, but a significant positive correlation occurred between water CO2 evasion and DIC concentration (R2 = 0.73, p < 0.01, Fig. 4d). Previous studies have demonstrated that CO2 outgassing can increase δ13CDIC values in the residual DIC in water (Deirmendjian and Abril 2018; Doctor et al. 2008; Spiro and Pentecost 1991). However, LY shows a significant negative correlation between CO2 evasion and δ13CDIC values (R2 = 0.57, p < 0.01, Fig. 4h), suggesting that CO2 outgassing is not the primary control of δ13CDIC values.
The diel cycle of DIC and DO concentrations is affected by in-stream metabolism in the GSS, including photosynthesis and respiration during sunny days with relatively stable and higher water level (Pu et al. 2017). These processes control DIC because photosynthesis releases O2 into water and consumes CO2 during the day, while respiration consumes O2 and releases CO2 into water during the night, making DO concentrations and pCO2 ideal for evaluating aquatic metabolic process (de Montety et al. 2011; Demars et al. 2015; Kurz et al. 2013). The inverse variation of DIC with DO at LY (Fig. 4c) thus reflects in-stream metabolism even during overcast periods, changing discharge, decreased temperature, and increased turbidity during precipitation. Even with these limiting environmental condition, the rate of photosynthesis in the stream exceeded respiration during the day, causing a net release of O2 and consumption of CO2, thereby increasing pH (Fig. 3). In contrast, respiration consumed O2 and released CO2 during the night thereby decreased the pH (Fig. 3). Therefore, metabolic processes are more important controls than temperature, turbidity, and discharge effects for DIC, pCO2, and DO diel cycle in the GSS.
The δ13CDIC values in stream water are controlled by CO2 sources (Marx et al. 2017). At LY, δ13CDIC has a positive correlation with DO concentrations (R2 = 0.35, p < 0.01; Fig. 4e) and a significantly negative correlation with pCO2 (R2 = 0.74, p < 0.01; Fig. S1), reflecting in-stream metabolic controls on δ13CDIC values. This control results from a 13C enrichment of residual pools of DIC during the day as DIC is consumed by photosynthesis and release of isotopically light biogenic CO2 during nighttime due to respiration of organic matter (Cavalli et al. 2012; Hasler et al. 2016; Pedersen et al. 2013; Zhao and Su 2014).
CO2 evasion fluxes at both CK and LY decreased with the rising discharge (Figs. 2 and 3), although LY was characterized by a diel pattern. According to Eq. 4, increasing discharge will decrease the k600 value, causing a decrease in CO2 evasion (Eq. 1). This decrease differs from CO2 evasion from the Santa Fe River, FL, USA (Khadka et al. 2014), and the Madeira River, the largest tributary of the Amazon River, Brazil (Almeida et al. 2017), where flooding was found to enhance CO2 evasion from water. However, CO2 evasion increased at LY after maximum discharge. Daytime CO2 evasion (mean = 129 mg/m2/h) is about 15.1% lower than nighttime CO2 evasion (mean = 152 mg/m2/h) because of differences in the production and consumption of CO2 during photosynthesis and respiration. Therefore, in-stream metabolism controls CO2 evasion at LY. Our results show that diel variations in CO2 evasion to atmosphere from streams and rivers should be taken account for a more accurate estimation of carbon budgets (Pu et al. 2017; Zhang et al. 2017a).
