Abstract
Sedimentary soil was selected as the original sample (SOS). The adsorption fractions were obtained by the removal of dissolved organic matter (SRDOM), removal of minerals (SRM), removal of free fat (SRLF), and removal of nonhydrolyzable organic carbon (SNHC) respectively to investigate the adsorption characteristic of oxytetracycline (OTC) by different fractions of organic matter in sedimentary soil. The adsorption mechanism was investigated by elemental analysis, infrared spectra, and UV-visible spectroscopy. The results showed that the DOM in the sedimentary soil inhibited the adsorption of OTC, but the adsorption of different fractions of organic matter was quite different. The sorption kinetics of OTC were fitted to the pseudo-second-order model and the adsorption capacity of each fraction was: SNHC≈SRDOM > SOS > SRLF> SRM. The adsorption processes of OTC by different fractions were spontaneous. Alkaline pH condition had an effect on the adsorption of four fractions except for SNHC, while neutral and acidic pH affects SOS and SRDOM more obviously, the SNHC fraction was almost free from pH varies. Mechanism analysis showed that the main factors determining the adsorption capacity were the aromaticity and polarity of organic matter fractions. For the organic matter–based fractions (SRM, SRLF, and SNHC), the adsorption coefficient was positively correlated with the aromaticity. Furthermore, for SOS and SRDOM based on inorganic minerals, it was not only related to aromaticity, but also the content and composition of inorganic minerals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
At present, the abuse of antibiotics has become a global problem (Briones et al. 2016). After antibiotics are ingested by the human or animal body, they cannot be completely absorbed. Up to 80–90% of the antibiotics are excreted with urine or feces, and then enter the environment (Kümmerer 2009). Due to the continued use of antibiotics, antibiotics remain in the environment for a long time, seriously endangering the environment and human health. Thus, it is of great significance to study the migration characteristics of antibiotics in the environment for a better understanding of the ultimate fate of antibiotics.
It is known that adsorption/desorption is an important process affecting the migration and transformation of antibiotics in the soil and sediments. A number of studies have shown that the physicochemical properties and components of soils and sediments can influence the adsorption behavior of antibiotics (ter Laak et al. 2006), such as soil and sediment composition (Sun and Zhang 2011), surface structure (Bao 2013; Okaikue-Woodi et al. 2018), charge characteristics (Jones et al. 2005; Leal et al. 2013; Tolls 2001), organic matter content (Zhao et al. 2011), etc. Related research showed that the organic matter in the soil played a key role in the adsorption of antibiotics (Figueroa et al. 2004; Figueroa and Mackay 2005; Mackay and Canterbury 2005; Wang et al. 2018b). Because the surface had a large number of functional groups, they would exhibit a certain polarity, causing the soil to carry a large amount of negative charge (Bao et al. 2009). Special functional groups such as -NH(CH3)2, -OH, and -CONH in antibiotics can be adsorbed to the surface of the soil by electrostatic interaction (Zhao et al. 2012), hydrogen bond (Kolz et al. 2005), synergistic interaction, and complexation mechanism (Gu et al. 2007). Therefore, it is necessary to study the behavior and mechanism of adsorption of antibiotics on the organic matter in the soil.
Related studies have shown that (Zhang et al. 2008) the content of organic matter was one of the decisive factors affecting the ability of the soil to adsorb antibiotics. Ren et al. (2008) showed that increasing the soil organic matter content can enhance the adsorption of PAHs in the soil. Kulshrestha et al. 2004 found that low concentration of organic matter promoted the adsorption of antibiotics onto the soil, while high concentrations of organic matter promoted desorption of antibiotics on the soil. However, some studies have shown that the amount of organic contaminants adsorbed by the original soil was significantly greater than that of the organic matter removed (Bao et al. 2009). In other words, the soil’s own DOM, i. e., the endogenous DOM, inhibited soil adsorption of organic pollutants (Yu et al. 2011). The above literature studies showed that although organic matter played an important role in the adsorption of pollutants, high organic matter contents did not necessarily promote the adsorption of organic pollutants. Perhaps it had a considerable relationship with its composition and structure. Further research is still needed.
The composition of organic matter in sediments is diverse and complex, which is composed of animal and plant residues and microorganisms and their decomposition and synthesis. At the beginning, LeBoeuf and Weber (1997) found that the humic acid organic matter in the soil/sediments can be divided into a rubbery state equivalent to a soft carbon organic component and a glassy state relative to a hard carbon organic component. Two different forms of organic matter components can be converted to each other under specific conditions. The adsorption and desorption of hydrophobic organic pollutants in a rubber state can be described by a linear distribution model, while the adsorption and desorption in glass state were non-linear. Later, people found that organic matter was mainly composed of proteinaceous substances, polysaccharides, fatty acids, and alkanes (Nebbioso and Piccolo 2013). Some scholars have also begun to pay attention to the adsorption behavior of different fractions of organic matter on pollutants (Wang et al. 2018c). Studies have shown that light fraction (LF) organic matter in soil or sediment had a much higher enrichment capacity for PAHS than a heavy fraction (HF) (Ni et al. 2008; Wu et al. 2004). Aiken found that the higher contents of aromatic components in the soil organic matter structure, the stronger the adsorption capacity of hydrophobic organic pollutants such as PAHs (Aiken et al. 1986). Ran et al. (2007) reported that fatty carbon contributed significantly to the adsorption of hydrophobic organic contaminants. However, most studies focused on the adsorption behavior of different organic fractions on hydrophobic organic pollutants (Zhao and Bi 2014). Antibiotics are hydrophilic organic pollutants, which are typical ionic organic compounds. According to the chemical structure, antibiotics can be divided into aminoglycosides, β-lactams, tetracyclines, quinolones, macrolides, polypeptides, amide alcohols, lincosamides, etc. (Cheng 2016). Although the adsorption properties of different fractions of sediments on pollutants such as sulfamethazine (Guo et al. 2016), ciprofloxacin (Mutavdžić Pavlović et al. 2017), and levofloxacin (Qin et al. 2018) have been exhibited, few studies have analyzed the behavior and mechanism of adsorption of tetracyclic antibiotics on different fractions of organic matter in sediments. It can be seen that the adsorption of tetracycline antibiotics by organic matter in different fractions of sedimentary soil can help to fully understand the adsorption behavior of organic matter on hydrophilic organic pollutants.
