Abstract
The concentrations, potential risk, and distributions of heavy metals in urban road runoff from different traffic density were determined and compared in Beijing, China. It showed that the concentrations of heavy metals in road runoff were strongly influenced by traffic density, resulting in total concentrations of Cu, Zn, Fe, Mn, and Pb in the runoff from highway higher than those from the road nearby campus. The potential ecological risk of heavy metals in the runoff from highway was higher than those from the road nearby campus. The distributions of heavy metals were not influenced by the traffic density. Cu, Zn, Cr, Cd, Pb, and Mn in road runoff transported predominantly in particulate-bound form and the dissolved form mainly distributed in colloidal fraction (1 kDa to 0.45 μm). Traffic density did not change the speciation of heavy metals in the road runoff, in which Cr and Zn mainly expressed in organic colloidal fraction while Fe, Mn, Cd, Pb, and Cu expressed in inorganic colloidal fraction. The traffic activities would contribute to the strong correlations between Fe, Zn, Mn, Cr, and Pb because of the similar sources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pollutants in urban street and highway runoff, such as heavy metals, nutrients, trace organics, and sediments, have been deemed as nonpoint pollution sources of urban aquatic environment. Among the pollutants, heavy metals have been of great concerns due to their potential harmfulness for the aquatic organisms, human and ecosystem (Chai et al. 2014; Wijesiri et al. 2016; Gill et al. 2017). Generally, heavy metals in urban road runoff are mainly generated from traffic activities, e.g., tires, automobile exhaust, road asphalt, fuel combustion, and parking dust (Revitt et al. 2014; LeFevre et al. 2015; Lintern et al. 2016). Besides, the behaviors (such as turning, gear shift, holding, and idling), types of vehicles and petrol used vary notably in the roads with different traffic density, which may influence the content and speciation of heavy metals in urban road runoff. Therefore, the concentrations and formations of heavy metals in urban road runoff related to the density of motor vehicle traffic are necessary to clarify for risk assessment and environmental behavior prediction, further for urban stormwater management.
It has been shown that the traditional fractionations of heavy metals in urban runoff are mostly divided into “dissolved” fraction (< 0.45 μm) and “particulate” fraction (> 0.45 μm) (Hargreaves et al. 2017; Yasutaka et al. 2017). However, the “dissolved” fraction does not represent the truly dissolved metal ions, which composed of free metals, complex ions, metals bound to a variety of ligands, forming molecules of various dimensions and chemical characteristics (Guéguen and Dominik 2003). Consequently, the “colloid” fraction is defined as the third phase, whose size ranged from 1 nm to 0.45 μm with an intermediate size or molecular weights. Colloids have received special attention because of their small size and large specific surface area, and thus, their strong adsorption capacity of other substances, as well as high diffusibility and mobility, which enhance heavy metals’ bioavailability and potential toxicity. It has been reported that the rapid migration and enrichment of colloidal heavy metals increase the pollution risk of groundwater due to colloids’ penetration in porous soil and fractured rocks (Aosai et al. 2014; Majumder et al. 2014). Therefore, understanding and evaluation of the heavy metals in different size fractions in the road runoff from different traffic density, especially colloidal fraction, are of fundamental importance for risk assessment and environmental behavior prediction of heavy metals, further for their control in various green infrastructures for stormwater management and the protection of the received water body.
The concentrations, variability, and distribution of heavy metals (e.g., particulate-bound and colloid-associated) in urban road runoff with different density of motor vehicle traffic were determined and compared in this study. Also, the ecological risk assessment was made and the speciation of colloid-associated fraction was detected.
