Abstract
The study was conducted to analyse the influence of three nutrient formulations, namely BG-11 medium, BBM and TAP medium, on growth potential and lipid yield of two microalgal genera (Botryococcus sp. and Chlorella sp.) and to study the roles of N, P and other major nutrients. The study focussed on the general patterns of starch and lipid synthesis and storage and to further assess how photosynthetic carbon partitioning into starch and lipid is altered by conditions in growth media such as N and C presence as seen in BG11 medium which are known to induce neutral lipid production and the lack of it in BBM and TAP medium. BG-11 medium performed better as compared to BBM and TAP medium in terms of biomass productivity and lipid yield. The lipid yield was highest in Botryococcus sp. (63.03% dry wt.) and Chlorella sp. (50.27% dry wt.) at 30th day of incubation. Mean biomass productivity was highest for Botryococcus in BBM medium (6.14 mg/L/day) and for Chlorella in BG-11 medium (4.97 mg/L/day). Mean lipid productivity (50.78% and 39.36%) was highest in BG11 medium for both Botryococcus and Chlorella species, respectively. A sharp decline in sugar content was observed in the late stationary phase of growth from 30th day to 45th day. Fatty acid methyl ester (FAME) profile of the extracted lipids showed predominantly oleic acid, followed by palmitic acid and stearic acid in both the strains when grown in BG-11 medium. The other biodiesel quality parameters were in accordance with the international standards. A complex relationship was found between chemical composition and biodiesel properties. Proximity analysis indicated that the fuel properties of biodiesels are determined by a number of parameters and by the combination of different chemical compositions. The results provide an insight into organic carbon partitioning into lipid compounds and how the organism’s lipid metabolism changes due to N-deplete culturing in TAP medium and inorganic carbon source availability as seen in BG-11 and BBM medium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae have been considered for biodiesel production, based on their ability to grow rapidly and accumulate large amounts of storage lipids (20–80% on dry weight basis), primarily in the form of triacylglycerides (TAG) (Chisti 2007). These organisms are reported to produce enhanced lipids under stressed conditions of limited nutrient inputs (Hu 2004). In addition, nitrogen source and their levels can also influence the growth as well as lipid production in these organisms (Cheng et al. 2014). Optimization of different nutrient formulations to enhance biomass productivity and lipid yield has been undertaken by researchers all over the world (Ruangsomboon 2015). Chlorella vulgaris (strain 2714) and two strains of Microcystis aeruginosa were grown in BG11, TAP and TP media, where Chlorella vulgaris exhibited the highest growth and productivity in TAP medium (Held and Raymond 2011). Extracted lipids from microalgae can be subjected to transesterification to yield fatty acid methyl esters, and the composition of fatty acid methyl ester (FAME) determines the biodiesel fuel quality. Differential fatty acid composition has been reported in literature (Gouveia and Oliveira 2009). Normally, when transesterification is performed with algal oil, the biodiesel yield is around 80% and all algal oils are not suitable for biodiesel production (El-Shimi et al. 2013).
Many investigators reported change in chemical structure of FAMEs when cultivated under different environmental conditions (Los and Murata 2004). FAME properties determine the quality of biodiesel, and these criteria can be compared with the ASTM grade biodiesel (Canaki and Sanli 2008). Biodiesel fuel properties can also be determined through physical parameters like cetane number, kinematic viscosity, oxidative stability and cold flow properties such as cloud point, cold-filter plugging point and lubricity which are influenced by fatty acid profile (Knothe 2008). In view of this, the present study was undertaken to evaluate the suitability of different nutrient formulations for growth of microalgae and to analyse their lipids as a source of biodiesel.
It is known that microalgae respond with physiological alterations to the nutrient composition where they grow. This behaviour can be viewed as a biotechnological attribute that can be manipulated in order to control the algae biochemical composition and growth focusing on specific compounds and higher productivity. Therefore, a cheap and promising media to improve microalgae production yield was selected. This study provides a future scope where algae can be cultivated in a minimal media or wastewater with desired components.
Material and methods
Cultural conditions
Chlorella sp. (MCC 7) and Botryococcus sp. (MCC 32) were procured from germplasm collection of CCUBGA, IARI. The cultures were grown and maintained in three different media recipes namely BG11 (Stanier et al. 1971), BBM (Nichols and Bold 1965) and TAP (Gorman and Levine 1965) (Table 1) under culture room conditions (temperature 28 ± 2 °C, light intensity 95 μE/m2/s and light/dark cycle 16:8 h).
Biomass harvesting and estimation of dry weight and sugar content
Microalgal biomass from the two genera (Chlorella sp. and Botryococcus sp.) was harvested at 15th, 30th and 45th day of growth. Dry weight was measured by filtering a known volume through pre-weighed GF/C filter paper (Whatman, Poole, UK) and dried at 80 °C till a constant weight was achieved (Sorokin 1959). The relative biomass productivity (P = g/L/day) was calculated as (N2 − N1) / t2 − t1 where N1 and N2 are the dry weight measurements (g/L) at time t1 and t2. A known volume of microalgal suspension was used for estimation of total sugars by the anthrone method using glucose as standard (Dubois 1956).
