Abstract
The presence of chemicals in laboratories and research centers exposes the staff working at such indoor environment to health risks. In this piece of research, a study was performed on the indoor environment of the Center for Environmental Engineering Research at Sahand University of Technology (Tabriz, Iran). For this purpose, the parameters affecting the dispersion of volatile organic compounds (VOCs), including ventilation rate, room temperature, pollution emission time, venting location, air flow regime within the indoor environment, and the number of vents, were simulated via CFD modeling. The CFD modeling was performed three-dimensionally in unsteady state. In case of turbulent flow within the indoor environment, k–ε turbulence model was used to obtain air velocity profile. Experimental data was used to validate the model. Results of the present research showed that when the venting location is on the ceiling, pollution concentration of 25 ppm can be achieved at some low temperature under a particular set of conditions. However, when the venting location was on the walls close to the pollution source, concentrations as low as 5 ppm and lower were observed within the laboratory indoor environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
People spend most of their time in indoor environments. This is while, according to studies performed by numerous researchers, the level of pollution in indoor environments is in many cases higher than that in outdoor environments (Goyal and Kumar 2013; Krugly et al. 2014; Lee et al. 1999; Loupa et al. 2007; Patnaik et al. 2018; Zhang et al. 2018). Being exposed to chemicals, laboratories are often more polluted than other indoor environments (e.g., residents, offices, schools, etc.) by several folds. Accordingly, the laboratory staff are exposed to a high level of health risk (Davardoost and Kahforoushan 2018; Gammage 2018; Lerner et al. 2018). Volatile organic compounds (VOCs) are among the most widely used chemicals in laboratories. Due to their high vapor pressure, low boiling point at room temperature, and high coefficient of diffusion into air, VOCs such as benzene, toluene, and formaldehyde tend to disperse through indoor environment air readily, thereby affecting indoor air quality (IAQ) (Edwards et al. 2001; Lewis 1991; Salthammer et al. 2018; Wolkoff 1995; Wolkoff and Nielsen 2001; Wolkoff et al. 2006). Exposure to chemicals such as VOCs, particularly benzene, can end up with numerous health problems (Bono et al. 2003; Capleton and Levy 2005; Norton et al. 2018; Sarigiannis et al. 2011). As such, it is necessary to evaluate the diffusion of such compounds in laboratory indoor environment accurately. Given the wide spectrum of already discovered VOCs, it is very difficult and time-intensive to consider every single one of them separately. Accordingly, in this research, in order to develop an understanding of the amount and dispersion mechanism of VOCs, benzene was selected as a representative VOC.
Following the same line of research, some researchers have concluded that smoking, surfactants, chemical solvents, dyes, sterilizing agents, and combustion products are among the sources of VOC emission (Annesi-Maesano et al. 2013; Baya et al. 2004; Begum et al. 2009; Fromme et al. 2008; Lagoudi et al. 1996; Lerner et al. 2018; Lewis 1991; Norton et al. 2018; Su et al. 2018; Wolkoff 1995; Yoon et al. 2011).
