Abstract
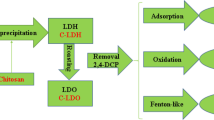
Chitosan/Co-Fe-layered double hydroxides (CS/LDHs) were prepared by coprecipitation method, which is a kind of composite material with excellent properties. The structure of CS/LDHs was characterized by SEM, FTIR, and XRD, which proved that chitosan (CS) was successfully induced into hydrotalcite and CS/LDHs still possess the structural characteristics of hydrotalcite. The adsorption of 2,4-dichlorophenol (2,4-DCP) was studied with CS/LDHs and LDHs as adsorbent separately. The activity of immobilized laccase (L-CS/LDHs) with CS/LDHs as carrier is significantly better than that of the one (L-LDHs) using LDHs as carrier. Under the optimum conditions (pH = 6, 55 °C, 48 h), L-CS/LDHs exhibited better removal performance for 2,4-DCP (81.53%, 100 mg/L) than LDHs (63.55%); the removal of 2,4-DCP by L-CS/LDHs is excellent, exceeding 97% as its initial concentration below 60 mg/L. It includes the catalytic action of laccase and dechlorination of Fe3+ and Co2+, and the adsorption can be ignored under the optimal conditions. After 5 cycles, it maintained 67% (L-CS/LDHs) and 54% (L-LDHs) of the original removal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chlorophenols, as important chemical raw materials, have been widely used in fungicides, herbicides, and preservatives (Xu et al. 2013a). Chlorophenols are toxic to any organism and can accumulate in the biosphere through the food chain (Zhou et al. 2011). 2,4-Dichlorophenol (2,4-DCP) has been designated as a priority pollutant by the Environmental Protection Agency (USA) in 1977 (Yang et al. 2015). Therefore, dealing effectively with 2,4-DCP pollutants has attracted worldwide attention. The removal methods of 2,4-DCP are mainly divided into physical, chemical, and bioremediation treatments (Li et al. 2010). Layered double Hydroxides (LDHs) are anionic double-hydroxyl compounds and can be represented as [MII1-xMIIIx(OH)2]x+(An-)x/nmH2O (Zhan et al. 2016a). In this formula, the hydroxide of MII and MIII constitutes the main layer-like structure, An− is interlayer anion, x is equal to MII/(MII+MIII) molar ratio, and m is the number of interlayer crystal water (Yang et al. 2014). The structure of LDHs determines the composition of intercalated metal ions, the type and number of intercalated anions, and the variability of interlayer spaces (Ling et al. 2016). LDHs have a layered host structure that can provide more active reaction centers and can improve or enhance the functionality of the host material by introducing functional guest molecules to provide superior performance in material applications (Zhan et al. 2016b; Ahmadzadeh and Dolatabadi 2018). In recent years, many researchers have attempted to insert other substances into the hydrotalcite interlayer by utilizing the special structure, resulting in a new type of composite material that is more excellent (Wang et al. 2016). Chitosan is a high molecular weight natural organic compound formed by deacetylation of chitin. The enriched –OH and –NH2 in chitosan molecules can form a lively interface and it is non-toxic, easy processing and degraded, environmental friendly organic materials (Li 2007; Rashid et al. 2015). The synthesis of chitosan and LDHs leads to the new organic-inorganic composite materials. Organic and inorganic materials can complement to have synergistic effect (Viswanathan and Meenakshi 2010; Liu et al. 2016). It overcomes the shortcomings of single inorganic material processing, low strength, and low stability of single organic materials, and has become the focus of material research now.