Although variations in stream discharge showed no relationship with DIC and DO concentrations (Fig. 4i, j) at CK, discharge had a negative relationship with DIC (R2 = 0.23, p < 0.01, Fig. 4i) and a significantly positive relationship with DO (R2 = 0.57, p < 0.01, Fig. 4j) at LY. We used a partial least-squares regression (PLS) model to evaluate to what extent discharge influences DIC diel variation at LY (Li et al. 2014; Paranaíba et al. 2018; Peter et al. 2014). PLS analyses included discharge, pH, SpC, DO, water temperature, DIC, Ca2+, δ13CDIC, pCO2, and CO2 evasion flux at LY only, using SIMCA 14.1 software (32-bit, Umetrics, Sweden). Due to a lack of any linear relationship between DIC, DO, and discharge, this PLS model did not analyze the data at CK. The PLS model performance is expressed in the terms R2Y and Q2. R2Y is comparable to R2 in linear regression and expresses how much of the variance in Y is explained by the X variables. Q2 is a measure of the predictive power of the PLS model. The model is more robust as Q2 approaches R2Y (Paranaíba et al. 2018; Peter et al. 2014; Sobek et al. 2003). Variable importance in projection (VIP) describes how much a variable contributes to explaining the Y variable (DIC). Highly important variables have VIP > 1.0, moderately important variables have 0.8 < VIP < 1.0, and unimportant variables have VIP < 0.8. In this study, the PLS model explained the variability in DIC well, with a R2Y of 0.99 and a Q2 of 0.98 (Table S2). The PLS models show that SIc, Ca2+, pCO2, δ13CDIC, CO2 evasion flux, and DO were important variables (VIP > 0.8; Table S2) to explain DIC concentrations. However, discharge was less important (VIP < 0.8; Table S2), reflecting limited control by discharge for the DIC diel cycle at LY. Consequently, in-stream metabolism remained an important driver of the DIC diel cycle, even during rising discharge following precipitation and the overcast nature of the study period.
Carbon sink produced by in-stream metabolism
Lower DIC concentrations at LY compared to CK (Figs. 2 and 3, Table S1) reflect carbon losses. The loss represents the carbon sink produced by in-stream metabolism, which could be buried in benthic sediments similar to the oceanic biological pump (McElroy 1983) and would represent a net loss of atmospheric CO2 to the sediments (Jiang et al. 2013; Liu et al. 2010, 2015; Liu and Dreybrodt 2015; Pu et al. 2017; Yang et al. 2015). A usual mass balance method was used to estimate the mass of carbon sink due to in-stream metabolism (de Montety et al. 2011; Liu et al. 2015; Liu and Dreybrodt 2015; Pu et al. 2017; Yang et al. 2015). The popular balance equation can be found below (Liu et al. 2015; Yang et al. 2015; Pu et al. 2017):
where FOC is the net mass of organic carbon formed in a day (mg/day), Q is the discharge of GS (L/s), △DIC is the amount of DIC loss between the CK and LY in each time step, 5.08 is a conversion factor between the molar mass of DIC and carbon (61.02/12.01), △Ca is the amount of CaCO3 lost between CK and LY in each time step, 3.337 is the conversion factor between the molar mass of calcium and carbon (40.08/12.011), F is the CO2 evasion flux from unit water surface area (mg/m2/h), 3.664 is the conversion factor between the molar mass of CO2 and carbon (44.01/12.01), and A is the GS water surface area during the study period (m2). The CO2 evasion flux from upstream to downstream regions showed strong spatial heterogeneity; however, the measured DIC concentration at CK was stable, suggesting that CO2 evasion flux cannot affect DIC concentrations at CK. Consequently, we use the evasion at LY for modeling. In Eq. 5, the first term on the right hand side of the equation denotes the total carbon loss/gain in GS over the study periods, the second term is the amount of carbon formed as calcite in GS, and the third term is the carbon loss via CO2 evasion from the GS to the atmosphere.
These calculations show that the total carbon loss was 15.19 kg C/day and the amount of carbon formed as calcite was 4.34 kg C/day. The carbon loss via CO2 evasion from the GS to the atmosphere was 5.21 kg C/day. Therefore, the carbon sink in the GSS channel produced by in-stream metabolism was around 5.6 kg C/day during the study period, which is about 6.1 times higher than it was in July, 2013 (sunny day, 0.91 kg C/day) (Pu et al. 2017). The result suggests that karst streams contribute to the terrestrial atmospheric carbon sink by stabilizing inorganic carbon originating from carbonate weathering process through the burial of organic carbon produced during aquatic photosynthesis (Liu and Dreybrodt 2015; Martin 2017; Pu et al. 2017).