Investigation and evaluation of air quality within different types of indoor environment have been the subject of numerous research works. Among others, a research performed in Norway explored the exposure to organic solvents in seven offset printing houses based on NIOSH-1501. The results indicated that the concentration of toluene and exposure to benzene were two to four and one to two times larger than the limits set in the NIOSH standard, respectively. Given that the organic solvent used in this study was alcohol-based, 10% iso-propanol was identified as the third dominant pollutant emitted when washing the printing rolls in the printing house (Svendsen and Rognes 2000). A research performed by Baya et al. to measure and evaluate the concentration of 16 VOCs in indoor environment of 25 houses in Athena, mean, median, maximum, and minimum values of the concentration of the 16 VOCs were estimated using a total l of 324 samples on seasonal, daily, and hourly bases. The obtained results indicated that 16 VOCs (including aliphatic and branched alkanes, aromatic and cyclic compounds) comprised 50% of the measured VOCs; it was further found that concentration of the VOCs was maximal in winter time (Baya et al. 2004). Guo et al. evaluated the health risk associated with exposure to VOCs in indoor environment in Hong Kong. For this purpose, four groups of people were selected, including the staff working at restaurant, office staff, housewives, and pupils. The main objective of their research was to identify the most risky location for the staff. The authors ended up concluding that the staff working at restaurant suffered from the highest risk of cancer when exposed to chloroform (Guo et al. 2004). Ramos et al. examined the contents of CO2 CO, VOC, and O3 at 11 fitness gyms across the city of Lisbon using a Wolfsense apparatus. The results indicated that, in order to reduce the athletes’ exposure to air pollutants as they practice, the HVAC system and ventilation rate shall be optimized (Ramos et al. 2014). Lee et al. evaluated the rate of pollutions emitted from office equipment, such as fax set, laser printers, inkjet printers, scanners, and photocopiers. The measured pollutants included VOCs, TVOC, ozone, PM10, m,p-xylene, and styrene. According to the results, among other VOCs, toluene, ethyl benzene, and styrene exhibited high concentrations. In addition, emission rate of ozone and VOC was higher from laser printers rather than inkjet ones (Lee et al. 2001). In a research where indoor air quality (IAQ) was investigated and compared between museums, printing industry, and offices, it was concluded that PM 2.5, benzene, toluene, SO2, and NO3 exhibit the highest concentrations in printing industry. Formaldehyde exhibited the highest concentration at museums, and O3 was the most concentrated pollutant in non-smoking offices. This was while the lowest concentration in printing industry was that of O3. It is worth mentioning that, in addition to the activities, machineries, and equipment used in these industries, the location within each environment is of paramount importance when it comes to the indoor air pollution (Saraga et al. 2011). Zhuang et al. presented a validated CFD model where IAQ was investigated within an office room with different ventilation and furniture layouts. According to the results presented based on this model, furniture layout inside the building acts as an important factor affecting the ventilation effectiveness and IAQ. Therefore, adjustment of the furniture layout can be very useful for improving air quality within breathing zone (Zhuang et al. 2014).
Working with chemicals, the staff working at research centers and laboratories are at serious health risks (Convertino et al. 2018; Spurgeon et al. 1997; Su et al. 2018). However, based on the abovementioned studies, it is inferred that, in contrary to the numerous research works performed on air pollution monitoring in indoor environments, smaller deals of attention have been paid to research centers and laboratories. As such, the present research discusses the factors affecting the dispersion of benzene, as an example of high-risk chemicals negatively impacting human health, in laboratory indoor environment. Using CFD modeling, effects of different parameters including openness/closeness of openings, ventilation rate, number of vents, venting location, and temperature on the dispersion of pollution were investigated. The k–ε turbulence model was used to model air flow under turbulent regime.
Methodology
Geometry of case study
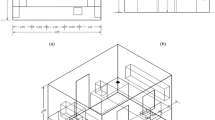
Figure 1 depicts the studied geometry. Dimensions of the room, door, windows, pollution source, and ventilation are given in Table 1. The symbols P, W1, W2, D, and V in Fig. 1 denote pollution source, window #1, window #2, door, and ceiling vent. It should be noted that the dimensions herein considered for the contamination source were not equal to the dimensions of the laboratory dishes containing the VOCs, but rather part of the cross-sectional area of the laboratory dishes and also compared to the test environment (the place into which the contamination is dispersed); the test pollutant source’s dimensions were negligible in terms of environment dimensions, and given that the alteration of dimensions for various test equipment is very small, then minor changes in the dimensions of leakage may not impose any significant effect on the amount and dispersion of VOCs.
Governing equations
Pollution dispersion proceeds mainly through molecular diffusion and convection mechanism. In order to simulate air flow under turbulent flow regime, k–ε turbulence model was used (Eqs. (1)–(5)) (Anderson and Wendt 1995; Bergman and Incropera 2011; Cussler 2009; Hirsch 2007; Hoffmann and Stein 2002; Kassomenos et al. 2008; Panagopoulos et al. 2011; Stathopoulou and Assimakopoulos 2008; Treybal 1980; Wendt 2008; Wilcox 1998; Wilcox 2008; Zhang and Niu 2004).