Laccases (EC1.10.3.2) are copper-containing blue hydroquinone oxidases (Hakulinen et al. 2002). In general, laccases have three roles: pigment formation, lignin degradation, and detoxification. Laccase has a remarkable catalytic oxidation of its substrate, not only can degrade anthraquinone, azo, and indigo dyes, but also can reduce the chlorophenols and steroid hormones and their derivatives, polycyclic aromatic hydrocarbons, chlorinated aromatic hydrocarbons, and can oxide-specific inorganic ions (Giardina et al. 2010;Youn et al. 1995; Papadopoulou et al. 2018). It is difficult for free laccase to be reused and recovered (Xu et al. 2013b). Free laccase cannot operate continuously and it drastically loses its activity due to microbial degradation or agglomeration. Immobilization of laccase can improve its characteristics to overcome above limitations.
In this study, CS/LDHs composite material was prepared to remove 2,4-DCP by the method of low saturation coprecipitation, introducing chitosan molecules into it. Based on our previous studies (Yang et al. 2013; Yang & Wang, 2014), laccase immobilization has been conducted by using CS/LDHs and LDHs as carrier respectively, glutaraldehyde as cross-linking agent. The removal of 2,4-DCP by immobilized laccase has been studied, and the adsorption of 2,4-DCP by the CS/LDHs and LDHs has also been discussed.
Experimental
Materials
2,4-DCP (Hangzhou Dayang Chemical Co., Ltd.), laccase (Ning Xia, derived from white rot fungi), FeCl3·6H2O (Analytical Pure, Tianjin Guangfu Fine Chemical Research Institute), CoCl2 (Analytical Pure, Tianjin Guangfu Fine Chemical Research Institute), chitosan (Shanghai Blue Quarter Technology Development Co., Ltd), glutaraldehyde (Analytical Pure, Tianjin Ruijin Special Chemical Co., Ltd.), 16 alkyltrimethylammonium bromide (CTBA, analytical grade), and other chemicals used in the experiment are analytical pure.
SHZ-82 water bath thermostat oscillator (Jiangsu Zhengji Instrument Co., Ltd.), Multiskan GO full-wavelength microplate reader (Thermo Fisher Scientific Corporation), LD-3 desktop electric centrifuge (Jintan Shenglan Instrument Manufacturing Co., Ltd.), GZX-9246 MBE digital blower dryer (Shanghai Bo News Industrial Co., Ltd. Medical Equipment Factory), and constant temperature magnetic stirrer.
Preparation of materials
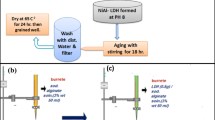
Preparation of LDHs
LDHs was prepared as described in the literature (Hang et al. 2013): the mixed salts of CoCl2 and FeCl3 (Co2+/Fe3+ mole ratio is 2) (Chaliha and Bhattacharyya 2009) and the alkali solution of Na2CO3 and NaOH were added to three-necked flask containing deionized water (100 mL), and maintained pH = 8–9, stirring (60 °C, 300 r/min, 18 h), then centrifuged, dried, and grinded.
Preparation of CS/LDHs
First, chitosan (3.0 g) was dissolved in a three-necked flask containing of 2% acetic acid (300 mL) and stirred at 60 °C until the solution became clear (yellowish). Then, CTAB (0.3 g) was added to above solution and Na2SO4 (60 mL) was dropped into it with a constant pressure dropping funnel, stirring until the solution was homogeneous, and centrifuged (4000 r/min, 10 min). The centrifuged solid dispersed in mixed metal salt solution (100 mL). The mixed solution and NaOH (1.5 mol/L) were added to three-necked flask containing deionized water (100 mL) and maintained pH = 8–9, stirring (60 °C, 300 r/min, 18 h), then centrifuged, dried, and grinded to obtain chitosan/Co-Fe-LDHs (CS/LDHs).
Characterization
FTIR spectra were recorded on a Nicolet iS 10 spectrometer (Thermoelectron corporation). X-ray diffractometer (XRD) patterns were characterized with a Rigaku powder equipped with Cu Ka radiation (k = 0.15405 nm). The morphology of products was analyzed by scanning electron microscopy (SEM, JSM-6700F).