Materials and methods
Sampling
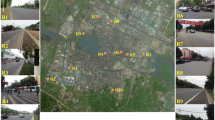
Two sites were selected for road runoff sampling. Site A is located at Chegongzhuang Street in Xicheng Region in Beijing, which is an arterial street with heavy traffic at downtown commercial district where the big cars (such as trucks, towed vehicles, etc.) are limited and only small cars are permitted. Comparatively, site B is located at the road nearby the campus of Beijing University of Civil Engineering and Architecture in Daxing Region with lighter traffic and diverse types of cars at the suburb in Beijing. The location of the sampling site A (39° 55′ 52.72″ N, 116° 20′ 10.44″ E) and site B (39° 44′ 58.90″ N, 116° 16′ 59.09″ E) are shown in Fig. 1. General characteristics of two sites are listed in Table 1. Urban road runoff was sampled with brown glass bottles at the curb (gutter) inlet on March 31, 2015, May 2 and 14, 2016, April 21, July 11, and August 5, 2018. The temporal precipitation of these six rainfall events are shown in Fig. 2, whose data are received from the closest meteorological station, which location was shown in Fig. 1 (20 km to site A and 40 km to site B). Before sampling, the bottles were thoroughly washed with acid solution (0.01 M HCl), followed by tap and deionized water sequentially. The samples were immediately transported to the laboratory and filtered through 0.45-μm cellulose filters and stored in the dark at 4 °C for ultrafiltration with decreasing pore size cutoff.
Colloid separation
To concentrate the solution with an operational colloidal particle size of 1 nm–0.45 μm, the separation of the colloidal fraction of the solution obtained from filtration through < 0.45 μm was performed by cascade ultra-filtration on membranes (regenerated cellulose) using a stirred cell (Amicon Stirred Cell Model 8200, Millipore, USA). Colloids of water samples were isolated of 30 kDa (3 nm), 10 kDa (2.5 nm), and 1 kDa (1 nm) nominal molecular cutoffs in concentration operation modes. Nitrogen gas at different pressures (0.4–2.7 kgf/cm2) was applied to the ultra-filtration cells directly. Constant stirring above the membrane surface was used to avoid the accumulation of solute molecules and minimize localized concentration effects at the membrane surface (Singhal et al. 2006). The schematic of the separation procedures employed is shown in Fig. 3.
The ultra-filtration membranes were pre-treated before using. Firstly, the membranes were soaked more than three times in a beaker filled with ultra-pure water for 3 days. Then, the membrane was installed into the ultra-filtration cell and rinsed twice with 0.01 M NaOH and 0.01 M HCl alternately, with the stirrer agitating more than 10 min each time (Du et al. 2014).
The organic carbon and heavy metal mass balance and recovery rate were checked as follows: (% recovery) = (CpVp + CrVr)/C0V0, where V0, Vp, and Vr are the volume of feed, permeate, and retentate samples, respectively, and C0, Cp, and Cr are the organic carbon (or heavy metals) concentrations of feed, permeate, and retentate samples, respectively (Wei et al. 2008). The organic carbon and heavy metal mass balance of ultra-filtration system in this study was 100 ± 5%.
Chemical analysis
Acid digestion of raw runoff samples was conducted with aqua regia prior to the measurement of Cu, Zn, Cr, Cd, Pb, and Mn. After acid digestion, the solutions were cooled and diluted to 100 mL with ultrapure water for Cu, Zn, Cr, Cd, Pb, and Mn determination to calculate total concentration in runoff. Cu, Zn, Cr, Cd, Pb, and Mn in different fractions were determined immediately after ultra-filtration. Concentrations of heavy metals in different fractions, the dissolved samples (< 0.45 μm) and the digested samples were determined by ICP–MS (NexION 300, PerkinElmer, Germany). Particulate-bound heavy metals were determined as the difference between the total and the dissolved concentrations in the runoff. Colloidal organic carbon (COC) was determined with a TOC analyzer (Jena multi N/C 3100, Germany).
To ensure the accuracy of the analyses, calibrations were performed after every tenth samples, and between these calibrations two internal standards were analyzed. The internal standards substances were Ge and Sc, both with the concentrations of 20 μg/L. Internal standards within ± 10% of a long-time average were accepted; otherwise, all ten samples run since the foregoing calibration sequence was reanalyzed.
Risk assessment
The quantitative approach developed by Hakanson (1980) was usually used to evaluate the potential ecological risk of multiple heavy metal pollution, whose potential ecological risk index (RI) is defined as follows.
where Eri is the risk factor, Tri is the toxic-response factor for a given substance (e.g., Cu = Pb = 5, Zn = 1, Cd = 30, Cr = 2) (Yang et al. 2009), Ci represents metal content, and C0 is the regional background value of heavy metals. The heavy metal content of class II standard of Surface Water Environmental Quality Standard (GB 3838-2002) in China (e.g., Cu = Zn = 1.0 mg/L, Cd = 0.005 mg/L, Cr = 0.05 mg/L, Pb = 0.01 mg/L) was chosen as the regional background value.
