Abstract
This research paper presents the results of an experimental investigation of the degradation of three different contaminants including progesterone (PGS), ibuprofen (IBU), and naproxen (NAP) using ZnO as the photocatalyst and ultraviolet (UV) light as a source for catalysts activation. Two operating parameters, namely, catalyst loading and initial concentration of contaminants, were tested in a batch photocatalytic reactor. To demonstrate the large-scale applications, experiments were also conducted in a submerged membrane photocatalytic reactor. It has proven that ZnO photocatalyst degraded the three contaminants very efficiently under almost all the studied experimental conditions, with efficiency rates of 92.3, 94.5, and 98.7 % for PSG, IBU, and NAP, respectively. The photodegradation kinetics study was performed to calculate the reaction rate constant, which is found to follow pseudo-first order kinetics. The membrane photocatalytic reactor was efficient to remove pollutants and it is observed that the degradation rate increases with increasing the membrane oscillation frequency approaching that of the stirred reactor.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The release of micro-pollutants such as hormones, pesticides, pharmaceutically active compounds, and personal care products to the environment has been a growing concern that attracted much attention from the public and the scientific community due to their adverse impact on human health, plants, soil, and aquatic systems. Many studies showed that their toxicological effects persist even at low concentrations of nano- to micrograms per liter and becomes further complicated by their presence as a mixture (Nigam et al. 2001; Wang et al. 2015). These compounds can be either synthetic or natural chemicals that generally include a class of chemical contaminants found in prescription medicines, over-the-counter therapeutic drugs, cosmetics, plastic additives, and other industrial products (Daughton and Ternes 1999; Mestre et al. 2007). The rapid increase in population together with the growth of urbanization and industrialization increases the demand for the production and consumption of such products and subsequently raises their levels in water sources due to improper disposal and treatment of municipal and industrial effluents (Rudd et al. 2016). Several research studies have confirmed the presence of pharmaceuticals and hormones in various aquatic systems such as rivers, lakes, groundwater, sewage treatment plant (STP) effluents, and consequently in drinking water (Bortey-Sam et al. 2015; Hanna-Attisha et al. 2015).
Among the micro-pollutants that have been identified as an ongoing issue in many water quality studies are endocrine-disrupting compounds (EDCs) as well as pharmaceutical products such as ibuprofen and naproxen (Han et al. 2012; Liu et al. 2013; Ohko et al. 2002). It has been shown that EDCs, such as estrone, (E1), 17β-estradiol (E2), estriol (E3), 17α-ethinylestradiol (EE2), and progesterone (PGS) can interfere with the normal functioning of the endocrine system resulting in adverse effects on human health that include reproductive and sexual abnormalities, decline in sperm count, neurological disorders, and increased incidents of testicular, prostate, ovarian, and breast cancer (Brody and Rudel 2003; Carlsen et al. 1992; Kavlock et al. 1996). Although many environmental studies have focused on the impact and treatment of streams polluted with estrogens, less research is done on other steroids such as progesterone, which has been detected recently in many rivers and agricultural watershed worldwide (Lin et al. 2008; Manickum and John 2014). Similarly, the release of pharmaceutical products such as ibuprofen (IBU) and naproxen (NAP) and their byproducts to the environment is of an equivalent concern to the health of mankind and the aquatic life. These compounds belong to the non-steroidal anti-inflammatory drugs (NSAIDs) characterized by the carboxylic aryl acid moiety that provides their acidic properties (Georgaki et al. 2014). Toxicity studies confirmed the potential health risk to human due to long-term exposure of such compounds that could include kidney failure and stomach injuries (Hu et al. 2013). Both IBU and NAP have been detected in hospital wastewater (Kümmerer 2001), sewage treatment plants effluents (Carballa et al. 2004), soil (Scheytt et al. 2006), and seawater (Lolić et al. 2015).