Practical implications
Much work on diel cycles of inorganic and organic carbon in rivers, streams, and creeks occurs during sunny weather and stable water level, and much of it reflects that in-stream metabolism (photosynthesis and respiration) is the primary control of diel cycle of carbon (de Montety et al. 2011; Jiang et al. 2013; Liu et al. 2015; Parker et al. 2005, 2007, 2014; Pu et al. 2017; Tobias and Boehlke 2011; Yang et al. 2015). Our results suggest that even with overcast conditions, precipitation, rising discharge, and elevated turbidity, in-stream metabolism results in diel cycles of DIC due to weak solar radiation. Consequently, the carbon sink produced by in-stream metabolism can occur in karst streams regardless of variations in environmental conditions. Karst terrains cover around 14.7% of earth’s surface (Chen et al. 2017), and most of headwater systems are sources of groundwater with elevated DIC concentrations. The stream outflows are also highly productive because of the generally clear water and solar radiation. The carbon sink produced by in-stream metabolism should be considered within the context of the global carbon cycle.
However, this study focuses only on a small subtropical stream fed by karst groundwater and a medium rainfall event (~ 22.8 mm) in summer. Additional similar studies in different climatic zones, land use types, rainfall events, and flow regimes could improve the understanding of the extent and causes of diel cycle of DIC.
Conclusions
This study demonstrates that overcast conditions, precipitation, and increased discharge and turbidity did not disrupt the diel cycle of DIC and carbon sink produced by in-stream metabolism (photosynthesis and respiration) in a karst headwater stream in southern China. Although diel conditions developed in ~ 1.3 km downstream of the chemostatic stream source, water chemistry showed significant diel variations. The variations resulted from metabolism of the submerged aquatic community and were not influenced by rainfall and rising discharge. Daytime photosynthesis caused a net release of O2 and consumption of DIC that when buried in the sediment was sequestered; at night, respiration consumed O2 and released CO2, resulting in an increase of DIC. The estimated organic carbon sink by in-stream metabolism is around 5.6 kg C/day during the study period, indicating that the submerged aquatic community in a karst stream can significantly stabilize carbon originating from karst weathering processes. Further study is needed to better understand the carbon sink produced by in-stream metabolism in different karst streams covering different climatic zone and land use types and to consider the influences derived from rainfall event and flow regimes.
References
Almeida RM, Pacheco FS, Barros N, Rosi E, Roland F (2017) Extreme floods increase CO2 outgassing from a large Amazonian river. Limnol Oceanogr 62:989–999. https://doi.org/10.1002/lno.10480
Atekwana EA, Krishnamurthy RV (1998) Seasonal variations of dissolved inorganic carbon and δ13C of surface waters: application of a modified gas evolution technique. J Hydrol 205:265–278. https://doi.org/10.1016/s0022-1694(98)00080-8
Atkins ML, Santos IR, Maher DT (2017) Seasonal exports and drivers of dissolved inorganic and organic carbon, carbon dioxide, methane and δ13C signatures in a subtropical river network. Sci Total Environ 575:545–563. https://doi.org/10.1016/j.scitotenv.2016.09.020
Cavalli G, Riis T, Baattrup-Pedersen A (2012) Bicarbonate use in three aquatic plants. Aquat Bot 98:57–60. https://doi.org/10.1016/j.aquabot.2011.12.007
Chen Z et al (2017) The World Karst Aquifer Mapping project: concept, mapping procedure and map of Europe. Hydrogeol J 25:771–785