Adsorption of 2,4-DCP
First, the adsorption experiments had been carried out to optimize the time of adsorption, dosage of the adsorbent, and pH. Then, the adsorption kinetic experiments were carried out in a 50 mL centrifuge tube with 10 mL of 2,4-DCP (20 mg/L). After adding 60 mg adsorbent, the stirred suspensions were centrifuged promptly at the various moments and the supernatant was available to determine the 2,4-DCP concentration. Finally, the adsorption isotherms were conducted through batch experiment in a centrifuge tube as above, with a series of initial concentrations of 2,4-DCP, ranging from 2 to 80 mg/L. The centrifuge tube was constantly shaken for 1.5 h in a thermostatic oscillator (25 ± 1 °C). Later, the supernatant was obtained by centrifuging for 5 min to analyze 2,4-DCP content.
Experimental for removing 2,4-DCP
Immobilization of laccase
The experiments were carried out in a 50 mL sealed centrifuge tube with glutaraldehyde (4 mL) and buffer (4 mL) solution, and the prepared material (0.1 g) was added as carrier. After the cross-linking reaction, excess glutaraldehyde was removed by centrifugation to obtain the activated carrier. Then, buffer solution (Na2HPO4-NaH2PO4, 4 mL) and laccase solution (4 mL) was added separately. After reaction, the immobilized laccase was flushed several times with buffer solution. The activity of immobilized laccase was determined spectrophotometrically, using o-tolidine (o-TD) as substrate. Oxidation of o-TD (5 mM) in 0.1 M acetate-acetate buffer (pH = 4.0) at 25 °C was monitored by following the increase of absorbance at 630 nm, using multiscan spectrum (Jasco V-630). One unit (U) of laccase activity was defined as the amount of enzyme required to oxidize 1 mmol of o-TD per minute.
Removal of 2,4-DCP
The experiment was carried out in a 50 mL sealed centrifuge tube, containing 10 mL of 2,4-DCP (100 mg/L), in the thermostatic oscillator at different temperature (25–65 °C). After the immobilized laccase was added, the suspensions were centrifuged promptly at the various moments and the supernatants was available, to determine the concentration of 2,4-DCP by spectrophotometric method.
After reaction, the supernatant (5 mL) was put into the colorimetric tube, diluted with distilled water to 50 mL; ammonium chloride-ammonia buffer solution (0.5 mL), 4-amino antipyrine solution (1 mL), and potassium cyanide (1 mL) were added, then mixed. After 10 min, the absorbance was measured at 510 nm. At the same time, the supernatant was replaced with distilled water as a blank.
Reaction kinetics study
The dynamics study was carried out in a 50 mL sealed centrifuge tube with 10 mL of 2,4-DCP (100 mg/L). After adding immobilized laccase, the centrifuge tube was put into the water bath thermostat oscillator and shaken for 48 h at the optimum temperature, then the mixed suspensions were centrifuged promptly at the various moments and the supernatant was obtained to analyze 2,4-DCP.
Reusability of the immobilized laccase
In this test, the immobilized laccase was repetitively used five recycle period for removing 2,4-DCP in optimum condition and removal efficiencies was obtained.
Results and discussion
Structural and morphology characterization of materials
In the SEM image of LDHs, the lamellar structure is obvious, the laminate area is large, and the crystallization effect of LDHs is excellent. Fig. 1B1 shows that CS/LDHs have lamellar structure obviously. After magnification of 10,000 times (Fig. 1B2), it is obvious that small pieces of hydrotalcite are filled with flocculent CS, forming a large group of composite material (CS/LDHs). However, the slab structure of CS/LDHs is not as clear as LDHs, indicating that chitosan hinders the growth of hydrotalcite crystals.