Results and discussion
Risk assessment of heavy metals in runoff
Metal content in runoff
The concentrations of the total, particulate-bound (> 0.45 μm), and dissolved heavy metals (< 0.45 μm) investigated in six runoff events at two sites are presented in Table 2. It was shown that the total heavy metal concentrations of Cu, Zn, Cr, Cd, Pb, and Mn in the six rainfall runoff events at site A were higher than those at site B, suggesting that the concentrations of heavy metals in road runoff were strongly influenced by traffic density. The concentrations of heavy metals in the road runoff are higher when traffic density is higher. The data also showed that the heavy metals in the runoff, from either highway or road nearby campus, predominantly expressed in particulate-bound form with the percentage of particulate-bound to the total heavy metals ranged from 63 to 99%. Obviously, the distributions of heavy metals had no evident difference at sites with different traffic density in the same runoff event, due to the particulate form associating with the suspended solids on the road surface (Lau et al. 2009; Gunawardana et al. 2015).
Additionally, the event mean concentrations (EMCs) of heavy metals observed in highway runoff in six runoff events at site A followed the order of Zn > Cu > Mn > Cr > Pb > Cd, which was similar to the results reported by Sim-Lin Lau (Lau et al. 2009). Although the concentrations order varied in different rainfall runoff events, the concentrations of Cu, Zn, Mn, and Cr were high enough to be paid attention in the six rainfall runoff events at both two sites. The dominant heavy metals of Zn and Cu in the road runoff could be caused by tire wear of vehicles for Zn (De Silva et al. 2016) and brake systems for Cu (Yu et al. 2016). The high concentration of Mn was probably due to the relatively low standard of Mn content (2 mg/L) in gasoline from national standard of Gasoline for Motor Vehicles (GB 17930-2013) in China (Standardization Administration of China 2013), which was consistent with the study of road dust in Beijing, China (Men et al. 2018). Cr may come from the braking lining wear in vehicles (Lee et al. 2011).
The concentrations of heavy metals in road runoff were compared with the Environmental Quality Standards for Surface Water in China (GB 3838-2002) (State Environmental Protection Administration 2002). The statistic data suggested that the total concentration of Cd in the road runoff exceeded the class V standard value (10 μg/L) slightly at both sites A and B on March 31, 2015, May 14, 2016, April 21, and August 5, 2018, seriously at site A on July 11, 2018, while the concentrations of Cr were 4–9 times the class V standard value (100 μg/L) in all rainfall runoff events at two sites, indicating the serious pollution of Cr at two sites. In addition, the concentrations of Pb in six runoff events were higher than the class V standard value only at site A. Although the concentrations of Cd and Pb were comparatively low, their potential toxicities may be high because of their low standard values. It was obvious that the long-term direct discharge of road runoff into surface water would trigger the heavy metal pollution and harmfulness for aquatic organisms. Therefore, the road runoff, especially from the high-density traffic road, should be treated for heavy metal removal prior to discharge to the waterbody.
Risk assessment of heavy metals
The following grade was used to assess the potential ecological risk of multiple heavy metal pollution indicated by the Er and RI values (Hsu et al. 2016; Feng et al. 2017):
The Er for different heavy metals and RI of runoff samples at sites A (highway) and B (road nearby campus) in six rainfall events are calculated and listed in Table 3.
As shown in Table 3, the Er values of all heavy metals (except Cd on May 2, 2016 and August 5, 2018, Cr on May 2, 2016 and July 11, 2018) in road runoff during six rainfall events at site A were higher than that at site B, suggesting higher potential ecological risk of heavy metals with higher traffic density. Universally, the Er values of Cd and Pb were higher than that of Cu, Zn, and Cr in the six runoff events at two sites. The Er values of Cu, Zn, and Cr in the runoff were generally lower than 40 at sites A and B, indicating that the potential ecological risks of these metals are low. The Er values of Cd were 252.39 on March 31, 2015, and 265.14 on July 11 at site A, 150.93 and 95.34 on May 14, 2016, 108.84 and 112.14 on August 5, 2018, at sites A and B, respectively, indicating a considerable and even high potential ecological effect for Cd in road runoff. Similarly, the Er values of Pb were higher than 80 at site A on March 31, 2015, and May 2, 2016, demonstrating a considerable potential ecological risk. Although the concentrations of Cd and Pb were lower than that of Cu, Zn, and Cr, the potential ecological risks of Cd and Pb were considerable and high in the road runoff, demonstrating that more attention need to be drawn to their source control and discharge reduction.