Ciais P et al (2013) Carbon and other biogeochemical cycles. In: Stocker TF et al (eds) Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge and New York, pp 465–570. https://doi.org/10.1017/CBO9781107415324.015
Clow DW, Mast MA (2010) Mechanisms for chemostatic behavior in catchments: implications for CO2 consumption by mineral weathering. Chem Geol 269:40–51. https://doi.org/10.1016/j.chemgeo.2009.09.014
Covington MD (2016) The importance of advection for CO2 dynamics in the karst critical zone: an approach from dimensional analysis. In: Feinberg JM, Gao Y, Alexander JEC (eds) Caves and Karst Across Time, vol Special Paper 516. Geological Society of America, pp 113–127. https://doi.org/10.1130/2015.2516(09)
de Montety V, Martin JB, Cohen MJ, Foster C, Kurz MJ (2011) Influence of diel biogeochemical cycles on carbonate equilibrium in a karst river. Chem Geol 283:31–43. https://doi.org/10.1016/j.chemgeo.2010.12.025
Deirmendjian L, Abril G (2018) Carbon dioxide degassing at the groundwater-stream-atmosphere interface: isotopic equilibration and hydrological mass balance in a sandy watershed. J Hydrol 558:129–143. https://doi.org/10.1016/j.jhydrol.2018.01.003
Demars BOL, Thompson J, Manson JR (2015) Stream metabolism and the open diel oxygen method: principles, practice, and perspectives. Limnol Oceanogr Methods 13:356–374. https://doi.org/10.1002/lom3.10030
Dinsmore KJ, Wallin MB, Johnson MS, Billett MF, Bishop K, Pumpanen J, Ojala A (2013) Contrasting CO2 concentration discharge dynamics in headwater streams: a multi-catchment comparison. J Geophys Res Biogeosci 118:445–461. https://doi.org/10.1002/jgrg.20047
Doctor DH, Kendall C, Sebestyen SD, Shanley JB, Ote N, Boyer EW (2008) Carbon isotope fractionation of dissolved inorganic carbon (DIC) due to outgassing of carbon dioxide from a headwater stream. Hydrol Process 22:2410–2423. https://doi.org/10.1002/hyp.6833
Godsey SE, Kirchner JW, Clow DW (2009) Concentration-discharge relationships reflect chemostatic characteristics of US catchments. Hydrol Process 23:1844–1864. https://doi.org/10.1002/hyp.7315
Gombert P (2002) Role of karstic dissolution in global carbon cycle. Glob Planet Chang 33:177–184. https://doi.org/10.1016/s0921-8181(02)00069-3
Hasler CT, Butman D, Jeffrey JD, Suski CD (2016) Freshwater biota and rising pCO2? Ecol Lett 19:98–108. https://doi.org/10.1111/ele.12549
Hess JW, White WB (1988) Storm response of the karstic carbonate aquifer of southcentral Kentucky. J Hydrol 99:235–252
Jiang ZC, Yuan DX (1999) CO2 source-sink in karst processes in karst areas of China. Episodes 22:33–35
Jiang Y, Hu Y, Schirmer M (2013) Biogeochemical controls on daily cycling of hydrochemistry and δ13C of dissolved inorganic carbon in a karst spring-fed pool. J Hydrol 478:157–168. https://doi.org/10.1016/j.jhydrol.2012.12.001
Johnson MS, Billett MF, Dinsmore KJ, Wallin M, Dyson KE, Jassal RS (2009) Direct and continuous measurement of dissolved carbon dioxide in freshwater aquatic systems-method and applications. Ecohydrology:n/a. https://doi.org/10.1002/eco.95
Karis T, Silvester E, Rees G (2016) Chemical regulation of alpine headwater streams during a storm event (Bogong High Plains, Victoria, Australia). J Hydrol 542:317–329. https://doi.org/10.1016/j.jhydrol.2016.09.014
Khadka MB, Martin JB, Jin J (2014) Transport of dissolved carbon and CO2 degassing from a river system in a mixed silicate and carbonate catchment. J Hydrol 513:391–402. https://doi.org/10.1016/j.jhydrol.2014.03.070
Kurz MJ, de Montety V, Martin JB, Cohen MJ, Foster CR (2013) Controls on diel metal cycles in a biologically productive carbonate-dominated river. Chem Geol 358:61–74. https://doi.org/10.1016/j.chemgeo.2013.08.042
Li Z, Zhang Z, Xiao Y, Guo J, Wu S, Liu J (2014) Spatio-temporal variations of carbon dioxide and its gross emission regulated by artificial operation in a typical hydropower reservoir in China. Environ Monit Assess 186:3023–3039. https://doi.org/10.1007/s10661-013-3598-0