Lipid extraction, transesterification and gas chromatography-mass spectroscopy (GC-MS) analyses
Dried microalgal biomass was pretreated with microwave (2450 MHz for 6 min) for efficient lipid extraction using a modified protocol (Rakesh et al. 2015). Lipids were extracted from 100 mg of dried biomass with chloroform and methanol (1:2 v/v) (Bligh and Dyer 1959), and the extracted lipids were calculated as per the equation:
Transesterification of lipids
The extracted lipids from 50 to 80 mg dry weight were transesterified using concentrated HCl (0.1 mL) as catalyst and methanol (100 mL). For this reaction, temperature was maintained at 60 °C for 3 h (Ruangsomboon et al. 2013). After shaking, the solution was kept for 16 h to separate the biodiesel and the sediment layers clearly. The separated upper layer having biodiesel was evaporated to release the excess methanol, and the remaining part was mixed with hexane to collect fatty acid methyl esters. The remaining catalyst was removed by successive rinsing with distilled water followed by filtration. The FAME thus obtained was evaporated to release the excess hexane using similar conditions. The yield of methyl esters (Y) was calculated as under
FAME were dissolved in known quantity of methanol and identified using GC-MS (Varian). Comparison was made using inbuilt standard mass spectra library system (NIST-05 and Wiley-8) of GC-MS.
Physical properties of biodiesel
Quality of biodiesel was assessed in terms of physical parameters namely cetane number (CN), saponification value (SV), iodine value (IV), degree of unsaturation (DU), long-chain saturation factor (LCSF), cold filter plugging point (CFPP), higher heating value (HHV), flash point and kinematic viscosity and density. These properties were calculated from the FAME profile (Knothe 2002, 2006; Krisnangkura 1986; Gopinath et al. 2009; Ramos et al. 2009; Ramírez-Verduzco et al. 2012; Demirbas 1998) through different scores as per the equations provided below.
The SV and IV for use in the above equation were calculated using the following equations:
where D is the number of double bonds in the fatty ester, M is the molecular mass of the fatty ester and N is the percentage of the particular fatty ester.
The DU was calculated as
where MUFA: monounsaturated and PUFA: polyunsaturated fatty acids present in the FAME.
The allylic position equivalents (APE) were calculated using the equations as under
bis-allylic position equivalents (BAPE)
where apn and bpn are the numbers of allylic and bis-allylic positions in fatty acid and Acn is the amount (mass %) of each fatty acid in the mixture.
The LCSF was estimated as
The CFPP was
The CP (Sarin et al. 2009) was calculated as
In which CP value is based on the C16:0 content (wt%) in the FA profile.
Other fuel parameters mentioned as under were calculated based upon previous reports (Ramírez-Verduzco et al. 2012).
The kinematic viscosity (υ, mm2/s) at 40 °C:
where Mwi is the molecular weight of a fatty acid, Ni is the percentage of the given fatty acid in the biodiesel and Di is the number of double bonds in the given fatty acid.
The density (ρ, g/cm3) of the biodiesel at 20 °C:
The HHV:
Proximity analysis
A proximity dissimilarity matrix based on Euclidean distance measurement of the biodiesel properties (both FAME profile and physical parameters) was studied for the two microalgal genera using SPSS.
Results and discussion
Effects of different nutrition recipes on biomass productivity, lipid yield and sugar content
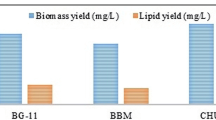
The comparative dry weight content in Botryococcus sp. (Table 2) incubated under three types of media namely BG-11, BBM and TAP showed mean dry weight of 429.5 mg/L in BG-11, 330.3 mg/L in BBM and 404.1 mg/L in TAP medium indicating thereby the BG-11 medium was most suitable followed by TAP and BBM medium. In general, the growth showed a gradual and linear increase through 15, 30 and 45 days of incubation. In all the three media, total sugars (38.05 mg/g; BBM) (60.11 mg/g; TAP) (47.13 mg/g; BG11) were maximum during 30th day of incubation. The main storage carbohydrate in green microalgae is sugars, which acts as a buffer on carbon availability fluctuations during growth (Tetlow et al. 2004). It has been proven that the growth rate is strongly correlated to the rate of starch degradation in microalgae and that protein synthesis represents a major carbon cost from starch during cell growth (Brányiková et al. 2011). Therefore, the observed carbohydrate accumulation could be the result of decreased starch-derived carbon demand for protein biosynthesis and growth.
When these media recipes were tested for the growth of Chlorella sp. (Table 2), BG-11 medium was best (409.7 mg/L) followed by the growth observed in TAP medium (363.4 mg/L) and BBM medium (263.7 mg/L). The growth showed a linear and increasing trend in all the three media. Sugars were highest (19.05 mg/g) in BG11 medium (9.1 mg/g, BBM) and (14.42 mg/g, TAP) observed at 30th day of incubation.
The composition of BG11, BBM and TAP media used in the study served to study the roles of N, P and other major nutrients. Carbon is only present in BG11 medium in the form of carbonate in Na2CO3 and as citric acid and Fe ammonium citrate. It is totally absent in BBM and TAP medium. A response to minimal N stress as seen in BBM and TAP medium was less well correlated to high rates of lipid productivity, presumably to preserve their physiological state at a higher level of function (e.g. maintenance of higher cellular protein concentrations) as has also been accounted by Adams et al. (2013). It has been also reported that the synthesis of these storage compounds would be primarily as a result of de novo carbon fixation by photosynthesis and its partitioning between synthesis pathways for lipids and starch (Fernandes et al. 2013; Hu et al. 2008). Fan et al. (2012) have speculated that lipid and starch syntheses are competitive pathways in nitrogen-starved cells of Chlamydomonas reinhardtii and carbon supply seems to be the major factor controlling carbon partitioning between starch and oil accumulation in this strain.