The XRD patterns of LDHs and CS/LDHs are shown in Fig. 2. LDHs and CS/LDHs have characteristic diffraction peaks including (003), (006), and (009). Obviously, the (003) and (006) diffraction peaks of CS/LDHs are not as good as LDHs, which indicate that the crystallinity of CS/LDHs is less than LDHs. For CS/LDHs, a peak between (003) and (006) is the characteristic diffraction peak of CS, indicating that CS and hydrotalcite have well compounded.
FTIR spectra are taken to investigate the structural property of CS/LDHs and LDHs (Fig. 3). CS/LDHs samples display the characteristic adsorption peaks of LDHs at about 600–650 cm−1 (MO), 1350–1385 cm−1 (CO32−), indicating the existence of hydrotalcite phase. Both of them have a strong absorption peak at 3421 cm−1 (superimposed by hydroxyl stretching vibration of water molecules in hydrotalcite). This strong absorption peak significantly shifts to the low wave number compared to the free state of the hydroxyl group. This indicates that the hydroxyl groups in hydrotalcite interlayers connect to H2O molecules and the anions in the layers by hydrogen bonds. The CS/LDHs samples exhibit the clear amino I (1625–1638 cm−1) and amino II (1562–1573 cm−1) of CS; it indicates CS combined with hydrotalcite successfully.
Adsorption of 2,4-DCP
Influencing factors
The adsorption efficiency of CS/LDHs and LDHs for 2,4-DCP (20 mg/L) was investigated at pH = 7, T = 25 °C, for 3 h (Fig. 4). As show in Fig. 4, both achieved adsorption balance in 90 min and the adsorption efficiency was 39% (CS/LDHs) and 25% (LDHs) respectively. Due to the introduction of chitosan molecules, the removal efficiency of 2,4-DCP by CS/LDHs is higher than that of LDHs.
The effect of dosage (CS/LDHs and LDHs) on the adsorption for 2,4-DCP (20 mg/L) were measured. As show in Fig. 5, with the increase of adsorbent, the adsorption is getting better and better. When the dosage is 60 mg, the adsorption capacity reaches the maximum (41% and 25%).
The effect of pH on 2,4-DCP adsorption at initial concentration of 20 mg/L was studied. As show in Fig. 6, pH is sensitive to the adsorption. When the pH < 2, the adsorption efficiencies of the both materials are almost close to zero. CS/LDHs and LDHs are alkaline materials, which is denatured or even dissolved in acidic condition.
Due to the introduction of chitosan, the adsorption effect of hydrotalcite on 2,4-DCP becomes better. The removal increases with the rise of pH, since –NH2 of chitosan forms –NH3+ in acidic solution, which resists the binding of –NH2 and 2,4-DCP. When pH = 7, the adsorption efficiency of both materials for 2,4-DCP reaches the maximum, since 2,4-DCP solution appears acidic, and the materials presents alkaline.
Adsorption dynamics study
Adsorption kinetics
The adsorption kinetics of LDHs and CS/LDHs for 2,4-DCP are shown in Fig. 7. In order to study the adsorption properties for 2,4-DCP by above materials, the data were fitted and analyzed by the pseudo-first-order dynamic equation and the pseudo-second-order dynamic equation.
Where qe is the sorption capacity at equilibrium (mg/g), qt is the adsorbed amount at a contact time t (mg/g), and k1 is the first-order adsorption rate constant (h−1), and k2 is the second-order adsorption rate constant (g mg−1 h−1). The fitting curve is shown in Fig. 8, and the fitting parameters are given in Table 1. According to the fitting results, the correlation coefficient R2 of the pseudo-first-order dynamic equation for the adsorption of 2,4-DCP by LDHs and CS/LDHs is 0.9896 and 0.9927, respectively. The equilibrium adsorption capacity obtained by the fitting result is very different from the equilibrium adsorption capacity obtained by the experiment. It is indicated that the pseudo-first-order kinetics is not suitable to describe the adsorption process of LDHs and CS/LDHs for 2,4-DCP. Therefore, the adsorption method of LDHs and CS/LDHs for 2,4-DCP is not physical adsorption. The correlation coefficient R2 is 0.9917 and 0.9964, which accords with the pseudo-second-order kinetics, indicating that the chemical adsorption dominates for the adsorption of LDHs and CS/LDHs to 2,4-DCP. The pseudo-second-order kinetic model is more suitable for describing this adsorption process.