The RI value was applied to assess the comprehensive ecological risk of multiple heavy metals at two sites. It was shown that the RI values in six runoff events at site A were higher than that at site B, indicating that the potential ecological risk is highly influenced by the traffic density. The RI values of runoff samples collected from site B were all lower than 300, which suggested a low or moderate risk in the road runoff nearby campus with lighter traffics. Whereas, the RI values of runoff samples collected from site A ranged from 300 to 600 on March 31, 2015, May 14, 2016, and July 11, 2018, indicating a considerable ecological risk of heavy metals in the highway runoff with heavy traffics.
Concentrations of metals during a runoff event
The variations of concentrations of heavy metals in runoff during the rainfall duration on May 2, 2016, at two sites are shown in Fig. 4. It was shown that the concentrations of all heavy metals in the runoff were higher at site A than those at site B during the entire rainfall duration except Cd and Cr in the first hour. At site A, the concentrations of all metals descended with the rainfall duration increasing, and apparent concentration peak could be observed around 30 min from the beginning, indicating the distinct first flush phenomenon (Kayhanian et al. 2012). However, the concentrations of all heavy metals had no evident first flush but fluctuations at site B during the entire rainfall duration, which could be caused by the relatively low concentrations of metals. Therefore, the concentrations of heavy metals were significantly influenced by the traffic density. The concentrations of heavy metals were higher, and an evident first flush effect was detected in the runoff during the rainfall duration at site A with higher traffic density. Conversely, the concentrations of heavy metals were low, and first flush effect was not obvious when traffic density was relatively low at site B, which is consistent with the result of TSS detection studied by Barrett (Barrett et al. 1998).
Partitioning of metals in runoff
The EMC distributions of different size fractions of Cu, Zn, Fe, Mn, Cd, Pb, and Cr in the six runoff events at two sites are shown in Fig. 5. Although the contents of heavy metals in different molecular weights varied with fluctuation, all of the heavy metals were found in different molecular weights in the six runoff events at sites A and B. Additionally, all of the heavy metals mainly distributed in colloidal fraction (1 kDa–0.45 μm) at sites A and B, suggesting that the molecular weight distributions of heavy metals were not influenced by the traffic density. It was shown that the colloidal fraction of heavy metals in the six runoff accounted for 68 to 99% at site A and 72 to 99% at site B, respectively. While, the content of truly dissolved fraction (< 1 kDa) only accounted for 1 to 32% at site A and 1 to 28% at site B, respectively. The temporal distributions in different molecular weights of the heavy metals in the runoff during the rainfall duration on May 2, 2016, were also investigated, and the results showed that all of the metals were mostly distributed in colloid fraction, with 74 to 99% at site A and 63 to 100% at site B (Fig. S1), respectively, further proving that the dissolved heavy metals mainly existed in colloidal form and the traffic density could not influence on the distribution of heavy metals. Therefore, the proportion of colloidal form to the total dissolved form of heavy metals would not be influenced by traffic density. Traffic density could just influence on the concentrations of heavy metals in road runoff. It also suggested that the dissolved heavy metals in road runoff could adhere to colloid substances (organic or inorganic), accumulate in colloid fraction and migrate as colloidal form. The colloidal heavy metals should be removed in the runoff control facilities such as bioretentions, grass swales, and wetlands.