A steep rise in sugar content in the logarithmic phase of growth from 15th to 30th day followed by a sharp decline was observed in the late stationary phase of growth from 30th day to 45th day. In Botryococcus sp., sugar content increased by 81.5% which subsequently decreased by 80.22% in BG11 medium, 84.2% increase followed by 92.3% decrease in BBM medium and 73% increase thereafter decreasing by 85.9% in TAP medium, while Chlorella sp. showed an increase of 77.5% and decreasing by 64.8% in BG11 medium, 54% increase followed by 37.3% decrease in BBM medium and 10.7% increase, which decreased by 64% in TAP medium. The lipid and carbohydrate syntheses are closely related and so compete for synthesis and storage. Under nutrient deprivation conditions, the starch degradation becomes inactive thus leading to the activation of lipid synthesis (Kona et al. 2017). Disruption of starch synthesis leading to neutral lipid over-accumulation in C. reinhardtii has been reported in literature (Fan et al. 2012; Li et al. 2010; Work et al. 2010), although mere antagonism between synthesis pathways of both storage compounds is not always sustained (Siaut et al. 2011). On the contrary, Li et al. (2011) showed that disruption of starch synthesis decreased TAG accumulation in Pseudochlorococcum sp. and suggested that in this strain, TAG could be synthesized, partially, at cost of previously assimilated starch.
The mean biomass productivity of 3.46 mg/L/day and 4.97 mg/L/day in BG-11 medium, 6.14 mg/L/day and 2.59 mg/L/day in BBM medium and 2.71 mg/L/day and 3.5 mg/L/day in TAP medium was observed for Botryococcus sp. and Chlorella sp. during the complete duration of the study (Fig. 1).
TAP medium was devoid of N, while BG11 contained six times more N in the form of nitrate as compared to BBM. The amount of phosphate in BG11 was considerably lesser as compared to BBM and TAP. Kapdan and Aslan (2008) reported that N/P ratio can influence the growth in terms of dry weight content and lipid yield. However, the interaction studies between N and P shows enhanced lipid content due to lack of P, rather than limitation of N as has also been reported by Hakkalin et al. (2014). Mayers et al. (2014) found that P limitation alongside N starvation had a subtle but minimal effect on bulk biochemical composition, but negatively influenced cell division and biomass productivity of Nannochloropsis sp.
Optimum nitrate concentration for enhanced biomass productivity has been reported to be between 0.5 and 0.7 g/L with biomass productivity 0.5 g/L and 0.65 g/L (Bhola et al. 2011; Lv et al. 2010; Chen et al. 2011). Fung et al. (2013) showed that NaNO3 concentration of 1.4 g/L decreases biomass productivity in Chlorella vulgaris, whereas our study showed enhanced biomass productivity in BG-11 medium with NaNO3 concentration of 1.5 g/L as compared to BBM medium (0.25 g/L of NaNO3) and TAP medium (NaNO3 absent). Bhola et al. (2011) have stated that nitrate can cause toxic conditions and provide adverse effects for the growth of microalgae. The cells may release N2 from photosynthetic pigments and utilize the same for other metabolic processes under nitrogen-deficient conditions.
In Botryococcus sp., BG-11 medium was most suitable for enhanced lipid yield (50.8%), followed by 33.1% in TAP medium and 31.9% in BBM medium. Chlorella sp. exhibited highest lipid yield in BG-11 medium (39.4%) and 21% in BBM and TAP medium. The mean lipid productivity on dry weight basis varied as 50.78% and 39.36% in BG11 medium, 31.95% and 21.28% in BBM medium and 33.12% and 21.06% in TAP medium in Botryococcus sp. and Chlorella sp. Large number of media formulations has been suggested for the growth of microalgae (Dayananda et al. 2007; Ge et al. 2011). The present study has established BG11 media (N 1.5 g/L; P 0.04 g/L) as more suitable for the growth of microalgae as potential biodiesel feedstock, as compared to BBM (N 0.25 g/L; P 0.17 g/L) and TAP media (N 0.0; P 0.03 g/L). This study supports the idea that it is the ratio of N and P that influences biomass productivity and lipid yield, as has also been reported by Kapdan and Aslan (2008).
Both Ca and Mg were found to be critical for biomass yield and lipid accumulation in microalgae by Gorain et al. (2013). These salts are found in much larger amounts in TAP media as compared to BBM and BG11 media. Cellular lipid content did not show significant rise under high Mg supplementation. The role of Mg ions in activating the enzyme acetyl-CoA carboxylase, catalysing the first step of fatty acid biosynthesis, is well established (Nelson and Cox 2008). Chloride was present in higher amounts in TAP media in the form of NH4Cl and CaCl2, while smaller amounts were present in BG11 and BBM. The role of Cl− as an essential micronutrient for oxygenic photosynthetic organisms is widely accepted (Raven 2016). It is also known that these roles of Cl− vary phylogenetically and with the N source (Raven 2016). The high concentrations of Cl− generally found in algal and plant cells, and particularly in their vacuoles, play a major role in turgor generation and in cell expansion (Franco-Navarro et al. 2015).