The fact that the such “emerging contaminants” are being detected in the environment with rising concentrations clearly indicates that current conventional wastewater treatment techniques such as biodegradation (Joss et al. 2006), coagulation (Boyd et al. 2003), sedimentation, and filtration (Matamoros et al. 2009) are ineffective in removing them from discharged effluents. Furthermore, and beside their high energy demand, these methods can also result in the production of toxic sludge (Eccles 1999). Therefore, there is an essential need to develop advanced techniques for the removal of these compounds from water sources and aquatic systems. Among the proposed advanced treatment systems which have better removal efficiency and less waste byproducts are those based on adsorption, membrane filtration, electrodialysis, and photodegradation (Barakat 2011). The latter, either applied alone or in presence of catalyst (photocatalysis), is considered to be a very promising advanced oxidation technique owing to its high efficiency, low operating and energy costs, and ease of handling (Han et al. 2012; Mai et al. 2008; Ollis et al. 1991).
Photocatalysis involves the presence of intermediate semiconductor catalysts that absorb light photons of sufficient energy, greater than or equal to the band gap energy of the semiconductor (hν ≥ Eg) which results in the production of electrons (ecb−) and holes (hcb+) in the conduction and valence bands as electron-hole pairs (Georgaki et al. 2014). Provided that recombination does not occur, electrons and holes that migrate to the semiconductor surface can produce hydroxyl and superoxide radicals that react with and transform organic molecules causing their degradation (Ghaedi et al. 2007; Han et al. 2012; Mai et al. 2008).
Although several semiconductors, such as ZnO, TiO2, Fe2O3, and ZnS, were investigated for the degradation of several organic pollutants, TiO2 and ZnO are the most extensively studied photocatalyst in recent years. This is mainly due to their non-toxicity, flat band structure (3.35 eV approx.), spectral overlap with sunlight emission (about 5%), low cost, biological and chemical stability, and easy applicability in ambient and harsh conditions (Dong et al. 2015; Martínez et al. 2011). Recently, however, ZnO was reported to have a superior advantage over TiO2 as demonstrated by a comparative study of photocatalytic degradation of estrone in water done by J. Han et al (Han et al. 2012). They found that ZnO exhibited markedly high UV absorbance than P25 TiO2 (benchmark photocatalyst, Aeroxide® TiO2 P25 (P25TiO2)) within the wavelength range of 320–385 nm as measured by diffuse reflectance spectroscopy. Moreover, their experimental results showed that ZnO enabled a 2.05–3.0 times higher degradation rate of estrone in water than P25 TiO2 under artificial UVA irradiation. The authors attributed that to the unique open structure of ZnO which provides many positively charged sites for scavenging photo-generated electrons, therefore preventing their recombination with the holes and facilitate the redox reactions.
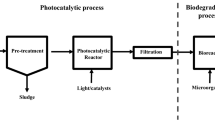
In this contribution, the photocatalytic degradation of PGS, IBU, and NAP using ZnO as a photocatalyst is investigated in a batch stirred reactor. To demonstrate the large-scale feasibility of applying the techniques for contaminant degradation, experiments were also conducted in a submerged membrane photocatalytic reactor. The latter is based on using a membrane to confine the photocatalyst within the treatment unit therefore achieving the advantages of suspended catalyst high interfacial area while eliminating the need for a catalyst recovery step. The studies involved the effect of the operating parameters on the degradation efficiency including catalyst loading and initial contaminant concentrations.
Experimental
Experimental protocol
Stock solutions of PGS, IBU, and NAP were prepared by adding 1 g of each into 1 L of methanol and stirring until complete dissolution then storing at − 5 °C for later use. The diluted solutions of micro-pollutants were prepared by adding the appropriated aliquot of the stock solution to 100 mL of deionized water to prepare (20, 40, and 80 ppm) diluted solutions. Prior to conducting the photocatalytic degradation tests, adsorption of PGS, IBU, and NAP on the catalyst (ZnO) was evaluated. In order to do so, all tests were started by adding the catalyst required amount to the diluted micro-pollutant solutions and stirring in darkness for 30 min to ensure adsorption equilibrium was achieved. All experiments were done at natural unmodified pH.
The batch stirred experiments were conducted in the reactor setup shown in Fig. 1. It consisted of a shielded 100-mL glass reactor (Pyrex) placed on top of a magnetic stirrer/heater to provide the necessary mixing to maintain a uniform catalyst suspension for the reaction. The UV lamp was placed horizontally above the reactor surface and was secured using fasteners.