Adsorption isotherm
The maximum adsorption capacity of CS/LDHs and LDHs was investigated in different 2,4-DCP concentration (Fig. 9). The adsorption isotherms data are fitted with the following Langmuir and Freundlich isotherm models:
-
Langmuir equation
-
Freundlich equation
Where qe is the equilibrium adsorption capacity (mg/g), Ce is the equilibrium concentration of 2,4-DCP in aqueous phase (mg/L), Qm is the maximum adsorption capacity (mg/g), KL is Langmuir constant (L/mg), and KF and n are Freundlich constants.
The fitting parameters are summarized in Table 2. The correlation coefficient of the Langmuir model for the adsorption isotherm fitting of LDHs and CS/LDHs to 2,4-DCP is greater than that of the Freundlich model, indicating that the isothermal fitting of the material for 2,4-DCP is more consistent with the Langmuir model. 1/n indicates the influence of the concentration on the adsorption amount, and the smaller the 1/n, the better the adsorption performance. From Tables 1 and 2, < 1/n < 2 indicates that the materials have weak adsorption and belong to the multi-layer adsorption between ions.
Immobilization of laccase
The laccase was immobilized on above materials by cross-linking method, and the concentration of glutaraldehyde, cross-linking time, immobilization time, pH, and concentration of laccase were optimized. The optimum conditions are as follows: glutaraldehyde concentration is 5% (v/v), the time of cross-linking is 2 h (CS/LDHs) and 3 h (LDHs), the immobilization time is 8 h, pH = 6, and the amount of laccase is 1 g/L (CS/LDHs) and 1.2 g/L (LDHs). Under the optimal conditions, the activity of immobilized laccase was 3126 U/g (L-CS/LDHs) and 2241 U/g (L-LDHs).
Removal of 2,4-DCP by immobilized laccase
Effect of time
In the experiment, immobilized laccase was used to degrade 2,4-DCP (100 mg/L), and reaction conditions were optimized (Fig. 10). As shown in Fig. 10, the removal increases with the reaction time lasting. After 36 h, the removal of 2,4-DCP by two materials remains basically stable, and the removal efficiency by L-CS/LDHs is about 12% higher than L-LDHs. It is obvious that the introduction of chitosan makes the removal of 2,4-DCP be improved. The removal of the two materials is 8.141 mg/g and 6.303 mg/g, respectively.
Effect of temperature
According to previous studies, the effect of temperature on the activity of laccase is relatively remarkable, so the reaction temperature of degradation 2,4-DCP by immobilized laccase was optimized (Fig. 11). The results show that the removal efficiencies of 2,4-DCP are the best at 55 °C and reach 80.63% (L-CS/LDHs) and 50.51% (L-LDHs). The optimum temperature for free laccase is 45 °C. After immobilizing, the partial active sites of laccase combined with the carrier, the molecular structure stability, and the adaptability to temperature increases, expanding the application of laccase.
Effect of pH
The impact of pH on the removal of 2,4-DCP is shown in Fig. 12. pH affects the removal significantly, concerning with the optimum pH of laccase and structure of the materials. 2,4-DCP removal reaches maximum when pH is 6. When pH > 6, laccase spatial structure will change, resulting in the loss of immobilized enzyme activity. At pH < 4, the carrier is partly dissolved, the removal of 2,4-DCP with CS/LHDs as carrier is more than 30%, better than LHDs. It is obvious that introduction of CS increases the acid resistance of carrier.