In general, under nutrient limited condition, the growth of the algae slows down, and there is a reduction in the requirement for the synthesis of new membrane compounds. However, fatty acid biosynthesis is not interrupted as photosynthesis continues. Therefore, the cell deposits these fatty acids in the form of triacylglycerols (Sharma et al. 2012). Furthermore, under normal culture condition, the two major components generated by photosynthesis are ATP and NADPH, which are used for producing biomass. As cell growth and proliferation are hampered under nutrient limitations, regeneration of NADP+ has been achieved by consuming NADPH for fatty acid biosynthesis.
The presence of Fe-EDTA corresponded to a marked influence on photosynthetic machinery and enhanced the production of ATP and NADP (H) (Martin et al. 2000). These energy carrier molecules help towards formation of carbohydrates through Benson and Calvin cycle (Briat et al. 2015). BBM and TAP media were devoid of Fe-EDTA. The higher iron-supplemented conditions in BG11 medium showed utilization of sugars which may be attributed to the active physiological state of the tested microalgal sp. with optimum iron supplementation condition, wherein the available carbon source is re-utilized in the formation of higher lipids. Nutrient/carbon-deprived condition in BBM and TAP medium re-utilizes the accumulated carbohydrate towards the lipid synthesis and other metabolic activities. Supplementation of Fe-EDTA in nutrient phase showed a positive influence on the photosynthesis mechanisms of Chlorella sp. under mixotrophic condition leading to the increase in biomass, chlorophyll, carbohydrates, proteins and lipids as reported by Kona et al. (2017). Fe-EDTA at specific concentration has quite diverse biomass productivity, lipid profile and metabolite synthesis. The higher concentration of Fe-EDTA, present in BG11 medium, showed marked influence on the oleic acid accumulation (Kona et al. 2017) which was also observed in the present study. Iron as an integral component of metallo-protein has a direct influence on the carbohydrate reserve which enables the chain elongation of fatty acid by activating the nitrate and nitrite reductase which directs the metabolism towards chain elongation and further leads to the unsaturation in stress phase (Oijen et al. 2004).
Fatty acid methyl ester profile
Lipids extracted from microalgal genera exhibit fatty acid profile mainly of C16 and C18 and, therefore, can be used for biodiesel production (Francisco et al. 2010; Converti et al. 2009). As the BG-11 medium was better in terms of growth and lipid content, it was used further to cultivate the two microalgal genera and to determine biodiesel quality parameters in terms of FAME analysis and physical properties (Table 3). Out of different fatty acids, oleic acid (C18:1) was predominant constituting 48.2% in Botryococcus sp. and 42.7% in Chlorella sp. In Botryococcus sp., the next prominent fatty acid was linolenic acid (C18:2; 20.1%) followed by palmitic acid (C16:0; 12.5%) and palmitoleic acid (10.1%). In Chlorella sp., oleic acid was followed by palmitic acid (17.4%) and linoleic acid (12.4%) and palmitoleic acid (11.1%). The total TGA were 77.9% in Botryococcus sp. and 78.7% in Chlorella sp. Over 80% of total fatty acid profile can be considered as an ideal source of lipids for biodiesel production (Liu et al. 2011). The saturated fatty acids in Botryococcus sp. was 19.6%, whereas these were 24.9% in Chlorella sp. The MUFA were 58.3% in Botryococcus sp., while Chlorella sp. showed 53.8% MUFA levels. The PUFA were similar in Botryococcus sp. (21.3%) and Chlorella sp. (20.1%). The composition of fatty acids can be influenced by the cultural conditions and the physiological potential of microalgae, their structure as well as the extraction procedure (Ho et al. 2012; Yeh and Chang 2012). The studies indicated an abundance of oleic acid, linoleic acid and palmitic acid in lipids extracted from Botryococcus sp. and Chlorella sp., and the ratio of saturated and unsaturated fatty acid determines the quality of biodiesel (Radakovits et al. 2010). Saturated fatty acids are resistant to degradation hence increase the longevity of biodiesel as well as its resistance to oxidation under hot climatic conditions. Converti et al. (2009) reported high values of palmitic acid and linoleic acid in microalgal oil, and composition was suggested to meet the requirements of European legislation of biodiesel. The biomass and neutral lipid productivity and nature of their corresponding fatty acid profile are both media and species specific (Sarpal et al. 2016). The study showed that proper selection of media and species was important to cultivate biomasses with desired characteristics either suitable for biodiesel or PUFA potential.
Physical parameters for biodiesel analysis
In addition to FAME profile, physical parameters such as CN, IV, density, viscosity and cold flow properties were calculated to assess quality of biodiesel. Both the tested microalgal strains showed good (> 51) cetane number (CN) which was at par with European standard and higher than ASTM standard. As per international standard of the ASTM D6751, the minimum CN must be 47; however, 51 is the minimum CN value of EN14214 (Europe) standards. The CN values reported for microalgal strains vary from 42.61 to 65.02, with an average value of 56.80 (Calixto et al. 2018).