In this study, the Weihe River sediment was selected as the original sample, and four kinds of fractions were obtained by fractional treatment. Oxytetracycline (OTC) was selected as the representative hydrophilic organic pollutants. The batch sorption experiments were conducted to learn adsorption kinetics, thermodynamics, and isotherm of different fractions. The elemental analysis, infrared spectra, and UV-visible spectroscopy were employed to reveal the mechanism and further understand the environmental behavior of hydrophilic organic pollutants in the soil.
Materials and methods
Sample analysis and treatment
Sedimentary soil (the sediment from the Weihe River bed) (34° 23′ 41.86″ N, 108° 52′ 38.99″ E) and humus soil (forest soil from plant debris) (34° 25′ 14.45″ N, 109° 1′ 1.46″ E) from 0 to 20 cm depth were collected as the original sample (OS). After the impurities in the sample were removed, it was placed in a ventilated dark place and dried naturally to constant weight then ground to pass through an 80-mesh sieve, sealed then stored in low-temperature before use (oxytetracycline was not detected in all samples). The different fractions of organic matter were obtained in sedimentary soil according to the reference literatures with some changes (Gélinas et al. 2001; Hu et al. 2006) and the brief process showed in Fig. 1.
Removal of DOM in the first step (water extraction): 30 g humus and sediments were weighed in a 500-mL conical flask and 300 mL of ultrapure water was added respectively, after shaken at 160 rpm for 24 h on an HSH-H shaker (Zhao et al. 2017), the mixture was centrifuged at 6000 rpm for 30 mins. The supernatant was passed through a 0.45-μm filter, the acquired filtrate was the soil DOM solution, and the DOM solution was placed in a brown bottle at a low temperature (4 °C). The centrifuged solid was dried at 50 °C that is the RDOM fraction.
The second step was to remove mineralization (carbonates and silicates): The obtained RDOM samples were first dissolved in 1 M HCl in a 500-mL conical flask. Carbonate was removed by shaking at 150 rpm for 24 h and centrifuged at 6000 rpm for 30 min. Silicate minerals were then removed using two successive 4-day shaking (150 rpm) with a mixture of 1 M HCl and HF 10% at room temperature. The samples were then centrifuged, and the supernatants after filtered were removed. The precipitate obtained by repeating these steps was washed with ultrapure water to neutral and then dried (50 °C) to obtain a demineralized fraction (RM).
The third step was to remove free fat (organic solvent extraction): The obtained HRM sample was extracted in the mixed solution (solid/liquid = 1:4, m:V) containing dichloromethane and methanol (2:1, V/V) for 15 min, centrifuged at 8000 rpm and the supernatant was then removed. The solid was obtained after these steps were repeated five times, and then dried (50 °C) to obtain a RLF fraction.
The fourth step was to remove sugar and protein (acid hydrolysis): The obtained RLF sample was hydrolyzed twice with 2 M trifluoroacetic acid (TFA, Acros) at 100 °C for 3 h in Teflon bottles, the process was conducted in duplicate. Subsequent 4 and 6 M TFA hydrolyzes were carried out at 100 °C for 18 h and centrifuged to remove the supernatant. Then, the residues hydrolyzes with 6 M HCl were carried out at 110 °C for 24 h; the centrifuged and the supernatant was then removed. Each hydrolyzes was conducted under the protection of N2, and the centrifugation was performed at 6000 rpm for 15 min every time. The obtained precipitate was washed with ultrapure water to neutral and then dried (50 °C) to obtain a NHC fraction. The total organic carbon (TOC) contents and pH values of different fractions are shown in Table 1.
Adsorption experiment
The batch sorption experiments were conducted by the Organization for Economic Co-operation and Development (OECD) method (OECD 2000). Specific operating procedures for adsorption kinetics, isothermal adsorption, and thermodynamic experiments were referred to the literature (Wang et al. 2018a).
Sorption kinetics
The 0.0500 ± 0.0005 g of different fractions samples were weighed into 10-mL tubes, and 5 ml of 10.0 mg/L OTC was added, then shaken at 150 rpm at constant temperature 303 K and pH = 7.0 in the dark. The samples were taken every 2, 4, 8, 12, 20, 24, 28, 36, and 48 h, and centrifuged at 8000 rpm for 15 min. All adsorption studies were conducted in triplicate, and a blank experiment was performed with the OTC solution. All sample operating procedures and conditions were identical to ensure the same experimental conditions.
Sorption isotherms and thermodynamics
The initial concentration of OTC ranged from 5 to 25 mg/L (5, 10, 15, 20, and 25 mg/L) for both sorption isotherms and thermodynamics experiments. The reaction temperature of the sorption thermodynamics experiment ranged from 293 K, 303 K, and 313 K, and based on the kinetic results, the adsorption reached equilibrium in 24 h. Other steps of sorption isotherms and thermodynamics and experiments were the same as the sorption kinetics described in the “Sorption kinetics” section.
Effect of pH on adsorption by different fractions
The 0.0500 ± 0.0005 g of different fraction samples were weighed into 10-mL tubes. Then, 0.1 M HCl or NaOH was added to adjust the solution pH to 2.0–10.0, and the solution pH was measured using a Mettler Toledo pH meter. Finally, 5 ml of 10.0 mg/L OTC was spiked and shook at 150 rpm at a constant temperature of 303 K.