Effect of concentrations
The effect of concentrations (10–200 mg/L) is shown in Fig. 13. When the concentration of 2,4-DCP is lower than 60 mg/L, the 2,4-DCP removal by L-CS/LDHs is more than 97%, and the removal of 2,4-DCP by L-LDHs is more than 88%.With the increase of the concentration, the removal of 2,4-DCP decreases. Since the active centers of laccase are occupied by 2,4-DCP fully and high concentrations of 2,4-DCP have toxic effect on laccase, leading to rapid decrease of 2,4-DCP degradation. The catalytic oxidation reaction of Fe3+ and Co2+ in the carrier has its equilibrium, leading to rapid decrease in the removal of 2,4-DCP.
Removal analysis
LDHs, CS/LDHs, and free laccase were used to deal with 2,4-DCP; the experimental results are shown in Table 3.
-
1.
Effect of concentration
When 2,4-DCP concentration is lower than 40 mg/L, the removal of 2,4-DCP is more than 95% by L-CS/LDHs and L-LDHs. As the concentration of 2,4-DCP is above 60 mg/L, the removal efficiency is obviously decreased by L-LDHs, but it is still exceeds 80% by L-CS/LDHs. It demonstrates the applicability of L-CS/LDHs to the degradation of 2,4-DCP. Due to the introduction of chitosan, hydrotalcite is entangled with it and the laccase connected with the material by flocculent chitosan; the laccase can be exposed and presents excellent activity.
-
2.
The removal of 2,4-DCP by immobilized laccase was significantly superior to carriers. It is obvious that the removal of 2,4-DCP (100 mg/L) by laccase on L-CS/LDHs is about 17%, and the removal of 2,4-DCP (100 mg/L) by laccase on L-LDHs is only 10%. According to Table 3, the efficiency is not as good as free laccase. The optimum pH of the free laccase is about 5, and both of carriers are alkaline, which have a certain effect on the activity of laccase. When the laccase is immobilized on surface of the carrier, the microenvironment of laccase active center is disturbed, and laccase partial structural changes lead to laccase partial inactivation.
-
3.
The removal of 2,4-DCP by the two carriers is more than 50%.
It is mainly due to the lamellar structure of LDHs and CS/LDHs is composed of Fe3+ and Co2+, and both cations have excellent dechlorination (Chaliha and Bhattacharyya 2009). So LDHs and CS/LDHs as carrier can remove 2,4-DCP greatly.
-
4.
Adsorption of materials can be ignored.
Though LDHs and CS/LDHs have some adsorption for 2,4-DCP with low concentration at 25 °C, the adsorption capacity is very small and the adsorption can be neglected at 55 °C.
-
5.
Comparison with literatures
Li Gan (Li et al. 2010) used electrochemical treatment to remove 2,4-DCP with an initial concentration of 3.25 mg/L, and the removal reached 99.21%. Saeid (Ahmadzadeh and Dolatabadi 2018; Gan et al. 2018) used iron-based nanoparticle adsorption and Fenton-like oxidation to remove 2,4-DCP with an initial concentration of 30 mg/L, and it was removed 67.5%. L-CS/LDHs had excellent removal effect on 2,4-DCP, and the removal efficiency is over 97% when the concentration is lower than 60 mg/L.
Reaction kinetics study
In Table 4, when pH = 6 and T = 55 °C, the initial concentration of 2,4-DCP is about 100 mg/L. The kinetic curves follow the first-order reaction: C=C0 exp. (k1t), in which C0 is the initial concentration of the reactant, C is its concentration at time t, and k1 is the reaction constant of the first-order reaction. The correlation coefficient (R2) for L-CS/LDHs is more than 0.96.
Reusability of the immobilized laccase
The immobilized laccase was used repeatedly for 5 times to degrade 2,4-DCP, which is shown in Fig. 14. During five batches, the removal of 2,4-DCP ranged from 82.14 to 55.34% by L-CS/LDHs and from 60.17 to 32.73% by L-LDHs, maintaining 67% (L-CS/LDHs) and 54% (L-LDHs) of the original removal.