The membrane reactor photocatalytic degradation experiments were conducted in the submerged membrane photocatalytic reactor shown in Fig. 2. It consisted of a rectangular cell charged with the continuous phase and an oscillatory 0.2-μm hydrophilic PVDF membrane (Microdyn Nadir GmbH) housed in a flat surface. The oscillatory motion provided both mixing for the catalyst suspension as well as surface shear to minimize catalyst deposition on the membrane surface. The UV lamp was placed in front of a quartz window located along the reactor side. A tubular arrangement was used to connect the membrane housing to a peristaltic vacuum pump driven by a variable speed motor to provide the necessary driving force for the permeate flow. The latter was measured by a McMillan flowmeter (McMillan company, model number S112) before circulating back to the reactor and was used to determine the membrane flux J. The transmembrane pressure (TMP) was maintained at 30 kPa by controlling the pump flow rate. Before each experiment, the UV lamp was warmed up for 15 min, then the reactor vessel was exposed to the warmed up lamp. The irradiation time was set at 120 min, during which multiple samples were taken at specified time intervals using a syringe filter to separate the ZnO particles from the samples before analysis.
Result and discussion
Photodegradation mechanism and pathway
The mechanism of photocatalysis of contaminants using ZnO photocatalyst in presence of UVA irradiation is shown in Fig. 3 and the associated equations (Shinde et al. 2011):
Previous studies of the different component degradation and reaction pathways under UV light irradiation showed that intermediate products are formed before near mineralization is achieved. For example, three main intermediate products have been identified to form during naproxen UV irradiation. These include 1-ethyl-6-methoxynaphthalene, 6-methoxy-2-1-ol-naphylethan, or 2-acetyl-6-methoxynaphthalene (Boscá et al. 1990; Consuelo Jiménez et al. 1997). Similarly, the investigation of ibuprofen UV degradation showed the formation of substituted phenolic compounds and aromatic carboxylic acids including 1-(4-isobutyl-phenyl)-ethanol and hydroxyl-ibuprofen (Jallouli et al. 2018; Lathasree et al. 2004). For the degradation of progesterone under direct and assisted UV exposure, the formation of intermediate products such as 4-hydroxyl butanoic acid, acetaldehyde, oxalic, and methanoic acids has been reported (Ifelebuegu et al. 2016; Méité et al. 2016).
The effect of pH
Since component decomposition takes place on the photocatalyst surface, the degradation process is strongly influenced by the component adsorption to the catalyst surface. The latter varies depending on the solution pH, the catalyst point of zero charge (PZC), and the component molecular structure. The interaction of a photocatalyst with cationic compounds would be favored at pH greater than the photocatalyst PZC, while anionic compounds would interact more favorably at pH less than the catalyst PZC. Since the PZC of ZnO is ~ 9.07, its surface is negatively charged above pH ~ 9. This would tend to have repulsive interactions of compounds such as naproxen and ibuprofen due to the deprotonation of both components at high pH (pKa ~ 4.2 and ~ 4.9 of naproxen and ibuprofen, respectively). For progesterone on the other hand, and since it is neutral, its adsorption will unlikely be affected by the change in pH. In view of the above, all experiments were done at neutral unmodified pH.
Batch stirred photocatalytic degradation
The effect of micro-pollutant initial concentration and catalyst loading was studied and discussed as the main parameters in heterogeneous photocatalysis. The photodegradation efficiency was calculated using the following equation:
where, co and c are the contaminant concentrations at initial time and time (t), respectively (mg/L). As can be seen from Fig. 4a–c, ZnO photocatalyst degraded the three contaminants very efficiently under almost all the studied experimental conditions with the efficiency rates of 92.3, 94.5, and 98.7 % for PSG, IBU, and NAP, respectively.
Effect of contaminant initial concentration
Figure 5a–c represents the concentration removal-time profiles of each contaminant as a function of initial concentration at a catalyst loading of 1 g/L ZnO. As shown in Fig. 5a–c, the photocatalytic degradation rate increases with increasing the initial contaminant concentration, which agrees with the results observed by previous investigators (Han et al. 2012).
Effect of catalyst loading
The effect of catalyst loading is an important factor in heterogeneous photocatalysis process. The concentration removal-time profiles of each contaminant as a function of catalyst loading at 2 g/L of contaminant initial concentration are presented in Fig. 6a–c. The results are in good agreement with previously published studies (Georgaki et al. 2014; Han et al. 2012). The photodegradation efficiency increases with increasing the catalyst loading due to the increase in the active sites in contact with the target contaminant. However, above the catalyst loading of ~ 1–1.5 g/L, the efficiency decreases due to several factors. These include a reduction in light transmission due to higher turbidity, increased light scattering, reduction in the surface area available for the light to promote the generation of h+/e− pairs due to particle agglomeration, and surface deactivation caused by particle collisions (Georgaki et al. 2014).