Analysis
The contents of oxytetracycline (OTC) were determined by waters ACQUITY UPLC H-class ultra-performance liquid chromatography. Elemental analyses (C, H, and N) was conducted via a Vario ELIII elemental analyzer. Tensor 27 infrared spectroscopy was used to characterize the samples. The absorption spectrum of DOM was determined by a UV-visible spectrophotometer. The concentration of TOC in the DOM was determined by the Elementar Vario TOC analyzer.
Results and discussion
Effect on adsorption kinetics of different fractions
The adsorption experiment results to explore the adsorption capacity (Qt) of different fractions with the reaction time shown in Fig. 2. The high-adsorption rate appeared at the first 12 h, and gradually decreased until equilibrium at about 24 h. Thus, the adsorption of different fractions to sedimentary soil can divide into two phases, that is, the first fast adsorption and the following slow phase. This was consistent with the results about the tetracycline adsorption to the soil obtained by Bao (2008). Fu et al. (1990) also reported that the adsorption kinetics process of contaminates was a two-speed process consisting of rapid diffusion and slow diffusion.
The pseudo-first-order and pseudo-second-order model were employed. The quasi-first-order and quasi-second-order kinetic models were used to fit the kinetic experimental data. The linearized form of the pseudo-first-order kinetic model is given as follows:
Where, Qe (mg/kg) and Qt (mg/kg) are the OTC adsorption amounts onto different fractions at equilibrium and at t time, respectively. K1 (1/h) is the rate constant of the pseudo-first-order kinetic model.
The linearized form of the pseudo-second-order kinetic model is also given as follows:
Where K2 (kg/mg·h) is the rate constant of the pseudo-second-order kinetic model. The corresponding regression analysis results are shown in Table 2. Results showed that the sorption kinetics of OTC was better fitted to pseudo-second-order model because of the higher R2 and lower value of RSS/dof, which indicated that adsorption of OTC by different fractions may contain multiple adsorptions. In addition, in the pseudo-second-order kinetic model, the adsorption rate constant K of SRM, SRLF, and SNHC is greater than SOS and SRDOM, which may be related to the specific surface area, microstructure, and composition of the sample after classification.
Effect on isothermal adsorption of different fractions
Effect of different fractions of organic matter on OTC adsorption
Freundlich and Langmuir isotherm models, which can be applicable to describe heterogeneous systems and reversible adsorption, respectively, were employed to fit the experimental data of OTC. Both Langmuir and Freundlich equations were suitable to fit the OTC sorption isotherms. The regression coefficients R2 are both greater than 0.92., while Langmuir isotherm model provided a better fit because of a greater regression coefficient (R2 > 0.94) and lower value of RSS/dof. The adsorption capacity of each fraction was: SNHC ≈ SRDOM > SOS > SRLF > SRM (Fig. 3, Table 3). Therefore, the adsorption of OTC in different fractions of organic matter in the sedimentary soil was closer to the monolayer adsorption theory. Zhao et al. reported that the isothermal adsorption of sediments to OTC can be better fitted by the Langmuir equation (Zhao 2013). The dimensionless constant RL can be used to illustrate the nature of Langmuir adsorption. The expression of RL is as follows (Wahab et al. 2010):
KL and C0 are the Langmuir constant (L/mg) and the initial concentration (mg/L) of the adsorption experiment, respectively. RL can be used to assess the adsorption process. When RL = 0, it indicates that the adsorption process is irreversible; when 0 < RL < 1, it means favorable adsorption; when RL = 1, it indicates that the adsorption process is linear adsorption; when RL > 1, it is not favorable to adsorption (Liu et al. 2010). In this study, all the calculated RL located at 0 < RL < 1, which indicated that the adsorption was conducive to the occurrence. The larger value of KL was favorable for the monolayer adsorption. The KL values of SOS, SRDOM, SRLF, and SRM SNHC were 0.0611, 0.0247, 0.0281, 0.0321, and 0.0196, respectively, so SOS was favorable for monolayer adsorption, while SNHC was difficult. This indicated that the mechanism of adsorption of OTC by different fractions of organic matter in sedimentary soil was different.
Effect of DOM on the adsorption of OTC
In order to further explore the mechanism of the adsorption of organic matter on OTC, humus soil was selected to compare with sedimentary soil. The detail sample information was mentioned in the “Sample analysis and treatment” section. The organic matter in two soils was extracted with water to obtain four samples: SOS, SRDOM, HOS (humus soil), and HRDOM (removal of DOM from humus soil). The adsorption experiments of OTC at different initial concentrations were carried out with four samples. The linear adsorption isotherms of the soil before and after the removal of DOM of humus and DOM of sedimentary soil were shown in Fig. 4. Comparing the adsorption isotherms of HOS and HRDOM, it can be clearly seen that with the increase of the initial concentration of OTC, the adsorption capacity of HOS containing DOM was higher than HRDOM, indicating that DOM in humus soil promoted the adsorption of OTC. However, comparing the adsorption isotherms of SOS and SRDOM, after removing DOM, SRDOM had a higher adsorption capacity for OTC than SOS, which indicated that DOM in sedimentary soil inhibited the adsorption of OTC. The reason for these two phenomena may be related to the functional group type, composition, and structure of the DOM of the humus and sedimentary soil. Some studies have also found that different sources and types of DOM have large differences in composition and structure, which resulted in different effects on the adsorption of pollutants. For example, Zhao et al. (Zhao and Bi 2014) showed that there were significant differences in the contents of hydrophilic and hydrophobic components in DOM from different sources.
Effect on adsorption thermodynamic of different fractions
Thermodynamic parameters including the Gibbs free energy (ΔG), enthalpy (ΔH), entropy (ΔS), and distribution coefficient (K0) were calculated to estimate the nature of OTC sorption. ΔH and ΔS were obtained from the slope and the intercept of the plots of log K0 versus 1/T (Fakhri and Adami 2014) (figure not given). The calculated thermodynamic parameters for OTC sorption on all investigated different fractions of organic matter in sedimentary soils were listed in Table 3. Thermodynamic parameters were determined by using the following equations.