Continuity equation:
Momentum conservation equation
Equation of continuity for species of benzene
Transport conservation equation for k and e
where ui is the air flow velocity along xi direction, ρ is air density, uj is air flow velocity along xj direction, t is time, p is air pressure, μ is viscosity, gj is gravitational acceleration along xj direction, C is molar concentration of air-benzene mixture, xα is molar fraction of benzene in air, Dαβ is diffusion coefficient of benzene into air, rα is volumetric reaction rate, k is turbulence kinetic energy (TKE), ε is TKE loss rate, μt is turbulent viscosity, Gk is the kinetic energy produced by buoyancy, and the constants Cε1 and Cε2 are herein assumed as 1.44 and 1.92, respectively (Anderson and Wendt 1995; Hoffmann and Stein 2002; Sada and Ichikawa 1993; Teodosiu et al. 2016; Wilcox 1998; Wilcox 2008).
The velocity equation was solved under steady state and no slip condition. In the meantime, continuity equation for benzene was solved in unsteady state considering the chemical reaction term, because of the assumption of no reaction between benzene and air compounds. Initial and boundary conditions are presented in the following (Eqs. (6)–(12)):
Equations (6)–(12) express the following, respectively: zero initial pollution, no pollution diffusion into walls, equality of the concentration on pollution source-air interface and the saturated concentration, zero velocity on the walls, input flow rate through the windows equal to Qi, output flow rate through vent(s) equal to Qj (13 m3/h), and zero pressure difference between the room and outdoor environment when the door is open. It is worth noting that, in some cases, the modeling was performed with the door close. In such cases, the air flow infiltrates from the room toward the outdoor environment through a very narrow gap between the door and the ground (~ 7 cm).
Meshing
In order to investigate the grid number independence of the results, four meshing layouts with different numbers of elements were considered to simultaneously solve the equations of momentum, continuity, and mass transfer. According to Fig. 2, no significant difference was observed in the results between the third and fourth meshing layouts. Figure 2a shows the CFD modeling results for laminar flow when only one of the windows is open and the door is closed with no ventilation. Figure 2b demonstrates the CFD modeling results for turbulent flow inside the room when all windows and door are open and still there is no ventilation. More details on the number and layout of the meshes are reported in Table 2.
Average concentration of acetone in room versus time. (a) CFD modeling results for laminar flow when only one of the windows is open and the door is closed with no ventilation. (b) CFD modeling results for turbulent flow inside the room when all windows and door are open and still there is no ventilation. Mesh 1: 1,075,112 elements, mesh 2: 2,440,389 elements, mesh 3: 7,495,534 elements, and mesh 4: 9,793,942 elements
CFD modeling was performed using COMSOL software (Guide 1998; Multiphysics 2012). Mass transfer and momentum equations were solved in fully coupled mode. In order to check the effects of different parameters on pollution dispersion in indoor environment, the model was run under different sets of conditions. Table 3 provides a list of the symbols and conditions investigated in each run.
The backward differential formula (BDF) was used to solve unsteady modeling. In this method, the values of initial step, maximum step, maximum BDF order, minimum BDF order, and fraction of initial step for backward Euler were found to be 0.001, 0.1, 2, 1, and 0.001, respectively.
Along the last column of Table 3 (the symbols), each run is designated by a five-character code. The characters indicate flow type (L = laminar, T = turbulent), openness/closeness of the window W1 (0 = closed, W = open), openness/closeness of the window W2 (0 = closed, W = open), openness/closeness of the door (0 = closed, D = open), and number of vents (0 = without ventilation, 1 = one ventilation at roof, 2 = two ventilations at roof).
Validation
In order to validate the model, acetone was used as a representative pollutant, because exposure to and working with benzene is known to raise serious health risks. Moreover, acetone is a VOC with volatility (diffusion coefficient and saturated concentration) close to benzene, widely available, and vastly used in different laboratories, making it a good candidate for validating the model. Governing equations (Eqs. (1)–(5)), boundary conditions, and initial conditions used to model the dispersion of acetone are the same as those adopted for benzene. In other words, similar to benzene, acetone is a volatile organic compound (VOC); the only differences were that, compared to benzene, the acetone was much safer in terms of testing and exposure. Experiments were performed in the temperature range of 25–27 °C (room temperature) under four sets of conditions representing different air flow regimes, ventilation rates, and numbers of vents in an indoor environment. Air flow and room temperature measurements were performed using hot wire anemometer, and acetone concentration was measured using an online VOC concentration measurement tool (PID) with the capability of storing the recorded data and transferring them to a PC. Measurements were performed at three points: (A) center of the room, (B) a point at 1-m distance to the point A along positive x-axis, and (C) a point at 1-m distance to the point A along positive y-axis. The three points were at the breathing height (1.5 m).