Reaction kinetics
The kinetics of photocatalytic degradation of organic dyes usually follows the Langmuir-Hinshelwood mechanism (Rahman et al. 2013):
in which k′ is the rate constant, Ka the adsorption constant of the reactant on the photocatalyst, c the reactant concentration, and t the time. At low concentration, Eq. (1) can be integrated as
where co is the initial concentration of the reactant and k = kKa is the apparent rate constant given by the slope of the graph of ln (co/c) versus t. The linear relations shown in Fig. 7d for the different components are in agreement with the Langmuir-Hinshelwood behavior model for describing the reaction kinetics and can be used to estimate the rate constant.
Figure 7b–d shows the change in k of the different components with the initial concentration. The results are also summarized in Table 1 for all the investigated experimental conditions. It can be seen that naproxen have the highest rate constant compared to progesterone and ibuprofen. Furthermore, the R2 values confirm that the reaction followed a pseudo-first-order kinetics with an increase in k with increasing the initial concentration for the range of experimental conditions used in this study.
It must be pointed however that such trend may not necessarily extend to higher concentration values since more available surface contact would be required between the targeted compounds and catalyst surface at elevated concentrations (Han et al. 2012; Xiang et al. 2016). As a result, the contaminants could occupy all the active sites on the catalyst surface which would lead to zero-order kinetics (Pouretedal et al. 2009). The fact that a pseudo-first-order kinetics was observed in this study indicates that the initial concentrations used were below the critical values which would have led to zero-order kinetics.
Component degradation in membrane photocatalytic reactor
The experimental measurements of the photocatalytic degradation of NAP, IBU, and PGS in an oscillatory membrane reactor are shown in Fig. 8 together with data from our previous studies of photocatalytic degradation of methylene blue dye contaminant (MEB) using the same experimental conditions and setup (Neppolian et al. 2002; Zhang et al. 2017). The experiments were conducted for a component initial concentration of 100 mg/L and catalyst loading of 1 g/L. The plot is presented in terms of the effect of the oscillation on the dimensionless degradation factor ξ defined as
in which kmr and kst are the component rate constants in the membrane and stirred reactors, respectively. The choice of the above ratio as a degradation factor is based on the assumption that the degradation rate in the stirred reactor would represent the maximum achievable rate under the specified experimental condition. This may be justified based on several factors. First is the higher energy dissipation per unit volume in the stirred reactor arrangement which results in efficient catalyst suspension and maximizes the catalyst-reactant interfacial area and consequently the reaction rates. Second is the near-full utilization of the catalyst load compared to the membrane reactor where catalyst unavailability occurs due to both sedimentation and membrane pore entrapments. Third is the the closer proximity of the UV light to the reaction medium and absence of barriers in the stirred reactor, which provides higher photonic energy per unit reactor volume. As can be seen, the data shown in the plot supports the above assumptions where the degradation rate in the membrane reactor is low at low oscillation intensities and increases with increasing the membrane oscillation frequency approaching that of the stirred reactor. Such an effect could not be attributed to mass transfer enhancement since the latter typically does not limit the rate of heterogeneous catalysis involving slurries of fine catalyst particles due to the very small diffusion length scale. Accordingly, the other most probable explanation for such observation would be related to the increase of the catalyst concentration fraction in suspension due to the decrease in both catalyst sedimentation as well as the deposited catalyst layer on the membrane surface. The latter is further supported by the observed increase in the membrane flux (as shown in Fig. 8), which increases with increasing the oscillation intensity indicating lower catalyst deposition on the membrane surface. Such dual augmenting effect on both permeate flux and the photocatalytic reaction rate would result in enhancing the overall process productivity.