Yu et al. (2004) reported that the change of free energy for physical adsorption was smaller than that for chemisorption. The former is in the range of 0 to − 20 kJ/mol and the latter is in the range of − 80 to − 400 kJ/mol. The value of ΔG ranged from − 3.25 to − 10.92 kJ/mol for the adsorption process under three temperatures (293 K, 303 K, and 313 K), illustrating that the adsorption process of OTC by different fractions of organic matter in sedimentary soil was spontaneous. The changes of ΔH may indicate the binding mechanisms including physisorption (ΔH < 40 kJ/mol) and chemisorption (ΔH > 40 kJ/mol) (Li et al. 2014). Most values of ΔH (ΔH < 40 kJ/mol, Table 4) indicated that OTC sorption onto sedimentary soil samples can be mainly attributed to physisorption. All the ΔH are greater than 0, indicating that the adsorption process is endothermic. In a word, raising the temperature will promote the reaction to move in a positive direction.
Effect of pH
The pH effect on adsorption showed in Fig. 5. Alkaline pH condition had an effect on the adsorption of four fractions except for SNHC, while neutral and acidic pH affected SOS and SRDOM more obviously, the SNHC fraction almost free from pH varies. The pH-dependent sorption characteristics of OTC to adsorptive fractions may cause by OTC speciation and the fractions surface charge. In general, OTC bears different charges on different sites depending on solution pH. At pH below 3.3, the dimethyl ammonium group was protonated, resulting in a cationic form of + 0 0. Between pH 3.3 and 7.7, it existed as a zwitterion, + − 0, due to the loss of proton from the phenolic diketone moiety. When pH was above 7.7, it was presented as a monovalent anion, + − −, or a divalent anion, 0 − −, due to the loss of protons from the tricarbonyl system and phenolic diketone moiety (Chang et al. 2009; Kulshrestha et al. 2004). In addition, the surface charge of soil and sediments also related to the pH value of the zero point of charge (pHzpc), that is, when the pH > pHzpc, soil, and sediment surfaces are negatively charged, while positively charged when the pH < pHzpc (Hendershot et al. 1979; Sakurai et al. 1989). The zero point of different adsorptive fractions was 5.12–5.65.
To the SOS sample, when the pH was lower than 3.00, the OTC existed as OTCH3+, the sample surface was positively charged, the electron static repulsive force between OTCH3+, and positively charged HOS would reduce the contact of OTC and sample, resulting in a lower sorption capacity of OTCH3+ at pH below pH 3.00. Similarly, when the pH was higher than 7.00, the electron static repulsive force would also lead to a reduce adsorption of OTC, OTC mostly exists as OTCH− and OTC2− and the SOS sample surface was the negatively charged this time. The maximum adsorption appeared at pH 6.0–7.0. However, the maximum adsorbed OTC came from the complexation between the OTC and the absorbent rather than the OTCH20 from the electrostatic attraction. The effect of pH on the SRDOM-adsorbed OTC was consistent with the SOS sample, which revealed that the DOM affects the adsorption of OTC with complexation, hydrogen bond, and hydrophobic partitioning rather than electrostatic interaction or charge change (Chen et al. 2017).
To the SRM sample, it had already known that the point of zero charge of most aluminosilicate minerals was around the pH 3.00 (Chen et al. 2012), and the electron static repulsive force would reduce the adsorption of OTC. Once it was removed, the electron static repulsive force between the mineral and contaminates no more existed, so the adsorption capacity would increase at a pH lower than 3.00. When the pH increased more than 3.00, the electron static attraction appears between the mineral and OTC, and the adsorption capacity would reduce when the minerals were removed, resulting in the adsorption capacity lower than SRDOM sample.
According to the adsorption capacity of SRLF and SNHC sample, it can be concluded that these two fractions could inhibit the adsorption of OTC. Fatty acid, polysaccharides, and protein contained a good number of oxygen-containing functional groups, such as carboxyl, phenolic hydroxyl, and lactone. Especially for the carboxyl group, as the pH further increased to 4–5, the carboxylate groups became dissociated, and water molecules might compete for the sorption sites through H-bond, leading to the sorption capacity of decreased OTC (Jia et al. 2013; Jia et al. 2014). When these groups were removed, the adsorption capacity would increase. As a result, the adsorption capacity of SRLF was higher than SRM, and the adsorption capacity of SNHC was obviously higher than SRLF when the pH increased to 4–5 or even higher. On the other hand, the sorption of OTCH2 by SNHC may be predominated by π–π electron donor–acceptor interaction of the protonated aniline ring with the π-electron rich graphene surface, referred to as π + −π EDA, rather than ordinary electrostatic cation exchange, which was not affected with pH, so the adsorption capacity at pH 2.0 ~ 10.0 was slightly changed.
Analysis of the mechanism of the adsorption
Elemental analysis
The mass composition of C, H, N, and O elements of different fractions of organic matter in the sedimentary soil was obtained by elemental analysis. The result of the atomic ratio of about H/C, (N + O)/C, and O/C are shown in Table 5. As seen from Table 5, the aroma and polarity of each fraction were significantly different.
Figure 6 showed that the SRM, SRLF, and SNHC fractions were dominated by organic matter; the adsorption coefficient Kd was positively correlated with aromaticity. In general, when the polarity increased, the adsorption coefficient should be reduced. However, as seen in Fig. 6, SNHC has increased polarity compared with SRM and SRLF, but its adsorption coefficient also increased, which may be the aromaticity of organic matter playing a leading role in adsorption. Chen et al. (2005) also showed that the adsorbent’s adsorption capacity was mainly dependent on its aromatic fraction. Although both aromaticity and polarity affected the adsorption capacity of the adsorbent, aromaticity played a leading role in the adsorption of pollutants. For SOS and SRDOM containing inorganic minerals, the adsorption capacity was not only related to aromaticity but also the content and composition of inorganic minerals. So they still show a high-adsorption capacity.