Unsaturated fatty acids usually lower the CN and increase the NOx emission. These are also prone to oxidation which in turn may affect the lubricity of a biodiesel (Saraf and Thomas 2007). The CN value can affect engine performance in terms of combustion and exhaust emissions, and higher CN correlates with lower NOx exhaust emissions (Ladommatos et al. 1996). Branching and chain length can affect CN as the number becomes smaller which decreases chain length and increases branching. Therefore, biodiesel should have high saturated and monounsaturated fatty acids and low polyunsaturated fatty acids, which may increase with the length of the unbranched carbon chain of the FAME (Knothe 2005). IV is also a biodiesel quality parameter included in EN 14214. This plays an important role in biodiesel oxidative stability and represents the DU by weighted sum of the masses of MUFA and PUFA. Polymerization of glycerides with formation of deposits may occur with unsaturation making it susceptible to oxidative attack (Francisco et al. 2010). IV of both the microalgal strains calculated was within the maximum limit of 120, and lower IV values of 57 and 68 g I2/100 g have been reported for Microcystis aeruginosa NPCD-1 and Trichormus sp. CENA77 (Da Ros et al. 2013). The CFPP, CP and pour point (PP) are the low-temperature flow properties for which no US or European standards are reported as each country can fix its own standard according to local climatic conditions. However, biodiesel fuels do suffer from cold flow properties more than mineral diesel fuel. If saturated FA are present in oils, crystallization occurs as saturated FA esters have higher melting points than unsaturated FA compounds. The levels of stearic acid were below 3.18% in tested strains which resulted in lower temperatures of CFPP (Table 4). LCSF of lipids is a critical parameter and determines oxidative stability, cetane number, IV and CFPP of the biodiesel. Unsaturated fatty acids are reported to enhance cold flow properties of biodiesel (Knothe 2008). The longer the biodiesel carbon chains, the worse are their low-temperature properties. Hence, this becomes an important parameter in determining the cold response of the biodiesel. LCSF values of 2.91 and 3.18 were shown in Botryococcus sp. and Chlorella sp. Other cyanobacterial strains namely M. aeruginosa NPCD-1, Synechococcus sp. PCC 7942 and Trichormus sp. CENA77 showed a higher LCSF values which was attributed to enhanced palmitic and stearic FAs (Da Ros et al. 2013). The unsaturated bonds are vulnerable to oxidation during storage, and this factor lowers the acceptability of any oil for biodiesel production (Behrens and Kyle 1996). The relative rates of oxidation given in the literature are 1 for oleate, 41 for linoleate and 98 linolineate (Frankel 1998). Thus, small amounts of more highly unsaturated fatty compounds have disproportionately strong effects.
Solid phase consisting mainly of the saturated methyl esters at the equilibrium point can affect CP value which can be accurately predicted by the amount of saturated methyl esters (C16:0 and C18:0), regardless of the unsaturated esters (Baig et al. 2016). CP values showed variations in the present study as reported in other studies (Sarin et al. 2009). PP is always lower than the cloud point, which is also shown in our study (Sarin et al. 2009). The oxidative stability of the biodiesel can be predicted by allylic position equivalents and bis-allylic position equivalents (Knothe 2002). No specifications are mentioned on the higher heating value in any of the reported standards. The energy content of fatty acid methyl esters is directly proportional to chain length (for pure fatty acids). The HHVs were within the set range (38–40.4 MJ/kg) in the two microalgal strains. For regular biodiesel, the range is normally 10–12% less than the petroleum-derived diesel (46 MJ/kg) (Ramírez-Verduzco et al. 2012). The filamentous nonheterocystous cyanobacterium Lyngbya kuetzingii showed HHV value of 41.5 (Song et al. 2013). Standard value has been set at 0.86–0.90 g/cm3 for density (ρ), according to EN 14214, which is another important parameter for biodiesel quality. Density calculated for both the microalgal strains was within the range, and similar q values were found in other microalgal and cyanobacterial species (Song et al. 2013). The appropriate kinematic viscosity (υ) in biodiesel ensures an adequate fuel supply which reaches injectors at different operating temperatures (Ramírez-Verduzco et al. 2012). “t” can affect the CFPP for engine operation at low temperatures as it is inversely proportional to temperature. Kinematic viscosity limits are 2.5–6.0 mm2/s, 1.9–6.0 mm2/s and 3.5–5.0 mm2/ s as per IS 15607, ASTM 6751-02 and EN14214. The microalgal strains tested were in the prescribed viscosity range of 1.48–4.66 mm2/s, hence meeting the standards.