Conclusions
In this study, the photodegradation of three pharmaceutical contaminants, progesterone (PGS), ibuprofen (IBU), and naproxen (NAP) in water, was studied be means of artificial UVA. Several experimental parameters, such as initial contaminant concentration and catalyst loading, were investigated. The results of the investigation showed that ZnO photocatalyst is highly efficient photocatalyst with photodegradation efficiency of up to 92.3, 94.5, and 98.7 % for PSG, IBU, and NAP after 120 min of UVA irradiation, respectively. The photodegradation kinetics followed a pseudo-first-order kinetics model with satisfactory regression coefficient. Moreover, it was found that the rate constant increases with increasing the initial contaminant concentration and decrease with increasing catalyst loading above an optimal value. By comparing the rate constant of the three contaminants, the NAP photocatalytic degradation was found to be overall faster than the two other remaining contaminants. This indicates that NAP is highly reactive under UVA irradiation. Finally, and based on results of the component degradation in a membrane photocatalytic reactor, it is concluded that the photocatalysis of pharmaceutical compounds may be considered as a promising advanced process for wastewater treatment applications.
References
Barakat MA (2011) New trends in removing heavy metals from industrial wastewater. Arab J Chem 4:361–377. https://doi.org/10.1016/j.arabjc.2010.07.019
Bortey-Sam N, Nakayama SMM, Ikenaka Y, Akoto O, Baidoo E, Mizukawa H, Ishizuka M (2015) Health risk assessment of heavy metals and metalloid in drinking water from communities near gold mines in Tarkwa, Ghana. Environ Monit Assess 187:397. https://doi.org/10.1007/s10661-015-4630-3
Boscá F, Miranda MA, Vañó L, Vargas F (1990) New photodegradation pathways for naproxen, a phototoxic non-steroidal anti-inflammatory drug. J Photochem Photobiol A Chem 54:131–134. https://doi.org/10.1016/1010-6030(90)87018-7
Boyd GR, Reemtsma H, Grimm DA, Mitra S (2003) Pharmaceuticals and personal care products (PPCPs) in surface and treated waters of Louisiana, USA and Ontario Canada. Sci Total Environ 311:135–149
Brody JG, Rudel RA (2003) Environmental pollutants and breast cancer. Environ Health Perspect 111:1007–1019
Carballa M, Omil F, Lema JM, Llompart Ḿ, Garcı́a-Jares C, Rodrı́guez I, Gómez M, Ternes T (2004) Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Res 38:2918–2926. https://doi.org/10.1016/j.watres.2004.03.029
Carlsen E, Giwercman A, Keiding N, Skakkebaek NE (1992) Evidence for decreasing quality of semen during past 50 years. Br Med J 305:609–613
Consuelo Jiménez M, Miranda MA, Tormos R (1997) Photochemistry of naproxen in the presence of β-cyclodextrin. J Photochem Photobiol A Chem 104:119–121. https://doi.org/10.1016/S1010-6030(97)00013-0
Daughton CG, Ternes TA (1999) Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ Health Perspect 107:907–938
Dong S, Feng J, Fan M, Pi Y, Hu L, Han X, Liu M, Sun J, Sun J (2015) Recent developments in heterogeneous photocatalytic water treatment using visible light-responsive photocatalysts: a review. RSC Adv 5:14610–14630. https://doi.org/10.1039/C4RA13734E
Eccles H (1999) Treatment of metal-contaminated wastes: why select a biological process? Trends Biotechnol 17:462–465. https://doi.org/10.1016/S0167-7799(99)01381-5
Georgaki I, Vasilaki E, Katsarakis N (2014) A study on the degradation of carbamazepine and ibuprofen by TiO2 and ZnO photocatalysis upon UV/visible-light irradiation. Am J Anal Chem 05:518–534. https://doi.org/10.4236/ajac.2014.58060
Ghaedi M, Ahmadi F, Shokrollahi A (2007) Simultaneous preconcentration and determination of copper, nickel, cobalt and lead ions content by flame atomic absorption spectrometry. J Hazard Mater 142:272–278. https://doi.org/10.1016/j.jhazmat.2006.08.012