Infrared spectra
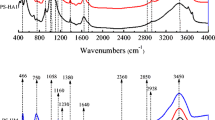
The infrared spectra of sedimentary soil before and after the adsorption of OTC are shown in Fig. 7, and the main changes in the adsorption of OTC after each fraction were: (1) Except for SNHC, the bending vibration of Si-O in all the samples between 440 and 460 cm−1 was shifted to high waves, which indicated that the N atoms in the OTC molecule may coordinate with the positively charged ions of the sample or the charge dipole occurs when the OTC molecule and the positive potential on the edge of the end face of the sample layer were electrostatically attracted. The bending vibration peak of SNHC at 455 cm−1 was shifted to 446 cm−1, and the intensity became large and the peak shape became sharp. This indicated that OTC and SNHC had a strong hydrogen bonding, which was one of the reasons for the better adsorption of SNHC (OuYang et al. 2003; Pan et al. 2011). (2) All samples (except SOS), the Al-O and Si-O bending vibration peaks between 515 and 526 cm−1, were shifted to high waves, indicating that coordination bonding, charge dipole, or charge transfer occurred. The bending vibration of Si-O or Al-O of SOS moved down to 520 at 525 cm−1, and it can be seen that OTC and SOS generated hydrogen bonding or complexation. (3) The C-H bending vibration absorption peak between 770 and 780 cm−1 was displaced downward or upward, and the C-O stretching vibration peak in the region of 990–1020 cm−1 was shifted downward, showing that the sedimentary soil in different fractions and the OTC had a hydrogen bonding or complexation and charge transfer. (4) The vibration absorption peaks of CH of SOS and SRDOM were shifted to 1474 and 1490 cm−1 at 1470 and 1477 cm−1 respectively, and the C=C stretching vibration absorption peak at 1600 cm−1 was also shifted to high waves, indicating the presence of charge transfer or coordination binding. (5) In SRM, SRLF, and SNHC, the C=C skeleton stretching vibration absorption peak shifted to high waves around 1600 and 1500 cm−1, indicating charge transfer or coordination binding. (6) In SRM, SRLF, and SNHC, the CH-outside the bending vibration peak at 690 cm−1 was displaced, and the SRM shifted to a low wave, and complexation or hydrogen bonding may occur, SRLF and SNHC moved toward high waves. Coordination or charge transfer may occur.
UV-visible spectroscopy
UV-visible spectroscopy is one of the important methods to analyze the structural characteristics of DOM in recent years, which provides a quick and convenient way to study the structure and properties of natural organic matter (Kalbitz et al. 2003). SUVA254 (specific ultraviolet absorbance 254 nm) value is widely used to characterize the contents of aromatic compounds in humus and unsaturated C=C bonds in DOM, and the larger the value, the higher the contents of humus and aromatic compounds in DOM (He et al. 2011; Shao et al. 2009). In Table 6, the SUVA254 values of HDOM and SDOM are 1.72 and 0.60, respectively, which indicated that the HDOM sample contained more aromatic substances and humus than SDOM samples, which further indicated that more aromatic compounds in the DOM of humus have an important influence on the adsorption of OTC.
The ratio of DOM absorbance at 253 to 203 nm, A253/A203, can be used to indicate the degree of substitution of the aromatic nucleus and the type of substituent. In general, when the substituent on the aromatic nucleus is mainly aliphatic chain, the ratio of A253/A203 is lower; and when the contents of carbonyl, carboxyl, hydroxyl, and ester in the substituent on the aromatic nucleus are high, the ratio of A253/A203 is higher (Korshin et al. 1997). In this study, the A253/A203 value of HDOM was 0.435, and the A253/A203 value of SDOM was 0.092. It can be seen that the aromatic nucleus substituent of HDOM contains more carbonyl, carboxyl, hydroxyl, and ester. These functional groups undergo hydrogen bonding and coordination with -C=O, -OH, -NH2, etc. in the oxytetracycline molecule, thereby the adsorption of OTC by humus soil was enhanced, while aromatic nucleus substituents of SDOM were mainly aliphatic chains. In comparison, it was not easy to interact with OTC, and it would occupy a certain adsorption site and inhibit the adsorption of OTC by sedimentary soil.
E2/E3 is the ratio of DOM absorbance at 250 nm and 365 nm, which can be used to indicate the proportion of humic acid and fulvic acid in DOM. Studies have shown that when E2/E3 < 3.5, the contents of humic acid in DOM are greater than the fulvic acid; when E2/E3 > 3.5, the contents of the fulvic acid in DOM are greater than the humic acid. The higher the E2/E3, the higher the proportion of fulvic acid (Peuravuori and Pihlaja 1997). The E2/E3 values of HDOM and SDOM were 3.89 and 4.75, respectively. It can be seen that the contents of fulvic acid in these two DOMs were greater than humic acid, and the contents of fulvic acid in SDOM were higher than HDOM. Both humic acid and fulvic acid are humic substances. Studies have found that humus not only increased the adsorption of organic pollutants by minerals as a new adsorption site but also inhibited the adsorption of organic pollutants by occupying adsorption sites and weakened the interaction between mineral surfaces and organic pollutants (Sheng et al. 2001). Therefore, HDOM promoted the adsorption of OTC as a new adsorption site, while SDOM inhibited OTC adsorption.
E4/E6 is the ratio of the absorbance of DOM at 465 to 665 nm, which can be used to indicate the degree of benzene ring C skeleton polymerization and carbonyl conjugate. When the value of E4/E6 was small, the degree of polymerization of the benzene ring C skeleton or the conjugate degree of carbonyl was higher (Strobel et al. 2001). The E4/E6 value of HDOM was 13.8, and the E4/E6 of SDOM was 6.00, which indicated that the SDOM had a higher degree of phenyl ring C skeleton polymerization or carbonyl condensation, so it was not conducive to the interaction with OTC and inhibited the adsorption of OTC. The degree of polymerization of benzene ring C skeleton or the conjugate degree of carbonyl of HDOM was relatively low, which was beneficial to its interaction with OTC molecules and promoted the adsorption of OTC.