For solving Eqs. (1)–(12), diffusion coefficient of acetone into air and saturated concentration of acetone were considered as 0.1049 × 10−4 m2/s and 11 mol/m3 (Lugg 1968; Pashley et al. 1998), respectively. Each test was conducted in three or four replications, and average values were reported. Moreover, experimental data were modeled and compared following 30 and 60 min of start of the pollution emission in indoor environment, with the results reported in Table 4. The results tabulated in Table 4 indicate that the experimental data and modeling results are in agreement. As such, the proposed model can be used to predict benzene concentration distribution in laboratory indoor environment.
Using Eqs. (13) and (14), the agreement between the model results and corresponding experimental data was examined based on R2 and normalized mean square error (NMSE) (Abdoli et al. 2018a, b; Roy 2010).
In the above equations, \( {C}_i^{exp} \) refers to the concentrations measured via experimentation, \( {C}_a^{exp} \) is average value of measured concentrations, \( {C}_i^{model} \) is the concentration predicted by the CFD model, and \( {C}_a^{model} \) is average value of the concentration predicted by the CFD model. The data given in Table 5 indicate the agreement between the experimental data and modeling results. That is, the proposed CFD model can be used to predict the dispersion of various VOCs under different operating conditions.
The simulation for benzene (based on the diffusion coefficient of benzene in the air = 0.0932 × 10−4 m2/s (Lugg 1968)) was performed under the same conditions as those applied for acetone, and the results are presented in Table 6. By comparing the data provided in Table 4 (experimental data and simulation results for acetone) against that in Table 6 (simulation results for benzene), one may observe that, with increasing the ventilation rate, the pollution concentration decreased, as per the simulation results, for both benzene and acetone. Moreover, the pollution concentration increased with time in both of the cases. In general, similar trends of changes in pollution concentration were seen both along the offset (the points A, B, and C) and time axes. The difference between the data reported in Tables 4 and 6 stems from the corresponding difference between diffusion coefficients of acetone and benzene through air as well as the difference between saturation concentrations of the two substances.
Results and discussion
The amount and evolution of pollution dispersion were investigated for 1 h. In order to investigate the factors affecting benzene dispersion in indoor environment, the proposed CFD model was run for 48 conditions.
Figure 3 shows the influence of closeness/openness of the windows, door, number of the vents at a temperature of 25 °C (298 K), and laminar flow on benzene pollution level at breathing height (1.5 m from the ground). According to Fig. 3, with increasing the number of vents on the ceiling of the laboratory, the level of pollution within breathing height was seen to increase; as such, a change guides the pollution toward the ceiling. The effect is less significant when the door is closed, because the air infiltration through the gap below the door leads the pollution downward to the ground. This parameter was more significantly observed when no vent is incorporated into the ceiling. Based on the results presented in Fig. 3, the pollution would be minimal with LW0D0 ventilation layout, with the pollution level reaching about 30 ppm after 1 h of the start of the emission.
According to OSHA standard, OEL-C, OEL-STEL, and OEL-TWA (indicating short-term exposure limit, 15-min exposure limit, and 8-h exposure limit) for benzene are 25, 5, and 1 ppm, respectively (Capleton and Levy 2005; Hygienists 1986; Values 1996; Williams 2014). Comparing the benzene concentration in the LW0D0 layout with the allowed limits, it is seen that the staff are at health risk upon either short-term or long-term exposure.
Based on Fig. 4, one can see the effects of closeness/openness of the windows, door, number of the vents at a temperature of 25 °C (298 K), and turbulent flow on benzene pollution level at breathing height (1.5 m from the ground). The figure indicates that the minimum benzene concentration within breathing zone is seen in the TWW02 layout (56 ppm), which is still higher than the allowed limits. As such, this ventilation layout cannot provide the laboratory with suitable working conditions for the staff.
By comparing Figs. 3 and 4, one can see the effect of air flow on pollution dispersion at 298 K. Upon changing the flow regime from laminar to turbulent, average concentration became uniform rapidly; that is, under turbulent flow regime, variations of average pollution concentration with time are hardly subtle.