Molecular weight distributions of heavy metals on March 31, 2015 (a), May 2, 2016 (c), May 14, 2016 (e), April 21, 2018 (g), July 11, 2018 (i), and August 5, 2018 (k) at site A; on March 31, 2015 (b), May 2, 2016 (d), May 14, 2016 (f), April 21, 2018 (h), July 11, 2018 (j), and August 5, 2018 (l) at site B
Moreover, Cu, Fe, Pb, and Cr were almost totally distributed in colloidal fraction in the six runoff events, which ranged from 85 to 99% at site A and 86 to 99% at site B, respectively. And the higher percentages of Cu, Fe, Pb, and Cr in colloidal size classes 1–30 kDa (from 45 to 71%) were also detected at two sites, further implying no influence by traffic density on the distribution of Cu, Fe, Pb, and Cr in the road runoff. Compared to Cu, Fe, Pb, and Cr, the distribution of Zn, Mn, and Cd in colloidal fraction were relatively low in six runoff events, but still ranged from 68 to 94% at site A, and 71 to 90% at site B, respectively. Based on the risk assessment of heavy metals in road runoff in Table 3, the potential toxicities of Cd and Pb were the highest among the heavy metals. In addition, the high content of colloidal fraction in the runoff may result in high diffusibility and mobility of Cd and Pb. Therefore, Cd and Pb in road runoff should be removed firstly to avoid their migration to aquatic environment.
Correlations between colloidal heavy metals and COC
To assess the speciation of colloidal metals and possible contamination from the similar sources, correlation analysis was conducted between heavy metals and COC (1 kDa to 0.45 μm) in runoff samples at two sites, as shown in Table 4. It was found that intensively significant correlations between colloidal Cr and COC at sites A (r = 0.936, p < 0.05) and B (r = 0.873, p < 0.05), as well as colloidal Zn and COC at site A (r = 0.893, p < 0.05) and site B (r = 0.798), respectively, which implied these colloidal metals in the road runoff were strongly bound to colloidal organic matters due to the functional groups with high affinity (Ren et al. 2010). While the weak correlations between Fe, Mn, Cd, Pb, Cu, and COC suggested that these metals mostly associated with inorganic colloidal substances. Traffic density did not change the speciation of heavy metals in the road runoff, in which Cr and Zn mainly expressed in organic colloidal fraction while Fe, Mn, Cd, Pb, and Cu expressed in inorganic colloidal fraction. It also suggested that most of Cu and Zn may migrate with organic matters synchronously in the runoff.
The significant correlations among colloidal Zn, Fe, Mn, Cr, and Pb (r > 0.80) were found in the road runoff at site A, as well as the strong correlations among colloidal Zn, Fe, Mn, Cr, and Pb (r > 0.50) in the road runoff at site B. The traffic activities would contribute to the strong correlations between Fe, Zn, Mn, Cr, and Pb because of the similar sources from tires, automobile exhaust, fuel combustion, etc. Moreover, the correlation coefficient was higher when traffic density was larger. No correlation was found between Cd and other metals in the runoff at two sites, suggesting that Cd may be not from the same sources of other metals.
Conclusions
The concentrations of Cu, Zn, Cr, Cd, Pb, and Mn in the six rainfall runoff events from highway were all higher than those from the road nearby campus, suggesting that the concentrations of heavy metals in road runoff are strongly influenced by traffic density. In view of multiple heavy metal contamination, the potential ecological risk of the heavy metals in the runoff from highway was higher than the road nearby campus, indicating a considerable ecological risk in the runoff from highway. Although the concentrations of Zn, Cu, Mn, and Cr were higher in the road runoff, the potential ecological risk of Cd and Pb exhibited considerable or high ecological risk. Cu, Zn, Cr, Cd, Pb, and Mn in road runoff transported predominantly in particulate-bound form (63 to 99%) and the dissolved form mainly distributed in colloidal fraction (68 to 99%), implying that the distribution of heavy metals was not influenced by the traffic density. Based on the higher potential toxicity and the high diffusibility and mobility of colloidal fraction, Cd and Pb in road runoff should be removed firstly to avoid their migrations to aquatic environment. Additionally, first flush effect was detected during the rainfall duration in the road runoff with higher traffic density, while without lighter traffic density. Traffic density did not change the speciation of heavy metals in the road runoff, in which Cr and Zn mainly expressed in organic colloidal fraction while Fe, Mn, Cd, Pb, and Cu expressed in inorganic colloidal fraction. The traffic activities would contribute to the strong correlations between Fe, Zn, Mn, Cr, and Pb because of the similar sources from tires, automobile exhaust, fuel combustion, etc. And the correlation coefficient was higher when traffic density was higher.