For the overall analysis, the adsorption of OTC on each fraction of sedimentary soil mainly involved complexation, coordination, charge transfer, and hydrogen bonding. The adsorption mechanism of OTC of each fraction was similar. Nevertheless, after the adsorption of OTC, the absorption peak position and vibration intensity of some characteristic functional groups and radical groups have changed to different degrees, which indicated that the binding intensity between OTC and different fractions was varied, leading to a different adsorption capacity.
Conclusions
The adsorption behavior and mechanism of OTC by different fractions of organic matter in sedimentary soil were investigated. The following conclusions were drawn:
-
1.
Adsorption studies showed that the sorption kinetics of OTC was fitted to the pseudo-second-order model. Both Langmuir and Freundlich equations were suitable to fit the OTC sorption isotherms. The adsorption capacity of each fraction was: SNHC ≈ SRDOM > SOS > SRLF > SRM and the different fractions of adsorbed OTC are spontaneous and endothermic.
-
2.
The main factors determining the adsorption capacity were the aromaticity and polarity of organic matter fractions. For the organic matter-based fractions (SRM, SRLF, and SNHC), the adsorption coefficient was positively correlated with the aromaticity. For SOS and SRDOM based on inorganic minerals, it was not only aromaticity but also related to the content and composition of inorganic minerals. The infrared spectra revealed that there were some differences in the functional groups and radical groups of the sedimentary soil. After the adsorption of OTC by SRDOM and SNHC, the absorption peak position and vibration intensity of some characteristic functional groups and radical groups changed strongly, indicating that the interaction strength with OTC was very strong. This was just positively related to the amount of adsorption. The UV-visible spectroscopy showed that the functional group characteristics of DOM and the hydrogen bonding and coordination between OTC were the key effects of OTC adsorption. The degree of polymerization of benzene ring C skeleton or the conjugate degree of carbonyl of HDOM was relatively low, the aromatic nucleus substituent contains more carbonyl, carboxyl, hydroxyl, and ester. These functional groups undergo hydrogen bonding and coordination with -C=O, -OH, -NH2, etc. in the oxytetracycline molecule, thereby enhancing the adsorption of OTC. While aromatic nucleus substituents of SDOM were mainly aliphatic chains, it was not easy to interact with OTC.
-
3.
Alkaline pH condition had an effect on the adsorption of four fractions except for SNHC, while neutral and acidic pH affects SOS and SRDOM more obviously, the SNHC fraction was almost free from pH varies. The pH-dependent sorption characteristics of OTC to adsorptive fractions expect for SNHC may be caused by OTC speciation and the fractions surface charge, while the sorption of OTCH2 by SNHC may be predominated by π–π electron donor–acceptor interaction of the protonated aniline ring with the π-electron rich graphene surface, rather than ordinary electrostatic cation exchange.
References
Aiken GR, Mcknight DM, Wershaw RL, Maccarthy P (1986) Humic substances in soil, sediment, and water: geochemistry, isolation and characterization. Soil Sci 142:323
Bao G (2013) Effects of different organic matter components in soil on environmental behavior of polycyclic aromatic hydrocarbons. Dissertation, Fujian Normal University (In Chinese)
Bao Y (2008) Environmental behavior and ecotoxicity of tetracycline antibiotics in soil. Dissertation, Nankai University (In Chinese)
Bao Y, Zhou Q, Wan Y, Xie X (2009) Effects of soil organic matter on adsorption-desorption of oxytetracycline in soil China. Environ Sci 29:651–655 (In Chinese)
Briones RM, Sarmah AK, Padhye LP (2016) A global perspective on the use, occurrence, fate and effects of anti-diabetic drug metformin in natural and engineered ecosystems. Environ Pollut 219:1007–1020
Chang P-H, Li Z, Jiang W-T, Jean J-S (2009) Adsorption and intercalation of tetracycline by swelling clay minerals. Appl Clay Sci 46:27–36. https://doi.org/10.1016/j.clay.2009.07.002
Chen B, Johnson EJ, Chefetz B, Zhu L, Xing B (2005) Sorption of polar and nonpolar aromatic organic contaminants by plant cuticular materials: role of polarity and accessibility. Environ Sci Technol 39:6138–6146
Chen B, Wu M, Zhang D, Ning P, Zhong Z, Mao Z (2012) Research advance in sorption mechanisms of antibiotics in soil inorganic minerals. Chem Indus Eng Progress 31:193–200 (In Chinese)
Chen K-L, Liu L-C, Chen W-R (2017) Adsorption of sulfamethoxazole and sulfapyridine antibiotics in high organic content soils. Environ Pollut 231:1163–1171. https://doi.org/10.1016/j.envpol.2017.08.011
Cheng R (2016) Two-stage model predict the time-dependent toxicity of antibiotics and mixtures to Chlorella pyrenoidosa Dissertation, Anhui Jianzhu University (In Chinese)
Fakhri A, Adami S (2014) Adsorption and thermodynamic study of Cephalosporins antibiotics from aqueous solution onto MgO nanoparticles. J Taiwan Inst Chem Eng 45:1001–1006. https://doi.org/10.1016/j.jtice.2013.09.028