It shows that the immobilized laccase can be used repeatedly. Although the removal efficiency is not as good as free enzyme, immobilized laccase can overcome the unstable shortcoming of free laccase, and its separation and recycling are convenient. Activity of laccase immobilized by inorganic materials (LDHs) reduces markedly, but the introduction of chitosan can effectively improve the inorganic carrier.
Conclusion
-
1.
In this study, CS/LDHs were successfully synthesized by introducing chitosan molecules into the special structure of hydrotalcite. It was confirmed by characterization of SEM, FTIR, and XRD.
-
2.
The adsorption efficiency of 2,4-DCP (20 mg/L) by above materials was studied. Under the optimum conditions, the adsorption capacity was 1.96 mg/g (CS/LDHs) and 1.33 mg/g (LDHs). The adsorption of LDHs and CS/LDHs has better fitting to the pseudo-second-order kinetics, and both are consistent with the Langmuir model and Freundlich model (R2 > 0.99).
-
3.
Above materials were used as carriers to immobilize laccase. Under the optimum conditions, the activity of immobilized laccase is 3126 U/g for L-CS/LDHs as carrier, 2241 U/g for L-LDHs.
-
4.
The removal efficiency of 2,4-DCP (100 mg/L) was studied by two kinds of immobilized laccase. Under the optimal conditions, the removal of 2,4-DCP is 81.53% (L-CS/LDHs) and 63.55% (L-LDHs) respectively. The reactions are more consistent with the first-order reaction kinetics. Immobilization has improved the stabilities of laccase and the immobilized laccases can retain 67% (L-CS/LDHs) and 54% (L-LDHs) of the original removal after 5 cycles.
-
5.
The removal of 2,4-DCP by L-CS/LDHs is excellent, exceeding 97% as its initial concentration below 60 mg/L. It includes the catalytic action of laccase and the dechlorination of Fe3+ and Co2+, and the adsorption can be ignored under the optimal conditions.
All in all, the study has a good application prospect for the removal of chlorophenols.
References
Ahmadzadeh S, Dolatabadi M (2018) In situ generation of hydroxyl radical for efficient degradation of 2,4-dichlorophenol from aqueous solutions. Environ Monit Assess 190(6):340
Chaliha S, Bhattacharyya KG (2009) Fe3+, Co2+ and Ni2+ impregnated MCM41 for wet oxidative destruction of 2,4-dichlorophenol in water. Catal Today 141(1–2):225–233
Gan L, Li B, Guo M et al (2018) Mechanism for removing 2,4-dichlorophenol via adsorption and Fenton-like oxidation using iron-based nanoparticles. Chemosphere 206:168–174
Giardina P, Faraco V, Pezzella C, Piscitelli A, Vanhulle S, Sannia G (2010) Laccases: a never-ending story. Cell Mol Life Sci:CMLS 67(3):369–385
Hakulinen N, Kiiskinen LL, Kruus K, Saloheimo M, Paananen A, Koivula A et al (2002) Crystal structure of a laccase from melanocarpus albomyces with an intact trinuclear copper site. Nat Struct Biol 9(8):601–605
Hang JZ, Zhao ZD, Fan Y, Wang L (2013) Adsorption performance and discoloring behavior of nickel-iron hydrotalcite-like compound for azo anionic dyes. Chem Res 24(2):149–154
Li M (2007). Study on the properties and application of chitosan. Sci Technol Inform: Sci Teach Res, (20): 52-53.