Proximity correlation analysis
A dissimilarity proximity matrix of the biodiesel properties of two microalgal genera understudy was plotted on SPSS (Table 5), to establish the proximity of the properties and the smaller values indicated closeness. Our observations showed that viscosity and density were closely associated with cloud point, long-chain saturation factor and had the least relationship with saponification value. Viscosity also showed close similarity with oxidative stability. Saponification value did not reveal likeness to CFPP, CP and LCSF. The iodine value showed a closeness to DU and APE, and the MUFA, PUFA and SFA were most distant to SV. The average chain length (ACL) was correlated with all the fuel properties investigated in this study. There was a very strong positive correlation with kinematic viscosity (KV). This is mainly due to the increase in carbon content, as well as random intermolecular interaction in the FAME, which consequently increased the KV. For the same reason, ACL was also found to have a strong positive correlation with density and HHV. The average number of double bonds in the biodiesel (which indicates the concentration of unsaturated fatty acid methyl esters) was found to be another influential factor affecting most of the biodiesel properties investigated in this study. The number of double bonds also affected KV, density, HHV and oxidation stability (OS). Although OS was observed to have a slight negative correlation with ACL, this property has a very strong negative correlation with number of double bonds. This is because a higher number of double bonds in the fatty acid chain of biodiesel makes it much more susceptible to oxidation. The number of double bonds also has a moderate positive correlation with density and HHV, but no correlation was found between the number of double bonds and KV. An average number of double bonds (ANDB) and an average chain length (ACL) may well be the most influential parameters affecting most of the properties of biodiesels. Parameters relating to biodiesel production and purification, such as free fatty acids and glycerol content, also influence certain biodiesel properties found in this study.
Conclusions
The present study involved the utilization of three nutritional formulations for the growth and lipid yield of two microalgal genera namely Botryococcus sp. and Chlorella sp. Out of the three media tested, BG-11 medium was the most suitable for dry weight content and lipid yield as compared to BBM and TAP medium. BG11 medium also showed better quality FAMEs due to high oleic acid content. A sharp decline in sugar content was observed in the late stationary phase of growth from 30th day to 45th day. The study in relation to FAME profile and physical parameters indicated that Botryococcus sp. as well as Chlorella sp. can be used as a suitable option for biodiesel production. As the biodiesel properties were in accordance with ASTM and EN standards, the appropriate ratio of SFA and UFA can be achieved by blending with other oil feedstocks so that the quality of biodiesel can be improved. A complex relationship was found between chemical composition and biodiesel properties. Proximity analysis indicated that the fuel properties of biodiesels are determined by a number of parameters and by the combination of different chemical compositions.
References
Adams C, Godfrey V, Wahlen B, Seefeldt L, Bugbee B (2013) Understanding precision nitrogen stress to optimize the growth and lipid content trade-off in oleaginous green microalgae. Bioresour Technol 131:188–194
Baig RN, Verma S, Nadagouda MN, Varma RS (2016) Room temperature synthesis of biodiesel using sulfonated graphitic carbon nitride. Sci Rep 6:39387
Behrens PW, Kyle DJ (1996) Microalgae as a source of fatty acids. J Food Lipids 3:259–272
Bhola V, Desikan R, Santosh SK, Subburamu K, Sanniyasi E, Bux F (2011) Effectsof parameters affecting biomass yield and thermal behaviour of Chlorella vulgaris. J Biosci Bioeng 111(3):377–382
Bligh EG, Dyer WJ (1959) Extraction of lipids in solutions by method of lipid extraction. Can J Biochem Physiol 37:911–917
Brányiková I, Maršálková B, Doucha J, Brányik T, Bišová K, Zachleder V, Vítová M (2011) Microalgae—novel highly efficient starch producers. Biotechnol Bioeng 108:766–776
Briat JF, Dubos C, Gaymard F (2015) Iron nutrition, biomass production and plant product quality. Trends Plant Sci 20:33–40
Calixto CD, da Silva Santana JK, Tibúrcio VP, da Costa Sassi CF, da Conceição MM, Sassi R (2018) Productivity and fuel quality parameters of lipids obtained from 12 species of microalgae from the northeastern region of Brazil. Renew Energy 115:1144–1152
Canaki M, Sanli H (2008) Biodiesel production from various feed stocks and their effects on the fuel properties. J Ind Microbiol Biotechnol 35:431–441
Chen CY, Yeh KL, Aisyah R, Lee DJ, Chang JS (2011) Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour Technol 102(1):71–81
Cheng P, Wang J, Liu T (2014) Effects of nitrogen source and nitrogen supply model on the growth and hydrocarbon accumulation of immobilized biofilm cultivation of B. braunii. Bioresour Technol 166:527–533
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculate and Chlorella vulgaris for biodiesel production. Chem Eng Process Process Intensif 48(6):1146–1151
Da Ros PCM, Silva CSP, Silva-Stenico ME, Fiore MF, De Castro HF (2013) Assessment of chemical and physicochemical properties of cyanobacterial lipids for biodiesel production. Mar Drugs 11(7):2365–2381
Dayananda C, Sarada R, Usha Rani M, Shamala TR, Ravishankar GA (2007) Autotrophic cultivation of Botryococcus braunii for the production of hydrocarbons and exopolysaccharides in various media. Biomass Bioenergy 31:87–93
Demirbas A (1998) Fuel properties and calculation of higher heating values of vegetable oils. Fuel 77(9/10):1117–1120
DuBois M, Gilles KA., Hamilton JK., Rebers PA, Smith F (1956) Colorimetric Method for Determination of Sugars and Related Substances. Anal Chem 28(3):350–356
El-Shimi HI et al. (2013) Biodiesel production from Spirulina-platensis microalgae by in-situ transesterification process. Journal of Sustainable Bioenergy Systems 3(3):224
Fan J, Yan C, Andre C, Shanklin J, Schwender J, Xu C (2012) Oil accumulation is controlled by carbon precursor supply for fatty acid synthesis in Chlamydomonas reinhardtii. Plant Cell Physiol 53:1380–1390. https://doi.org/10.1093/pcp/pcs082
Fernandes B, Teixeira J, Dragone G, Vicente AA, Kawano S, Bišová K, Přibyl P, Zachleder V, Vítová M (2013) Relationship between starch and lipid accumulation induced by nutrient depletion and replenishment in the microalga Parachlorella kessleri. Bioresour Technol 144:268–274
Francisco EC, Neves DB, Jacob-Lopes E, Franco TT (2010) Microalgae as feedstock for biodiesel production: carbon dioxide sequestration, lipid production and biofuel quality. J Chem Technol Biotechnol 85:395–403
Franco-Navarro JD, Brumós J, Rosales MA, Cubero-Font P, Talón M, and Colmenero-Flores JM (2015) Chloride regulates leaf cell size and water relations in tobacco plants. J Exp Bot 67(3):873-891.