Effects of closeness/openness of the windows, door, number of the vents at a temperature of − 5 °C (268 K), and laminar flow on benzene pollution level at breathing height (1.5 m from the ground) can be observed in Fig. 5. According to the figure, the minimum benzene concentration within the indoor environment is seen in the LW0D0 layout (about 6 ppm, OEL-STEL), in which case it is still hard to definitely confirm desirable working conditions for the staff.
The reason behind the similarity between the trends of changes in concentration with time in Figs. 3 and 5 is the similarity in air flow field in the two cases. This is while average concentration at 25 °C is higher than that at − 5 °C under the same air flow regime, which can be explained by the reduction in coefficient of diffusion and saturated concentration of benzene with decreasing the temperature.
Figure 6 shows the influence of closeness/openness of the windows, door, number of the vents at a temperature of − 5 °C (268 K), and turbulent flow regime on benzene pollution level at breathing height (1.5 m from the ground). According to Fig. 6, the lowest benzene concentration across the laboratory was obtained with TWW01 layout, in which case the benzene content of air reduced to 12 ppm. Comparing Figs. 4 and 6, it can be concluded that an increase in room temperature affects indoor air pollution directly.
According to Figs. 3, 4, 5, and 6, average concentration of the pollution was lower than the allowed limit per OEL-Ceiling (25 ppm) only at 268 K and with L0W00, L0WD0, LW000, LW0D0, LWWD0, LWW01, T0W00, TW000, TW0D0, TWW00, TWWD0, TW001, and TWW01 layouts; that is, short-term exposure under the abovementioned conditions is free of health risk for the staff. Given that the pollution concentration within breathing zone was not below the OEL-TWA and OEL-STEL in all of the layouts, another exhausted ventilation layout is considered in the following.
According to Fig. 7, vents were considered on the two walls close to the pollution source. In order to investigate the effect of layout change in different air flow regimes and at various temperatures, modeling was performed according to Table 7. The designation practiced in Table 7 follows the same rules used in Table 3, except that the last character in Table 7 refers to the normal vector of the plane in which the exhausted ventilation resides.
Table 8 provides the modeling results when the ventilation is located on the walls close to the pollution source (Fig. 7). The concentrations in breathing zone (1.5 m from the ground) presented in Table 8 refer to 30 and 60 min after the start of emission at 298 and 268 K. According to Table 8, it is observed that, in all layouts where laminar flow is developed in the room, the pollution concentration is less than OEL-STEL (5 ppm).
Comparing the results obtained from Figs. 3, 4, 5, and 6 and those reported in Table 8, it is observed that the change of exhausted ventilation position from the ceiling to the walls close to the pollution source decreases the pollution content within the breathing zone. If the exhausted ventilation layout is similar to that demonstrated in Fig. 7, the presence and activities of the staff and students in the laboratory will be free of health risk for limited time spans (about 15 min); however, since the pollution concentration is still above OEL-TWA (1 ppm), the presence and activities of the staff and students in the laboratory for long times may end up with health risks for them.
Conclusions
In the present research, in order to investigate the effects of temperature, ventilation layout, number of vents, time, and air flow velocity within a room, three-dimensional CFD modeling was performed in unsteady state. The Environmental Engineering Research Center at Sahand University of Technology (Tabriz, Iran) was selected as an experimental indoor environment, representing similar laboratories and research centers. Moreover, knowing that the governing equations for the selected indoor environment hold true for other indoor environments, the results of this research can be generalized to other laboratories and research centers. Differences in the dimensions of similar laboratory environments, as compared to Environmental Engineering Research Center at University of Sahand, may solely affect the geometry rather than the theories and equations governing the dispersion of contamination in the laboratory environment. As such, one can use the governing equations, boundary conditions, modeling approach, and the general results obtained herein for other laboratories and research centers.
Once finished with simulating the model using experimental data, the model results were used to compare the obtained concentrations with the allowed limits by OSHA.