References
Aosai D, Yamamoto Y, Mizuno T, Ishigami T, Matsuyama H (2014) Size and composition analyses of colloids in deep granitic groundwater using microfiltration/ultrafiltration while maintaining in situ hydrochemical conditions. Colloids Surf A Physicochem Eng Asp 461:279–286. https://doi.org/10.1016/j.colsurfa.2014.08.007
Barrett ME, Irish LB Jr, Mmalina JF Jr, Charbeneau RJ (1998) Characterization of highway runoff in Austin, Texas, area. J Environ Eng 124:131–137. https://doi.org/10.1061/(ASCE)0733-9372(1998)124:2(131)
Chai HX, Shen SB, Bin HX et al (2014) Effect of baffled water-holding garden system on disposal of rainwater for green building residential districts. Desalin Water Treat 52:2717–2723. https://doi.org/10.1080/19443994.2013.807057
De Silva S, Ball AS, Huynh T, Reichman SM (2016) Metal accumulation in roadside soil in Melbourne, Australia: effect of road age, traffic density and vehicular speed. Environ Pollut 208:102–109. https://doi.org/10.1016/j.envpol.2015.09.032
Du X, Xu Z, Li J, Zheng L (2014) Characterization and removal of dissolved organic matter in a vertical flow constructed wetland. Ecol Eng 73:610–615. https://doi.org/10.1016/j.ecoleng.2014.09.098
Feng J, Zhu X, Wu H, Ning C, Lin G (2017) Distribution and ecological risk assessment of heavy metals in surface sediments of a typical restored mangrove–aquaculture wetland in Shenzhen, China. Mar Pollut Bull 124:1033–1039. https://doi.org/10.1016/j.marpolbul.2017.01.004
Gill LW, Ring P, Casey B, Higgins NMP, Johnston PM (2017) Long term heavy metal removal by a constructed wetland treating rainfall runoff from a motorway. Sci Total Environ 601–602:32–44. https://doi.org/10.1016/j.scitotenv.2017.05.182
Guéguen C, Dominik J (2003) Partitioning of trace metals between particulate, colloidal and truly dissolved fractions in a polluted river: the Upper Vistula River (Poland). Appl Geochem 18:457–470. https://doi.org/10.1016/S0883-2927(02)00090-2
Gunawardana C, Egodawatta P, Goonetilleke A (2015) Adsorption and mobility of metals in build-up on road surfaces. Chemosphere 119:1391–1398. https://doi.org/10.1016/j.chemosphere.2014.02.048
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14:975–1001. https://doi.org/10.1016/0043-1354(80)90143-8
Hargreaves AJ, Vale P, Whelan J, Constantino C, Dotro G, Campo P, Cartmell E (2017) Distribution of trace metals (Cu, Pb, Ni, Zn) between particulate, colloidal and truly dissolved fractions in wastewater treatment. Chemosphere 175:239–246. https://doi.org/10.1016/j.chemosphere.2017.02.034
Hsu LC, Huang CY, Chuang YH, Chen HW, Chan YT, Teah HY, Chen TY, Chang CF, Liu YT, Tzou YM (2016) Accumulation of heavy metals and trace elements in fluvial sediments received effluents from traditional and semiconductor industries. Sci Rep 6:1–12. https://doi.org/10.1038/srep34250
Kayhanian M, Fruchtman BD, Gulliver JS, Montanaro C, Ranieri E, Wuertz S (2012) Review of highway runoff characteristics: comparative analysis and universal implications. Water Res 46:6609–6624. https://doi.org/10.1016/j.watres.2012.07.026
Lau S-L, Han Y, Kang J-H, Kayhanian M, Stenstrom MK (2009) Characteristics of highway stormwater runoff in Los Angeles: metals and polycyclic aromatic hydrocarbons. Water Environ Res 81:308–318. https://doi.org/10.2175/106143008X357237
Lee JY, Kim H, Kim Y, Han MY (2011) Characteristics of the event mean concentration (EMC) from rainfall runoff on an urban highway. Environ Pollut 159:884–888. https://doi.org/10.1016/j.envpol.2010.12.022
LeFevre GH, Paus KH, Natarajan P et al (2015) Review of dissolved pollutants in urban storm water and their removal and fate in bioretention cells. J Environ Eng 141:1–23. https://doi.org/10.1061/(ASCE)EE.1943-7870.0000876.