Liu Z, Dreybrodt W (2015) Significance of the carbon sink produced by H2O-carbonate-CO2-aquatic phototroph interaction on land. Science Bulletin 60:182–191. https://doi.org/10.1007/s11434-014-0682-y
Liu Z, Zhao J (2000) Contribution of carbonate rock weathering to the atmospheric CO2 sink. Environ Geol 39:1053–1058. https://doi.org/10.1007/s002549900072
Liu Z, Chris G, Yuan D, Meiman J (2004) South China karst aquifer storm-scale hydrochemistry. Ground water 42:491–499
Liu Z, Li Q, Sun H, Wang J (2007) Seasonal, diurnal and storm-scale hydrochemical variations of typical epikarst springs in subtropical karst areas of SW China: soil CO2 and dilution effects. J Hydrol 337:207–223. https://doi.org/10.1016/j.jhydrol.2007.01.034
Liu Z, Dreybrodt W, Wang H (2010) A new direction in effective accounting for the atmospheric CO2 budget: considering the combined action of carbonate dissolution, the global water cycle and photosynthetic uptake of DIC by aquatic organisms. Earth-Sci Rev 99:162–172. https://doi.org/10.1016/j.earscirev.2010.03.001
Liu H, Liu Z, Macpherson GL, Yang R, Chen B, Sun H (2015) Diurnal hydrochemical variations in a karst spring and two ponds, Maolan karst experimental site, China: biological pump effects. J Hydrol 522:407–417. https://doi.org/10.1016/j.jhydrol.2015.01.011
Looman A, Santos IR, Tait DR, Webb JR, Sullivan CA, Maher DT (2016) Carbon cycling and exports over diel and flood-recovery timescales in a subtropical rainforest headwater stream. Sci Total Environ 550:645–657. https://doi.org/10.1016/j.scitotenv.2016.01.082
Lynch JK, Beatty CM, Seidel MP, Jungst LJ, DeGrandpre MD (2010) Controls of riverine CO2 over an annual cycle determined using direct, high temporal resolution pCO2 measurements. J Geophys Res Biogeosci 115. https://doi.org/10.1029/2009jg001132
Mann PJ, Spencer RGM, Dinga BJ, Poulsen JR, Hernes PJ, Fiske G, Salter ME, Wang ZA, Hoering KA, Six J, Holmes RM (2014) The biogeochemistry of carbon across a gradient of streams and rivers within the Congo Basin. J Geophys Res Biogeosci 119:687–702. https://doi.org/10.1002/2013jg002442
Martin JB (2017) Carbonate minerals in the global carbon cycle. Chem Geol 449:58–72
Martin JB, Brown A, Ezell J (2013) Do carbonate karst terrains affect the global carbon cycle? Acta Carsol 42:187–196
Martin JB, Kurz MJ, Khadka MB (2016) Climate control of decadal-scale increases in apparent ages of eogenetic karst spring water. J Hydrol 540:988–1001. https://doi.org/10.1016/j.jhydrol.2016.07.010
Marx A, Dusek J, Jankovec J, Sanda M, Vogel T, van Geldern R, Hartmann J, Barth JAC (2017) A review of CO2 and associated carbon dynamics in headwater streams: a global perspective. Rev Geophys 55:560–585. https://doi.org/10.1002/2016rg000547
McElroy MB (1983) Marine biological-controls on atmospheric CO2 and climate. Nature 302:328–329. https://doi.org/10.1038/302328a0
Mo X (2015) Change processes and influence factors of dissolved inorganic carbon in a surface creek fed by a karst subterranean stream in subtropical karst area, SW China. Southwest University
Nimick DA, Gammons CH, Parker SR (2011) Diel biogeochemical processes and their effect on the aqueous chemistry of streams: a review. Chem Geol 283:3–17. https://doi.org/10.1016/j.chemgeo.2010.08.017
Paranaíba JR, Barros N, Mendonça R, Linkhorst A, Isidorova A, Roland F, Almeida RM, Sobek S (2018) Spatially resolved measurements of CO2 and CH4 concentration and gas-exchange velocity highly influence carbon-emission estimates of reservoirs. Environ Sci Technol 52:607–615. https://doi.org/10.1021/acs.est.7b05138
Parker SR, Poulson SR, Gammons CH, DeGrandpre MD (2005) Biogeochemical controls on diel cycling of stable isotopes of dissolved O2 and dissolved inorganic carbon in the big Hole River, Montana. Environ Sci Technol 39:7134–7140. https://doi.org/10.1021/es0505595