Results of the present research show that, when the vents are located on the ceiling of a room and air flows within the room under either laminar or turbulent regime, pollution concentration within breathing zone may not decrease, due to the driving force generated in such layout. Moreover, with increasing the temperature and air flow velocity in an indoor environment, concentration of pollution within breathing zone increases. In order to achieve pollution concentrations below the OEL-Ceiling (25 ppm), the room temperature should set at 268 K and the ventilation layout should be one of the following: L0W00, L0WD0, LW000, LW0D0, LWWD0, LWW01, T0W00, TW000, TW0D0, TWW00, TWWD0, TW001, and TWW01. This is while, under none of the mentioned layouts, average pollution concentration within breathing zone does not fall below OEL-TWA or OEL-STEL. As such, another exhausted ventilation layout was considered in this study.
Results of the present research can be used to develop occupational health standards for various types of laboratories and research centers, so as to present not only allowable exposure limits for different occupations but also safe positions within indoor spaces.
According to the results of the present research, if the exhausted ventilation layout is positioned on the walls close to the pollution source, average pollution concentration within breathing zone will be less than 5 ppm, so that the presence and activities of the staff and students in the laboratory will be free of health risk for limited time spans (about 15 min); however, since the pollution concentration is still above OEL-TWA (1 ppm), the presence and activities of the staff and students in the laboratory for longer periods are not recommended.
References
Abdoli SM, Shafiei S, Raoof A, Ebadi A, Jafarzadeh Y (2018a) Insight into Heterogeneity Effects in Methane Hydrate Dissociation via Pore-Scale Modeling. Transp Porous Media:1–19
Abdoli SM, Shafiei S, Raoof A, Ebadi A, Jafarzadeh Y, Aslannejad H (2018b) Water flux reduction in microfiltration membranes: a pore network study. Chem Eng Technol 41(8):1566–1576
American Conference of Governmental Industrial Hygienists (1986) Documentation of the threshold limit values and biological exposure indices. 5th ed Cincinnati, OH: American Conference of Governmental Industrial Hygienists
Anderson JD, Wendt J (1995) Computational fluid dynamics, vol 206. McGraw-Hill, New York
Annesi-Maesano I, Baiz N, Banerjee S, Rudnai P, Rive S, Group S (2013) Indoor air quality and sources in schools and related health effects. J Toxicol Environ Health B Crit Rev 16:491–550. https://doi.org/10.1080/10937404.2013.853609
Baya MP, Bakeas EB, Siskos PA (2004) Volatile organic compounds in the air of 25 Greek homes. Indoor Built Environ 13:53–61. https://doi.org/10.1177/1420326x04036007
Begum BA, Paul SK, Dildar Hossain M, Biswas SK, Hopke PK (2009) Indoor air pollution from particulate matter emissions in different households in rural areas of Bangladesh. Build Environ 44:898–903. https://doi.org/10.1016/j.buildenv.2008.06.005
Bergman TL, Incropera FP, DeWitt DP, Lavine AS (2011) Fundamentals of heat and mass transfer. John Wiley & Sons
Bono R, Scursatone E, Schilirò T, Gilli G (2003) Ambient air levels and occupational exposure to benzene, toluene, and xylenes in northwestern Italy. J Toxicol Environ Health A 66:519–531
Capleton AC, Levy LS (2005) An overview of occupational benzene exposures and occupational exposure limits in Europe and North America. Chem Biol Interact 153:43–53
Convertino M et al (2018) Stochastic pharmacokinetic-pharmacodynamic modeling for assessing the systemic health risk of perfluorooctanoate (PFOA). Fundam Appl Toxicol 163:293–306
Cussler EL (2009) Diffusion: mass transfer in fluid systems. Cambridge University Press
Davardoost F, Kahforoushan D (2018) Health risk assessment of VOC emissions in laboratory rooms via a modeling approach. Environ Sci Pollut Res:1–11
Edwards RD, Jurvelin J, Saarela K, Jantunen M (2001) VOC concentrations measured in personal samples and residential indoor, outdoor and workplace microenvironments in EXPOLIS-Helsinki, Finland. Atmos Environ 35:4531–4543