Han J, Liu Y, Singhal N, Wang L, Gao W (2012) Comparative photocatalytic degradation of estrone in water by ZnO and TiO2 under artificial UVA and solar irradiation. Chem Eng J 213:150–162. https://doi.org/10.1016/j.cej.2012.09.066
Hanna-Attisha M, LaChance J, Sadler RC, Champney Schnepp A (2015) Elevated blood lead levels in children associated with the flint drinking water crisis: a spatial analysis of risk and public health response. Am J Public Health 106:283–290. https://doi.org/10.2105/AJPH.2015.303003
Hu Y, Song C, Liao J, Huang Z, Li G (2013) Water stable metal-organic framework packed microcolumn for online sorptive extraction and direct analysis of naproxen and its metabolite from urine sample. J Chromatogr A 1294:17–24. https://doi.org/10.1016/j.chroma.2013.04.034
Ifelebuegu AO, Ukpebor J, Nzeribe-Nwedo B (2016) Mechanistic evaluation and reaction pathway of UV photo-assisted Fenton-like degradation of progesterone in water and wastewater. Int J Environ Sci Technol 13:2757–2766. https://doi.org/10.1007/s13762-016-1103-3
Jallouli N, Pastrana-Martínez LM, Ribeiro AR, Moreira NFF, Faria JL, Hentati O, Silva AMT, Ksibi M (2018) Heterogeneous photocatalytic degradation of ibuprofen in ultrapure water, municipal and pharmaceutical industry wastewaters using a TiO2/UV-LED system. Chem Eng J 334:976–984. https://doi.org/10.1016/j.cej.2017.10.045
Joss A, Zabczynski S, Göbel A, Hoffmann B, Löffler D, McArdell CS, Ternes TA, Thomsen A, Siegrist H (2006) Biological degradation of pharmaceuticals in municipal wastewater treatment: Proposing a classification scheme. Water Res 40:1686–1696. https://doi.org/10.1016/j.watres.2006.02.014
Kavlock RJ et al (1996) Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the U.S. EPA-sponsored workshop. Environ Health Perspect 104:715–740
Kümmerer K (2001) Drugs in the environment: emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources – a review. Chemosphere 45:957–969. https://doi.org/10.1016/S0045-6535(01)00144-8
Lathasree S, Rao AN, SivaSankar B, Sadasivam V, Rengaraj K (2004) Heterogeneous photocatalytic mineralisation of phenols in aqueous solutions. J Mol Catal A Chem 223:101–105. https://doi.org/10.1016/j.molcata.2003.08.032
Lin AY-C, Yu T-H, Lin C-F (2008) Pharmaceutical contamination in residential, industrial, and agricultural waste streams: risk to aqueous environments in Taiwan. Chemosphere 74:131–141. https://doi.org/10.1016/j.chemosphere.2008.08.027
Liu S, Ying G-G, Liu Y-S, Peng F-Q, He L-Y (2013) Degradation of norgestrel by bacteria from activated sludge: comparison to progesterone. Environ Sci Technol 47:10266–10276. https://doi.org/10.1021/es304688g
Lolić A, Paíga P, Santos LHMLM, Ramos S, Correia M, Delerue-Matos C (2015) Assessment of non-steroidal anti-inflammatory and analgesic pharmaceuticals in seawaters of North of Portugal: occurrence and environmental risk. Sci Total Environ 508:240–250. https://doi.org/10.1016/j.scitotenv.2014.11.097
Mai J, Sun W, Xiong L, Liu Y, Ni J (2008) Titanium dioxide mediated photocatalytic degradation of 17β-estradiol in aqueous solution. Chemosphere 73:600–606. https://doi.org/10.1016/j.chemosphere.2008.05.073
Manickum T, John W (2014) Occurrence, fate and environmental risk assessment of endocrine disrupting compounds at the wastewater treatment works in Pietermaritzburg (South Africa). Sci Total Environ 468–469:584–597. https://doi.org/10.1016/j.scitotenv.2013.08.041
Martínez C, Canle L M, Fernández MI, Santaballa JA, Faria J (2011) Kinetics and mechanism of aqueous degradation of carbamazepine by heterogeneous photocatalysis using nanocrystalline TiO2, ZnO and multi-walled carbon nanotubes–anatase composites. Appl Catal B Environ 102:563–571. https://doi.org/10.1016/j.apcatb.2010.12.039