Figueroa RA, Leonard A, Mackay AA (2004) Modeling tetracycline antibiotic sorption to clays. Environ Sci Technol 38:476–483
Figueroa RA, Mackay AA (2005) Sorption of oxytetracycline to iron oxides and iron oxide-rich soils. Environ Sci Technol 39:6664–6671
Fu X, Sheng W, Yao T (1990) Physical chemistry (fourth edition). Higher education press (In Chinese), Beijing
Gélinas Y, Prentice KM, Baldock JA, Hedges JI (2001) An improved thermal oxidation method for the quantification of soot/graphitic black carbon in sediments and soils. Environ Sci Technol 35:3519–3525. https://doi.org/10.1021/es010504c
Gu C, Karthikeyan KG, Sibley SD, Pedersen JA (2007) Complexation of the antibiotic tetracycline with humic acid. Chemosphere 66:1494–1501
Guo X et al (2016) Sorption mechanisms of sulfamethazine to soil humin and its subfractions after sequential treatments. Environ Pollut 221:266
He X, Xi B, Wei Z, Guo X, Li M, An D, Liu H (2011) Spectroscopic characterization of water extractable organic matter during composting of municipal solid waste. Chemosphere 82:541–548
Hendershot WH, Singleton GA, Lavkulich LM (1979) Variation in surface charge characteristics in a soil chronosequence soil. Sci Soc Am J 43:387–389. https://doi.org/10.2136/sssaj1979.03615995004300020030x
Hu J, Zhang H, Peng PA (2006) Fatty acid composition of surface sediments in the subtropical Pearl River estuary and adjacent shelf, Southern China. Estuar Coast Shelf Sci 66:346–356. https://doi.org/10.1016/j.ecss.2005.09.009
Jia M, Wang F, Bian Y, Jin X, Song Y, Kengara FO, Xu R, Jiang X (2013) Effects of pH and metal ions on oxytetracycline sorption to maize-straw-derived biochar. Bioresour Technol 136:87–93
Jia MY, Wang F, Bian YR, Yang XL, Cheng gang GU, Song Y, Jiang X (2014) Influencing factors of cu~ (2+) sorption to straw-derived. Biochar Soils 46(In Chinese):489–497
Jones AD, Bruland GL, Agrawal SG, Vasudevan D (2005) Factors influencing the sorption of oxytetracycline to soils. Environ Toxicol Chem 24:761–770
Kalbitz K, Schmerwitz J, Schwesig D, Matzner E (2003) Biodegradation of soil-derived dissolved organic matter as related to its properties. Geoderma 113:273–291. https://doi.org/10.1016/S0016-7061(02)00365-8
Kolz AC, Ong SK, Moorman TB (2005) Sorption of tylosin onto swine manure. Chemosphere 60:284–289
Korshin GV, Li C-W, Benjamin MM (1997) Monitoring the properties of natural organic matter through UV spectroscopy: a consistent theory. Water Res 31:1787–1795. https://doi.org/10.1016/S0043-1354(97)00006-7
Kulshrestha P, Jr GR, Aga DS (2004) Investigating the molecular interactions of oxytetracycline in clay and organic matter: insights on factors affecting its mobility in soil. Environ Sci Technol 38:4097–4105
Kümmerer K (2009) Antibiotics in the aquatic environment – a review – part I. Chemosphere 75:417–434. https://doi.org/10.1016/j.chemosphere.2008.11.086
Leal RM, Alleoni LR, Tornisielo VL, Regitano JB (2013) Sorption of fluoroquinolones and sulfonamides in 13 Brazilian soils. Chemosphere 92:979–985
LeBoeuf EJ, Weber WJ (1997) A distributed reactivity model for sorption by soils and sediments. 8. Sorbent organic domains: discovery of a humic acid glass transition and an argument for a polymer-based model. Environ Sci Technol 31:1697–1702. https://doi.org/10.1021/es960626i
Li H, Zhang D, Han X, Xing B (2014) Adsorption of antibiotic ciprofloxacin on carbon nanotubes: pH dependence and thermodynamics. Chemosphere 95:150–155
Liu Q-S, Zheng T, Wang P, Jiang J-P, Li N (2010) Adsorption isotherm, kinetic and mechanism studies of some substituted phenols on activated carbon fibers. Chem Eng J 157:348–356. https://doi.org/10.1016/j.cej.2009.11.013
Mackay AA, Canterbury B (2005) Oxytetracycline sorption to organic matter by metal-bridging. J Environ Qual 34:1964–1971
Mutavdžić Pavlović D, Ćurković L, Grčić I, Šimić I, Župan J (2017) Isotherm, kinetic, and thermodynamic study of ciprofloxacin sorption on sediments. Environ Sci Pollut Res 24:10091–10106. https://doi.org/10.1007/s11356-017-8461-3
Nebbioso A, Piccolo A (2013) Molecular characterization of dissolved organic matter (DOM): a critical review. Anal Bioanal Chem 405:109–124
Ni JZ, Luo YM, Wei R, Li XH (2008) Distribution of polycyclic aromatic hydrocarbons in particle-size separates and density fractions of typical agricultural soils in the Yangtze River Delta, East China. Eur J Soil Sci 59:1020–1026. https://doi.org/10.1111/j.1365-2389.2008.01066.x
OECD (2000) Adsorption - desorption using a batch equilibrium method. OECD Guidelines for the Testing of Chemicals 1:1–44
Okaikue-Woodi FEK, Kelch SE, Schmidt MP, Enid Martinez C, Youngman RE, Aristilde L (2018) Structures and mechanisms in clay nanopore trapping of structurally-different fluoroquinolone antimicrobials. J Colloid Interface Sci 513:367–378. https://doi.org/10.1016/j.jcis.2017.11.020
OuYang T, Zhao Z, Gu X, Li X (2003) FTIR Study on the adsorption of bensulfuron-methyl by goethite. Spectroscopy and Spectral Analysis 23:1097–1100 (In Chinese). https://doi.org/10.3321/j.issn:1000-0593.2003.06.017
Pan P, Yang J, Deng S, Jiang H, Zhang J, Li L, Shen F (2011) Heavy metals and pesticides co-contamination in. Environ J Agro-Environ Sci 30:1925–1929 (In Chinese)