Li FL, Peng HZ, Feng LY (2010) Adsorptive removal of 2,4-dcp from water by fresh or regenerated chitosan/ACF/TiO2, membrane. Sep Purif Technol 70(3):354–361
Ling F, Fang L, Lu Y, Gao J, Wu F, Zhou M et al (2016) A novel Co Fe layered double hydroxides adsorbent: high adsorption amount for methyl orange dye and fast removal of Cr(vi). Microporous Mesoporous Mater 234:230–238
Liu XZ, Li Y, Liang YX, Zhang RF (2016) Laccase immobilization with silica/chitosan macroporous composites and its application in 2,4-dichlorophenol degradation. J Chem Eng Chin Univ 30(1):201–209
Papadopoulou AA, Tzani A, Polydera AC, Polydera AC, Katapodis P, Voutsas E, Detsi A, Stmatis H (2018) Green biotransformations catalysed by enzyme-inorganic hybrid nanoflowers in environmentally friendly ionic solvents. Environ Sci Pollut Res 25(27):26707–26714
Rashid S, Shen C, Chen X, Li S, Chen Y, Wen Y et al (2015) Enhanced catalytic ability of chitosan–Cu–Fe bimetal complex for the removal of dyes in aqueous solution. RSC Adv 5(110):90731–90741
Viswanathan N, Meenakshi S (2010) Selective fluoride adsorption by a hydrotalcite/chitosan composite. Appl Clay Sci 48(4):607–611
Wang J, Li Y, Chen W, Peng J, Hu J, Chen Z et al (2016) The rapid coagulation of graphene oxide on la-doped layered double hydroxides. Chem Eng J 309:445–453
Xu J, Tan L, Baig SA, Wu D, Lv X, Xu X (2013a) Dechlorination of 2,4-dichlorophenol by nanoscale magnetic Pd/Fe particles: effects of pH, temperature, common dissolved ions and humic acid. Chem Eng J 231(17):26–35
Xu R, Zhou Q, Li F, Zhang B (2013b) Laccase immobilization on chitosan/poly(vinyl alcohol) composite nanofibrous membranes for 2,4-dichlorophenol removal. Chem Eng J 222(15):321–329
Yang B, Wang XM (2014) Study on the optimum condition for immobilization and properties of laccase on coordinated chitosan-Cu2+. Adv Mater Res 864-867:1262–1265
Yang B, Du D, Sun Y, Wang XM (2013) Decolorization of reactive black KNB and direct red dye by laccase. Chin J Environ Eng 7(12):4835–4840
Yang K, Yan LG, Yang YM, Yu SJ, Shan RR, Yu HQ et al (2014) Adsorptive removal of phosphate by Mg–Al and Zn–Al layered double hydroxides: kinetics, isotherms and mechanisms. Sep Purif Technol 124(124):36–42
Yang J, Huang Y, Yang Y, Hongming Y et al (2015) Cagelike mesoporous silica encapsulated with microcapsules for immobilized laccase and 2,4-DCP degradation. J Environ Sci 38(12):52–62
Youn HD, Hah YC, Kang SO (1995) Role of laccase in lignin degradation by white-rot fungi. FEMS Microbiol Lett 132(3):183–188
Zhan T, Song Y, Li X, Hou W (2016a) Electrochemical sensor for bisphenol a based on ionic liquid functionalized Zn-Al layered double hydroxide modified electrode. Mater Sci Eng C 64:354
Zhan TR, Song Y, Yang Q, Hou W (2016b) Structure and catalytic activity of hemoglobin assembled with layered double hydroxide nano sheets by coprecipitation using a T-shaped microreactor. Chem Eng J 306:1143–1150
Zhou Y, Jin XY, Lin H, Chen ZL (2011) Synthesis, characterization and potential application of organobentonite in removing 2,4-DCP from industrial wastewater. Chem Eng J 166(1):176–183
Funding
This study is supported by the National key R & D projects of China (2016YFC0208100).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Rights and permissions
About this article
Cite this article
Yang, B., Liu, J., Liu, Z. et al. Preparation of chitosan/Co-Fe-layered double hydroxides and its performance for removing 2,4-dichlorophenol. Environ Sci Pollut Res 26, 3814–3822 (2019). https://doi.org/10.1007/s11356-018-3886-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3886-x