Matamoros V, Arias C, Brix H, Bayona JM (2009) Preliminary screening of small-scale domestic wastewater treatment systems for removal of pharmaceutical and personal care products. Water Res 43:55–62. https://doi.org/10.1016/j.watres.2008.10.005
Méité L, Soro BD, Aboua NK, Vr M, Traoré KS, Mazellier P, Laat JD (2016) Qualitative determination of photodegradation products of progesterone and testosterone in aqueous solution. Am J Anal Chem Vol.07(01):22–33. https://doi.org/10.4236/ajac.2016.71003
Mestre AS, Pires J, Nogueira JMF, Carvalho AP (2007) Activated carbons for the adsorption of ibuprofen. Carbon 45:1979–1988. https://doi.org/10.1016/j.carbon.2007.06.005
Neppolian B, Choi HC, Sakthivel S, Arabindoo B, Murugesan V (2002) Solar/UV-induced photocatalytic degradation of three commercial textile dyes. J Hazard Mater 89:303–317. https://doi.org/10.1016/S0304-3894(01)00329-6
Nigam R, Srivastava S, Prakash S, Srivastava MM (2001) Cadmium mobilisation and plant availability – the impact of organic acids commonly exuded from roots. Plant Soil 230:107–113. https://doi.org/10.1023/A:1004865811529
Ohko Y, Iuchi KI, Niwa C, Tatsuma T, Nakashima T, Iguchi T, Kubota Y, Fujishima A (2002) 17β-Estradiol degradation by TiO2 Photocatalysis as a means of reducing estrogenic activity. Environ Sci Technol 36:4175–4181. https://doi.org/10.1021/es011500a
Ollis DF, Pelizzetti E, Serpone N (1991) Photocatalyzed destruction of water contaminants. Environ Sci Technol 25:1522–1529. https://doi.org/10.1021/es00021a001
Pouretedal HR, Eskandari H, Keshavarz M, Semnani A (2009) Photodegradation of organic dyes using nanoparticles of cadmium sulfide doped with manganese, nickel and copper as nanophotocatalyst. Acta Chimica Slovenica 56(2):353–361
Rahman QI, Ahmad M, Misra SK, Lohani M (2013) Effective photocatalytic degradation of rhodamine B dye by ZnO nanoparticles. Mater Lett 91:170–174. https://doi.org/10.1016/j.matlet.2012.09.044
Rudd ND, Wang H, Fuentes-Fernandez EMA, Teat SJ, Chen F, Hall G, Chabal YJ, Li J (2016) Highly Efficient luminescent metal-organic framework for the simultaneous detection and removal of heavy metals from water. ACS Appl Mater Interfaces 8:30294–30303. https://doi.org/10.1021/acsami.6b10890
Scheytt TJ, Mersmann P, Heberer T (2006) Mobility of pharmaceuticals carbamazepine, diclofenac, ibuprofen, and propyphenazone in miscible-displacement experiments. J Contam Hydrol 83:53–69. https://doi.org/10.1016/j.jconhyd.2005.11.002
Shinde SS, Shinde PS, Bhosale CH, Rajpure KY (2011) Zinc oxide mediated heterogeneous photocatalytic degradation of organic species under solar radiation. J Photochem Photobiol B Biol 104:425–433. https://doi.org/10.1016/j.jphotobiol.2011.04.010
Wang S, Dong X, Dai B, Pan M, He S, Wang J (2015) Determination of V, Cr, Cu, As, and Pb ions in water and biological samples by combining ICP-MS with online preconcentration using impregnated resin. J AOAC Int 98:218–224. https://doi.org/10.5740/jaoacint.11-476
Xiang Y, Fang J, Shang C (2016) Kinetics and pathways of ibuprofen degradation by the UV/chlorine advanced oxidation process. Water Res 90:301–308. https://doi.org/10.1016/j.watres.2015.11.069
Zhang C, Sabouni R, Shao Y, Gomaa HG (2017) Performance of submerged oscillatory membrane photoreactor for water treatment. J Environ Chem Eng 5:3330–3336. https://doi.org/10.1016/j.jece.2017.06.046
Funding
The authors are grateful for the American University of Sharjah for the financial support Grant # FRG15-R-41.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Suresh Pillai
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM1
(TIF 4551 kb)
Rights and permissions
About this article
Cite this article
Sabouni, R., Gomaa, H. Photocatalytic degradation of pharmaceutical micro-pollutants using ZnO. Environ Sci Pollut Res 26, 5372–5380 (2019). https://doi.org/10.1007/s11356-018-4051-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-4051-2