Peuravuori J, Pihlaja K (1997) Isolation and characterization of natural organic matter from lake water: comparison of isolation with solid adsorption and tangential membrane filtration. Environ Int 23:441–451. https://doi.org/10.1016/S0160-4120(97)00049-4
Qin X, Du P, Chen J, Liu F, Wang G, Weng L (2018) Effects of natural organic matter with different properties on levofloxacin adsorption to goethite: experiments and modeling. Chem Eng J 345:425–431. https://doi.org/10.1016/j.cej.2018.03.125
Ran Y, Sun K, Yang Y, Xing B, Zeng E (2007) Strong sorption of phenanthrene by condensed organic matter in soils and sediments. Environ Sci Technol 41:3952–3958
Ren L, Ling W-T, Gao Y (2008) Enhanced fixation of phenanthrene in soils amended with exotic organic materials. Chin J Appl Ecol 19:647–652 (In Chinese)
Sakurai K, Ohdate Y, Kyuma K (1989) Potentiometric automatic titration (PAT) method to evaluate zero point of charge (ZPC) of variable charge soils. Soil Sci Plant Nutr 35:89–100. https://doi.org/10.1080/00380768.1989.10434740
Shao ZH, He PJ, Zhang DQ, Shao LM (2009) Characterization of water-extractable organic matter during the biostabilization of municipal solid waste. J Hazard Mater 164:1191–1197
Sheng G, Johnston CT, Teppen BJ, Boyd SA (2001) Potential contributions of smectite clays and organic matter to pesticide retention in soils. J Agric Food Chem 49:2899–2907
Strobel BW, Hansen HCB, Borggaard OK, Andersen MK, Raulund-Rasmussen K (2001) Composition and reactivity of DOC in forest floor soil solutions in relation to tree species and soil type. Biogeochemistry 56:1–26. https://doi.org/10.1023/a:1011934929379
Sun H, Zhang W (2011) Existing state of hydrophobic organic compounds in soils and sediments. Environ Chem 30:231–241 (In Chinese)
ter Laak TL, Wouter AG, Tolls J (2006) The effect of pH and ionic strength on the sorption of sulfachloropyridazine, tylosin, and oxytetracycline to soil. Environ Toxicol Chem 25:904–911
Tolls J (2001) Sorption of veterinary pharmaceuticals in soils: a review. Environ Sci Technol 35:3397–3406
Wahab M, Jellali S, Jedidi N (2010) Ammonium biosorption onto sawdust: FTIR analysis, kinetics and adsorption isotherms modeling. Bioresour Technol 101:5070–5075
Wang D, Xu HY, Yang SK, Wang WK, Wang YH (2018a) Adsorption property and mechanism of oxytetracycline onto willow residues. Int J Env Res Public Health 15(11). https://doi.org/10.3390/ijerph15010008
Wang RZ, Yang SK, Fang J, Wang ZZ, Chen YY, Zhang D, Yang CY (2018b) Characterizing the interaction between antibiotics and humic acid by fluorescence quenching method. Int J Environ Res Public Health 15:13. https://doi.org/10.3390/ijerph15071458
Wang ZZ, Jiang QL, Wang RZ, Yuan XY, Yang SK, Wang WK, Zhao YQ (2018c) Effects of dissolved organic matter on sorption of oxytetracycline to sediments. Geofluids 2018:1–12. https://doi.org/10.1155/2018/1254529
Wu Q, Mai B, Yang Q, Peng P, Fu J (2004) The distribution state of PAHs and organochlorine pesticides in sediments. China Environ Sci 24:89–93 (In Chinese). https://doi.org/10.3321/j.issn:1000-6923.2004.01.021
Yu H, Huang GH, An CJ, Wei J (2011) Combined effects of DOM extracted from site soil/compost and biosurfactant on the sorption and desorption of PAHs in a soil-water system. J Hazard Mater 190:883–890
Yu Y, Zhuang YY, Wang ZH, Qiu MQ (2004) Adsorption of water-soluble dyes onto modified resin. Chemosphere 54:425–430
Zhang M, Wang L, Zheng S (2008) Adsorption and transport characteristics of two exterior-source antibiotics in some agricultural soils. Acta Ecol Sin 28:761–766 (In Chinese). https://doi.org/10.3321/j.issn:1000-0933.2008.02.038
Zhao L, Wang C, Yang Z, Zhen X (2017) Ultraviolet-visible and fluorescence characteristics of dissolved organic matter in the fallen leaves of Populus tomentosa. Environ Sci Technol 40:98–102
Zhao X, Bi E (2014) Effects of dissolved organic matter on the sorption of organic pollutants to soils. Environ Chem 33:256–261 (In Chinese). https://doi.org/10.7524/j.issn.0254-6108.2014.02.019
Zhao Y (2013) Study on the adsorption behaviors of ppcps onto sediment in the Weihe River. Dissertation, Chang’an University (In Chinese)
Zhao Y, Geng J, Wang X, Gu X, Gao S (2011) Adsorption of tetracycline onto goethite in the presence of metal cations and humic substances. J Colloid Interface Sci 361:247–251
Zhao YP, Gu XY, Gao SX, Geng JJ, Wang XR (2012) Adsorption of tetracycline (TC) onto montmorillonite: cations and humic acid effects. Geoderma 183:12–18. https://doi.org/10.1016/j.geoderma.2012.03.004
Funding
This work was supported by the National Natural Science Foundation of China [grant number 41672224], [grant number 41372259], [grant number 41807457]; the National Key Research and Development Program of China [grant number 2016YFC0400701]; and the Henan Province Transportation Science and Technology Project [grant number 2017 J4-1].
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, D., Yang, S., Wang, Y. et al. Adsorption characteristics of oxytetracycline by different fractions of organic matter in sedimentary soil. Environ Sci Pollut Res 26, 5668–5679 (2019). https://doi.org/10.1007/s11356-018-4028-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-4028-1