Fromme H, Diemer J, Dietrich S, Cyrys J, Heinrich J, Lang W, Kiranoglu M, Twardella D (2008) Chemical and morphological properties of particulate matter (PM10, PM2.5) in school classrooms and outdoor air. Atmos Environ 42:6597–6605. https://doi.org/10.1016/j.atmosenv.2008.04.047
Gammage RB (2018) Evaluation of changes in indoor air quality occurring over the past several decades. In: Indoor air and human health. CRC Press, pp. 19–52
Goyal R, Kumar P (2013) Indoor–outdoor concentrations of particulate matter in nine microenvironments of a mix-use commercial building in megacity Delhi. Air Qual Atmos Health 6:747–757
Guide, COMSOL Multiphysics User’S (1998) Comsol Multiphysics
Guo H, Lee S, Chan L, Li W (2004) Risk assessment of exposure to volatile organic compounds in different indoor environments. Environ Res 94:57–66
Hirsch C (2007) Numerical computation of internal and external flows: The fundamentals of computational fluid dynamics. Elsevier
Hoffmann AC, Stein LE (2002) Computational fluid dynamics. In: Gas cyclones and swirl tubes. Springer, pp 123–135
Kassomenos P, Karayannis A, Panagopoulos I, Karakitsios S, Petrakis M (2008) Modelling the dispersion of a toxic substance at a workplace. Environ Model Softw 23:82–89. https://doi.org/10.1016/j.envsoft.2007.05.003
Krugly E, Martuzevicius D, Sidaraviciute R, Ciuzas D, Prasauskas T, Kauneliene V, Stasiulaitiene I, Kliucininkas L (2014) Characterization of particulate and vapor phase polycyclic aromatic hydrocarbons in indoor and outdoor air of primary schools. Atmos Environ 82:298–306. https://doi.org/10.1016/j.atmosenv.2013.10.042
Lagoudi A, Loizidou M, Asimakopoulos D (1996) Volatile organic compounds in office buildings: 2. Identification of pollution sources in indoor air. Indoor Built Environ 5:348–354. https://doi.org/10.1177/1420326x9600500607
Lee S-C, Chang M, Chan K-Y (1999) Indoor and outdoor air quality investigation at six residential buildings in Hong Kong. Environ Int 25:489–496. https://doi.org/10.1016/S0160-4120(99)00014-8
Lee S, Lam S, Fai HK (2001) Characterization of VOCs, ozone, and PM10 emissions from office equipment in an environmental chamber. Build Environ 36:837–842
Lerner JEC, Elordi ML, Orte MA, Giuliani D, Gutierrez M d l A, Sanchez EY, Sambeth JE, Porta AA (2018) Exposure and risk analysis to particulate matter, metals, and polycyclic aromatic hydrocarbon at different workplaces in Argentina. Environ Sci Pollut Res 25(9):8487–8496
Lewis C (1991) Sources of air pollutants indoors: VOC and fine particulate species. J Expo Anal Environ Epidemiol 1:31–44
Loupa G, Kioutsioukis I, Rapsomanikis S (2007) Indoor-outdoor atmospheric particulate matter relationships in naturally ventilated offices. Indoor Built Environ 16:63–69. https://doi.org/10.1177/1420326x06074895
Lugg G (1968) Diffusion coefficients of some organic and other vapors in air. Anal Chem 40:1072–1077
Multiphysics C (2012) Comsol multiphysics user guide (version 4.3 a) COMSOL, AB: 39–40
Norton AE, Doepke A, Nourian F, Connick WB, Brown KK (2018) Assessing flammable storage cabinets as sources of VOC exposure in laboratories using real-time direct reading wireless detectors. J Chem Health Saf
Panagopoulos IK, Karayannis AN, Kassomenos P, Aravossis K (2011) A CFD simulation study of VOC and formaldehyde indoor air pollution dispersion in an apartment as part of an indoor pollution management plan. Aerosol Air Qual Res 11:758–762
Pashley EL, Zhang Y, Lockwood PE, Rueggeberg FA, Pashley DH (1998) Effects of HEMA on water evaporation from water–HEMA mixtures. Dent Mater 14:6–10
Patnaik A, Kumar V, Saha P (2018) Importance of Indoor Environmental Quality in Green Buildings. In: Environmental Pollution. Springer, Singapore, pp 53–64
Ramos C, Wolterbeek H, Almeida S (2014) Exposure to indoor air pollutants during physical activity in fitness centers. Build Environ 82:349–360
Roy RK (2010) A primer on the Taguchi method. Society of Manufacturing Engineers