Lintern A, Leahy PJ, Heijnis H, Zawadzki A, Gadd P, Jacobsen G, Deletic A, Mccarthy DT (2016) Identifying heavy metal levels in historical flood water deposits using sediment cores. Water Res 105:34–46. https://doi.org/10.1016/j.watres.2016.08.041
Majumder S, Nath B, Sarkar S, Chatterjee D, Roman-Ross G, Hidalgo M (2014) Size-fractionation of groundwater arsenic in alluvial aquifers of West Bengal, India: the role of organic and inorganic colloids. Sci Total Environ 468–469:468–469. https://doi.org/10.1016/j.scitotenv.2013.08.087
Men C, Liu R, Xu F, Wang Q, Guo L, Shen Z (2018) Pollution characteristics, risk assessment, and source apportionment of heavy metals in road dust in Beijing, China. Sci Total Environ 612:138–147. https://doi.org/10.1016/j.scitotenv.2017.08.123
Ren H, Liu H, Qu J, Berg M, Qi W, Xu W (2010) The influence of colloids on the geochemical behavior of metals in polluted water using as an example Yongdingxin River, Tianjin, China. Chemosphere 78:360–367. https://doi.org/10.1016/j.chemosphere.2009.11.018
Revitt DM, Lundy L, Coulon F, Fairley M (2014) The sources, impact and management of car park runoff pollution: a review. J Environ Manag 146:552–567. https://doi.org/10.1016/j.jenvman.2014.05.041
Singhal RK, Preetha J, Karpe R, Tirumalesh K, Kumar SC, Hegde AG (2006) The use of ultra filtration in trace metal speciation studies in sea water. Environ Int 32:224–228. https://doi.org/10.1016/j.envint.2005.08.015
Standardization Administration of China (2013) Gasoline for motor vehicles (GB 17930-2013)
State Environmental Protection Administration (2002) Environmental quality standard for surface water (GB 3838-2002)
Wei Q, Wang D, Wei Q, Qiao C, Shi B, Tang H (2008) Size and resin fractionations of dissolved organic matter and trihalomethane precursors from four typical source waters in China. Environ Monit Assess 141:347–357. https://doi.org/10.1007/s10661-007-9901-1
Wijesiri B, Egodawatta P, McGree J, Goonetilleke A (2016) Influence of uncertainty inherent to heavy metal build-up and wash-off on stormwater quality. Water Res 91:264–276. https://doi.org/10.1016/j.watres.2016.01.028
Yang Z, Wang Y, Shen Z, Niu J, Tang Z (2009) Distribution and speciation of heavy metals in sediments from the mainstream, tributaries, and lakes of the Yangtze River catchment of Wuhan, China. J Hazard Mater 166:1186–1194. https://doi.org/10.1016/j.jhazmat.2008.12.034
Yasutaka T, Imoto Y, Kurosawa A, Someya M, Higashino K, Kalbe U, Sakanakura H (2017) Effects of colloidal particles on the results and reproducibility of batch leaching tests for heavy metal-contaminated soil. Soils Found 57:861–871. https://doi.org/10.1016/j.sandf.2017.08.014
Yu Y, Li Y, Li B, Shen Z, Stenstrom MK (2016) Metal enrichment and lead isotope analysis for source apportionment in the urban dust and rural surface soil. Environ Pollut 216:764–772. https://doi.org/10.1016/j.envpol.2016.06.046
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant number 51878024), the Natural Science Foundation of Beijing Municipality (grant number 8162016), Science and Technology Projects of Beijing Municipal Education Commission (grant number KM201810016008), the Beijing Advanced Innovation Center of Urban Design for Future Cities: Sponge City Development and Water Quantity & Quality Risk Control (grant number UDC2016040100), and the Research Founds of Beijing University of Civil Engineering and Architecture (grant number KYJJ2017030).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Fig S1
(DOCX 393 kb)
Rights and permissions
About this article
Cite this article
Du, X., Zhu, Y., Han, Q. et al. The influence of traffic density on heavy metals distribution in urban road runoff in Beijing, China. Environ Sci Pollut Res 26, 886–895 (2019). https://doi.org/10.1007/s11356-018-3685-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3685-4