Parker SR, Gammons CH, Poulson SR, DeGrandpre MD (2007) Diel variations in stream chemistry and isotopic composition of dissolved inorganic carbon, upper Clark Fork River, Montana, USA. Appl Geochem 22:1329–1343. https://doi.org/10.1016/j.apgeochem.2007.02.007
Parker SR, Darvis MN, Poulson SR, Gammons CH, Stanford JA (2014) Dissolved oxygen and dissolved inorganic carbon stable isotope composition and concentration fluxes across several shallow floodplain aquifers and in a diffusion experiment. Biogeochemistry 117:539–552. https://doi.org/10.1007/s10533-013-9899-0
Pedersen O, Colmer TD, Sand-Jensen K (2013) Underwater photosynthesis of submerged plants—recent advances and methods. Front Plant Sci 4:140. https://doi.org/10.3389/fpls.2013.00140
Peter H, Singer GA, Preiler C, Chifflard P, Steniczka G, Battin TJ (2014) Scales and drivers of temporal pCO2 dynamics in an Alpine stream. J Geophys Res Biogeosci 119:1078–1091. https://doi.org/10.1002/2013jg002552
Pu J, Yuan D, Zhao H, Shen L (2014) Hydrochemical and PCO2 variations of a cave stream in a subtropical karst area, Chongqing, SW China: piston effects, dilution effects, soil CO2 and buffer effects. Environ Earth Sci 71:4039–4049
Pu J, Li J, Khadka MB, Martin JB, Zhang T, Yu S, Yuan D (2017) In-stream metabolism and atmospheric carbon sequestration in a groundwater-fed karst stream. Sci Total Environ 579:1343–1355. https://doi.org/10.1016/j.scitotenv.2016.11.132
Raymond PA, Zappa CJ, Butman D, Bott TL, Potter J, Mulholland P, Laursen AE, McDowell WH, Newbold D (2012) Scaling the gas transfer velocity and hydraulic geometry in streams and small rivers. Limnol Oceanogr Fluids Environ 2:41–53. https://doi.org/10.1215/21573689-1597669
Rice EW, Baird RB, Eaton AD, Clesceri LS (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Public Health Association, the American Water Works Association and the Water Environment Federation, Washington D.C
Shin WJ, Chung GS, Lee D, Lee KS (2011) Dissolved inorganic carbon export from carbonate and silicate catchments estimated from carbonate chemistry and δ13CDIC. HESS 15:2551–2560. https://doi.org/10.5194/hess-15-2551-2011
Shuster ET, White WB (1971) Seasonal fluctuations in the chemistry of lime-stone springs: a possible means for characterizing carbonate aquifers. J Hydrol 14:93–128
Sobek S, Algesten G, Bergstrom AK, Jansson M, Tranvik LJ (2003) The catchment and climate regulation of pCO2 in boreal lakes. Glob Chang Biol 9:630–641
Spiro B, Pentecost A (1991) One day in the life of a stream—a diurnal inorganic carbon mass balance for a travertine-depositing stream (waterfall beck, Yorkshire). Geomicrobiol J 9:1–11. https://doi.org/10.1080/01490459109385981
Tobias C, Boehlke JK (2011) Biological and geochemical controls on diel dissolved inorganic carbon cycling in a low-order agricultural stream: implications for reach scales and beyond. Chem Geol 283:18–30. https://doi.org/10.1016/j.chemgeo.2010.12.012
Tobias C, Böhlke JK (2010) Biological and geochemical controls on diel dissolved inorganic carbon cycling in a low-order agricultural stream: implications for reach scales and beyond. Chem Geol. https://doi.org/10.1016/j.chemgeo.2010.12.012
Vesper D, White W (2003a) Storm pulse chemographs of saturation index and carbon dioxide pressure: implications for shifting recharge sources during storm events in the karst aquifer at Fort Campbell, Kentucky/Tennessee, USA. Hydrogeol J 12. https://doi.org/10.1007/s10040-003-0299-8
Vesper DJ, White WB (2003b) Metal transport to karst springs during storm flow: an example from Fort Campbell, Kentucky/Tennessee, USA. J Hydrol 276:20–36. https://doi.org/10.1016/s0022-1694(03)00023-4