Sada K, Ichikawa Y (1993) Simulation of air flow over a heated flat plate using anisotropic k-ε model A2. In: Murakami S (ed) Computational Wind Engineering, vol 1. Elsevier, Oxford, pp 697–704. https://doi.org/10.1016/B978-0-444-81688-7.50077-2
Salthammer T, Zhang Y, Mo J, Koch HM, Weschler CJ (2018) Assessing human exposure to organic pollutants in the indoor environment. Angew Chem Int Ed
Saraga D, Pateraki S, Papadopoulos A, Vasilakos C, Maggos T (2011) Studying the indoor air quality in three non-residential environments of different use: a museum, a printery industry and an office. Build Environ 46:2333–2341
Sarigiannis DA, Karakitsios SP, Gotti A, Liakos IL, Katsoyiannis A (2011) Exposure to major volatile organic compounds and carbonyls in European indoor environments and associated health risk. Environ Int 37:743–765. https://doi.org/10.1016/j.envint.2011.01.005
Spurgeon A, Harrington JM, Cooper CL (1997) Health and safety problems associated with long working hours: a review of the current position. Occup Environ Med 54:367–375
Stathopoulou OI, Assimakopoulos VD (2008) Numerical study of the indoor environmental conditions of a large athletic hall using the CFD code PHOENICS. Environ Model Assess 13:449–458. https://doi.org/10.1007/s10666-007-9107-5
Su M, Sun R, Zhang X, Wang S, Zhang P, Yuan Z, Liu C, Wang Q (2018) Assessment of the inhalation risks associated with working in printing rooms: a study on the staff of eight printing rooms in Beijing, China. Environ Sci Pollut Res:1–7
Svendsen K, Rognes KS (2000) Exposure to organic solvents in the offset printing industry in Norway. Ann Occup Hyg 44:119–124
Teodosiu C, Ilie V, Teodosiu R (2016) Modelling of volatile organic compounds concentrations in rooms due to electronic devices. Process Saf Environ. https://doi.org/10.1016/j.psep.2016.06.013
Treybal RE (1980) Mass transfer operations. New York
Values TL, Biological Exposure Indices (BEIs) (1996) threshold limit values for chemical substances and physical agents biological exposure indices. American Conference of Governmental Industrial Hygienists (ACGIH), Cincinnati
Wendt JF (ed) (2008) Computational fluid dynamics: an introduction. Springer Science & Business Media
Wilcox DC (1998) Turbulence modeling for CFD, vol 2. DCW industries, La Canada
Wilcox DC (2008) Formulation of the k-w turbulence model revisited. AIAA J 46:2823–2838. https://doi.org/10.2514/1.36541
Williams PR (2014) An analysis of violations of Osha’s (1987) occupational exposure to benzene standard. J Toxicol Environ Health B Crit Rev 17:259–283
Wolkoff P (1995) Volatile organic compounds sources, measurements, emissions, and the impact on indoor air quality. Indoor Air 5:5–73
Wolkoff P, Nielsen GD (2001) Organic compounds in indoor air—their relevance for perceived indoor air quality? Atmos Environ 35:4407–4417. https://doi.org/10.1016/S1352-2310(01)00244-8
Wolkoff P, Wilkins C, Clausen P, Nielsen G (2006) Organic compounds in office environments–sensory irritation, odor, measurements and the role of reactive chemistry. Indoor Air 16:7–19
Yoon C, Lee K, Park D (2011) Indoor air quality differences between urban and rural preschools in Korea. Environ Sci Pollut Res Int 18:333–345. https://doi.org/10.1007/s11356-010-0377-0
Zhang LZ, Niu JL (2004) Modeling VOCs emissions in a room with a single-zone multi-component multi-layer technique. Build Environ 39:523–531. https://doi.org/10.1016/j.buildenv.2003.10.005
Zhang N, Huang H, Su B, Ma X, Li Y (2018) A human behavior integrated hierarchical model of airborne disease transmission in a large city. Build Environ 127:211–220
Zhuang R, Li X, Tu J (2014) CFD study of the effects of furniture layout on indoor air quality under typical office ventilation schemes. In: Building Simulation, vol 7, no. 3. Springer Berlin Heidelberg, pp. 263–275
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Davardoost, F., Kahforoushan, D. Evaluation and investigation of the effects of ventilation layout, rate, and room temperature on pollution dispersion across a laboratory indoor environment. Environ Sci Pollut Res 26, 5410–5421 (2019). https://doi.org/10.1007/s11356-018-3977-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3977-8