Frankel EN (1998) Lipid oxidation. Dundee: The Oily Press. Vol 10
Fung KS, Liew EWT, Ngu HLN (2013) Optimization of nutrient media composition for microalgae biomass production using central composite design. Chemeca 2013: Challenging Tomorrow, p.278
Ge Y, Liu J, Tian G (2011) Growth characteristics of Botryococcus braunii 765 under high CO2 concentration in photobioreactor. Bioresour Technol 102(1):130–134
Gopinath A, Puhan S, Nagarajan G (2009) Relating the cetane number of biodiesel fuels to their fatty acid composition: a critical study. Proc Inst Mech Eng D: Journal of Automobile Engineering 223(4):565–583
Gorain PC, Bagchi SK, Mallick N (2013) Effects of calcium, magnesium and sodium chloride in enhancing lipid accumulation in two green microalgae. Environ Technol 34(13–14):1887–1894
Gorman DS, Levine RP (1965) Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A 54:1665–1669
Gouveia L, Oliveira AC (2009) Microalgae as a raw material for biofuels production. J Ind Microbiol Biotechnol 36(2):269–274
Hakkalin NLS, Paz AP, Aranda DAG, Moraes LMP (2014) Enhancement of cell growth and lipid content of a freshwater microalga Scenedesmus sp. by optimizing nitrogen, phosphorus and vitamin concentrations for biodiesel production. Nat Sci 6:1044–1054
Held P, Raymond K, (2011) Determination of algal cell lipids using Nile red—using microplates to monitor neutral lipids in Chlorella vulgaris
Ho SH, Chen CY, Chang JS (2012) Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour Technol 113:244–252
Hu Q (2004) Environmental effects on cell composition. In: Richmond, A. (Ed.), Handbook of Microalgal Culture: Biotechnology and Applied Phycology. Blackwell Science, Victoria, pp. 83–93
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639. https://doi.org/10.1111/j.1365-313X.2008.03492
Kapdan IK, Aslan S (2008) Application of the Stover–Kincannon kinetic model to nitrogen removal by Chlorella vulgaris in a continuously operated immobilized photobioreactor system. J Chem Technol Biotechnol 83:998–1005
Knothe G (2002) Structure indices in FA chemistry: how relevant is the iodine value? J Am Oil Chem Soc 79:847–854
Knothe GH (2005) Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process Technol 86:1059–1070
Knothe G (2006) Analyzing biodiesel: standards and other methods. J Am Oil Chem Soc 83:823–833
Knothe G (2008) “Designer” biodiesel: optimizing fatty ester composition to improve fuel properties. Energy Fuel 22:1358–1364
Kona R, Hemlatha M, Srivastav KV, Mohan SV (2017) Regulatory effect of Fe-EDTA on mixotrophic cultivation of Chlorella sp. towards biomass growth and metabolite production. Bioresour Technol 244:1227–1234
Krisnangkura KA (1986) A simple method for estimation of cetane index of vegetable oil methyl esters. J Am Oil Chem Soc 63:552–553
Ladommatos N, Parsi M, Knowles A (1996) The effect of fuel cetane improver on diesel pollutant emissions. Fuel 75(1):8–14
Li Y, Han D, Hua G, Dauvillee D, Sommerfeld M, Ball S, Hu Q (2010) Chlamydomonas starchless mutant defective in ADP-glucose pyrophosphorylase hyper-accumulates triacylglycerols. Metab Eng 12:387–391. https://doi.org/10.1016/j.ymben.2010.02.002
Li Y, Han D, Sommerfeld M, Hu Q (2011) Photosynthetic carbon partitioning and lipid production in the oleaginous microalga Pseudochlorococcum sp. (Chlorophyceae) under nitrogen-limited conditions. Bioresour Technol 102:123–129. https://doi.org/10.1016/j.biortech.2010.06.03
Liu X, Sheng J, Curtiss R III (2011) Fatty acid production in genetically modified cyanobacteria. Proc Natl Acad Sci U S A 108:6899–6904. https://doi.org/10.1073/pnas.1103014108
Los DA, Murata N (2004) Membrane fluidity and its roles in the perception of environmental signals. Biochim Biophys Acta Biomembr 1666(1):142–157
Lv JM, Cheng LH, Xu XH, Zhang L, Chen HL (2010) Enhanced lipid production of Chlorella vulgaris by adjustment of cultivation conditions. Bioresour Technol 101(17):6797–6804
Martin W, Scheibe R, Schnarenberger C (2000) The Calvin cycle and its regulation. In: Leegood RC, C R, Shakey TD, von Caemmerer S (eds) Photosynthesis: Physiology and Metabolism. Kluwer Academic Publishers, Dordrecht, pp 9–51
Mayers JJ, Flynn KJ, Shields RJ (2014) Influence of the N:P supply ratio on biomass productivity and time-resolved changes in elemental and bulk biochemical composition of Nannochloropsis sp. Bioresour Technol 169:588–595