Vesper DJ, White WB (2004) Storm pulse chemographs of saturation index and carbon dioxide pressure: implications for shifting recharge sources during storm events in the karst aquifer at Fort Campbell, Kentucky/Tennessee, USA. Hydrogeol J 12:135–143
Wigley TML (1977) WATSPEC: a computer program for determining the equilibrium speciation of aqueous solutions. Geo-Abstracts for the British Geomorphological Research Group
Yang R, Chen B, Liu H, Liu Z, Yan H (2015) Carbon sequestration and decreased CO2 emission caused by terrestrial aquatic photosynthesis: insights from diel hydrochemical variations in an epikarst spring and two spring-fed ponds in different seasons. Appl Geochem 63:248–260. https://doi.org/10.1016/j.apgeochem.2015.09.009
Yuan D (1997) The carbon cycle in karst. Z Geomorphol, Zeitschrift für Geomorphologie 108(Suppl):91–102
Zeng C, Liu ZH, Zhao M, Yang R (2016) Hydrologically-driven variations in the karst-related carbon sink fluxes: insights from high-resolution monitoring of three karst catchments in Southwest China. J Hydrol 533:74–90
Zhang C, Wang J, Pu J, Yan J (2012) Bicarbonate daily variations in a karst river: the carbon sink effect of subaquatic vegetation photosynthesis. Acta Geol Sin (Engl Ed) 86:973–979. https://doi.org/10.1111/j.1755-6724.2012.00721.x
Zhang T, Li J, Pu J, Martin JB, Khadka MB, Wu F, Li L, Jiang F, Huang S, Yuan D (2017a) River sequesters atmospheric carbon and limits the CO2 degassing in karst area, southwest China. Sci Total Environ 609:92–101. https://doi.org/10.1016/j.scitotenv.2017.07.143
Zhang T, Pu J, Li J, Yuan D, Li L (2017b) Stable isotope and aquatic geochemistry of a typical subtropical karst subterranean stream in southwest China. Carbonates Evaporites 32:415–430. https://doi.org/10.1007/s13146-017-0356-3
Zhao B, Su Y (2014) Process effect of microalgal-carbon dioxide fixation and biomass production: a review. Renew Sust Energ Rev 31:121–132. https://doi.org/10.1016/j.rser.2013.11.054
Acknowledgements
Special thanks are given to Wen Liu and Xue Mo for their help in field and lab works. Heartfelt thanks are also given to Qiang Zhang for his YSI and ISCO autosampler instruments.
Funding
The study is financially supported by National Natural Science Foundation of China (No. 41572234, No. 41202185, No. 41702271), the Special Fund for Basic Scientific Research of Chinese Academy of Geological Sciences (YYWF201636), the Guangxi Natural Science Foundation (2016GXNSFCA380002, 2017GXNSFFA198006), the Geological Survey Project of CGS (DD20160305-03), and the Special Fund for Basic Scientific Research of Institute of Karst Geology (No. 2017006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Fig. S1
Cross-plot between (a) DO and water temperature, (b) CO2 evasion flux and water temperature and (c) δ13CDIC and pCO2 at extractive 24-h timescale from 0600 h on August 18 to 0600 h on August 19. (DOCX 120 kb)
Table S1
Summary of hydrochemical parameters measured at CY and LY site in GSS on August 17–19, 2013 (DOCX 21 kb)
Table S2
Parameters of PLS models explaining the variability in DIC at LY site. Highly important variables have VIP > 1.0 (marked bold), moderately important variables have VIP 0.8–1.0 (marked italic), and unimportant variables have VIP <0.8. R2Y is comparable to R2 in linear regression and expresses how much of the variance in Y is explained by X. Q2 is a measure of the predictive power of the PLS model. The closer Q2 to R2Y, the better and more robust is the model. (DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Pu, J., Li, J., Zhang, T. et al. Diel-scale variation of dissolved inorganic carbon during a rainfall event in a small karst stream in southern China. Environ Sci Pollut Res 26, 11029–11041 (2019). https://doi.org/10.1007/s11356-019-04456-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04456-z