Nelson DL, Cox MM (2008) Lipid biosynthesis. In: Principles of biochemistry, 4th edn. W. H. Freeman and Company, New York, pp 805–845
Nichols HW, Bold HC (1965) Trichosarcina polymorpha gen. et sp. nov. J Phycol 1(1):34–38
Oijen VT, Leeuwe VMA, Gieskes WW, de Baar HJ (2004) Effects of iron limitation on photosynthesis and carbohydrate metabolism in the Antarctic diatom Chaetoceros brevis (Bacillariophyceae). Eur J Phycol 39(2):161–171
Radakovits R, Jinkerson RE, Darzins A, Posewitz MC (2010) Genetic engineering of algae for enhanced biofuel production. Eukaryot Cell 9:486–501
Rakesh S, Dhar DW, Prasanna R, Saxena AK, Saha S, Shukla M, Sharma K (2015) Cell disruption methods for improving lipid extraction efficiency in unicellular microalgae. Eng Life Sci 15(4):443–447. https://doi.org/10.1002/elsc.201400222
Ramírez-Verduzco LF, Rodriguez-Rodriguez JE, Jaramillo-Jacob AR (2012) Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel 91:102–111
Ramos MJ, Fernández CM, Casas A, Rodríguez L, Pérez Á (2009) Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol 100(1):261–268
Raven JA (2016) Chloride: essential micronutrient and multifunctional beneficial ion. J Exp bot 68(3):359–367.
Ruangsomboon S (2015) Effects of different media and nitrogen sources and levels on growth and lipid of green microalga Botryococcus braunii KMITL and its biodiesel properties based on fatty acid composition. Bioresour technol 191:377–384
Ruangsomboon S, Ganmanee M, Choochote S (2013) Effects of different nitrogen, phosphorus, and iron concentrations and salinity on lipid production in newly isolated strain of the tropical green microalga, Scenedesmus dimorphus KMITL. J Appl Phycol 25:867–874
Saraf S, Thomas B (2007) Influence of feedstock and process chemistry on biodiesel quality. Process Saf Environ Prot 85:360–364
Sarin A, Arora R, Singh NP, Sarin R, Malhotra RK, Kundu K (2009) Effect of blends of palm-jatropha-pongamia biodiesels on cloud point and pour point. Energy 34:2016–2021
Sarpal AS, Costa ICR, Teixeira CMLL, Filocomo D, Candido R et al (2016) Investigation of biodiesel potential of biomasses of Microalgaes chlorella, spirulina and tetraselmis by NMR and GC-MS techniques. J Biotechnol Biomater 6:220
Sharma KK, Schuhmann H, Schenk PM (2012) High lipid induction in microalgae for biodiesel production. Energies 5:1532–1553
Siaut M, Cuine S, Cagnon C, Fessler B, Naguyen M, Carrier P, Beyly A, Beisson F, Triantaphylidès C, Li-Beisson Y, Gilles P (2011) Oil accumulation in the model green alga Chlamydomonas reinhardtii: characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnol 11:7
Song M, Pei H, Hu W, Ma G (2013) Evaluation of the potential of ten microalgal strains for biodiesel production. Bioresour Technol 141:245–251
Sorokin C (1959) Tabular comparative data for the low-and high-temperature strains of chlorella. Nature 184:613–614
Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35:171–205
Tetlow IJ, Morell MK, Emes MJ (2004) Recent developments in understanding the regulation of starch metabolism in higher plants. J Exp Bot 55:2131–2145
Work VH, Radakovits R, Jinkerson RE, Meuser JE, Elliott LG, Vinyard DJ, Laurens LML, Dismukes GC, Posewitz M (2010) Increased lipid accumulation in the Chlamydomonas reinhardtii sta7-10 starchless isoamylase mutant and increased carbohydrate synthesis in complemented strain. Eukaryot Cell 9:1251–1269. https://doi.org/10.1128/EC.00075-10
Yeh KL, Chang JS (2012) Effects of cultivation conditions and media composition on cell growth and lipid productivity of indigenous microalga Chlorella vulgaris ESP-31. Bioresour Technol 105:120–127
Acknowledgements
The authors are grateful to Director, IARI, New Delhi, for essential facilities.
Funding
The study is financially supported by the Department of Biotechnology, Govt. of India under Indo-Denmark Collaborative Project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vishwakarma, R., Dhar, D.W. & Saxena, S. Influence of nutrient formulations on growth, lipid yield, carbon partitioning and biodiesel quality potential of Botryococcus sp. and Chlorella sp.. Environ Sci Pollut Res 26, 7589–7600 (2019). https://doi.org/10.1007/s11356-019-04213-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04213-2