Abstract
Guava leaves (Psidium guajava, GL), a high-phenolic- and flavonoid-containing plant resource capable of substituting the high-quality forage, may help in mitigating ruminal methane (CH4) emission without adverse impact on nutrient degradability if supplemented at an appropriate level. In order to test this hypothesis, rumen fermentation, CH4 production, and nutrient degradability of GL either solely or as a substitute of berseem hay (Trifolium alexandrinum, BH) were evaluated in a diet containing 50:50 concentrate to roughage. Five different levels of GL (0, 12.5, 25, 37.5, and 50%) were tested in vitro after 24 h incubation using a semi-automated gas production (GP) system. The current findings indicated that merely the presence of GL resulted in significantly lower values for cumulative GP (P < 0.001), CH4 emission (P < 0.05), truly degraded dry matter (TDDM; P < 0.001), truly degraded organic matter (TDOM; P < 0.001), and ammonia nitrogen (NH3-N) concentration (P < 0.001); however, pH (P < 0.001) and partitioning factor (P < 0.001) were higher. The total and individual volatile fatty acid (VFA) concentrations were drastically declined with GL as compared to BH (P < 0.05). A negative linear correlation was recorded between the levels of GL and GP including CH4 production (P < 0.05). The addition of GL up to 25% did not pose any negative effect on both TDDM and TDOM values along with NH3-N concentration. In addition, the inclusion of GL up to 25% did not affect the total or individual VFA concentration. Conclusively, in a medium concentrate diet, use of 25% GL and 25% BH in animal diet could be a promising alternative for mitigating the CH4 production without any deleterious effect on nutrient degradability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In most of the tropics as well as sub-tropics, high-quality forage (i.e., berseem or clover hay) is considered as the main livestock diet during the dry season. Unfortunately, due to the high methanogenic potential (Adjorlolo et al. 2016), it significantly contributes to the global warming by producing up to 18% of the anthropogenic greenhouse gases, representing 37% of the total anthropogenic methane (CH4) emission (FAO 2006). In addition, unwanted emission of CH4 may account up to 12% loss of the gross energy of feeds (Eckard et al. 2010). Currently, various CH4 mitigation strategies in ruminants are being explored worldwide by nutritionists in order to achieve economic as well as environmental benefits. For instance, essential oils, saponins, condensed tannins, nitrates, and sulfates have been assessed to alleviate the CH4 production in vitro and in vivo (Patra and Yu 2014; Wu et al. 2015).
In the previous studies, direct inclusion of plants containing tannins in ruminant diets efficiently lowered the CH4 emission (Tiemann et al. 2008; Hariadi and Santoso 2010). Furthermore, these tannins have been reported to be associated with reduced ruminal degradation of proteins and carbohydrates (Ramírez-Restrepo et al. 2004), increased microbial protein synthesis (Hernández et al. 2014), increased nitrogen retention, reduced urinary N2O emission, and fiber fermentation (Soltan et al. 2013). However, a major drawback of implementing diets with tannin-rich plants aimed at reducing CH4 emissions is often associated with a decline in the digestibility of the feeds along with lower productivity of the animals (Waghorn 2008). Guava (Psidium guajava) is an important fruit crop and medicinal plant belonging to the genus Psidium of the Myrtaceae family (Gutiérrez et al. 2008). Flavonoids and tannins are the predominant polyphenolic compounds in guava leaves (GL) (Badan 2004).
To the best of our knowledge, there is no report regarding the optimal proportion of GL that could limit methanogenesis without deterring the nutrient digestibility toward achieving economic and environmental goals as well. Therefore, it was postulated that the understanding of gas production kinetics and nutrient degradability is very vital to obtain the optimal concoction of Trifolium alexandrinum (berseem hay; BH) and GL in total mixed ration (TMR) to justify more suitably balanced diets along with alleviating the negative impact of CH4 production. We hypothesized that GL, at a proper level, could help in reducing ruminal CH4 emission without adverse impact on nutrient degradability. Hence, the study was aimed at assessing the effect of replacement of the high-quality forage (i.e., BH) by different levels of GL in a medium concentrate ration on CH4 emission, true degradation of dry matter (DM), organic matter (OM), and microbial efficiency via an in vitro gas production system.
Materials and methods
The present study was accomplished at the Advanced Laboratory of Animal Nutrition, Department of Animal and Fish Production, Faculty of Agriculture (El-Shatby), Alexandria University. All the experimental procedures including test animals followed the scientific and ethical regulations recommended by the EEC Directive of 1986 (86/609/EEC). The experimentation guidelines of the Internal Commission for Environmental and Ethics of Alexandria University approved all protocols involving animals.
Experimental forages and proximate analysis
The guava leaves were collected from a private orchard in Alexandria, dried at 50 °C in a vacuum oven for 48 h, finely powdered in a Willey mill (1 mm), and stored in a tightly closed container. Berseem hay (T. alexandrinum, BH) was used as the reference forage because it is a high-quality forage and it is a popular conventional roughage source (Sallam 2005).
The browses and experimental diet were analyzed for dry matter (DM), organic matter (OM), crude protein (CP), and ether extract (EE) according to the Association of Official Analytical Chemists (AOAC 2006). Neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL) were determined according to Van Soest et al. (1991) using an ANKOM 220 fiber analyzer (ANKOM Technology Corporation, Macedon, NY, USA). The NDF analysis was accomplished with the addition of thermostable α-amylase. Besides, the samples were also analyzed for tannins (TT), condensed tannins (CT), and total phenols (TP). The TPs were determined with the Folin-Ciocalteau reagent using tannic acid as standard (Makkar et al. 1993). The TTs were estimated as the difference between TPs (measured by Folin-Ciocalteau reagent) before and after tannin removal by insoluble polyvinylxpolypyrrolidone (PVPP) (Makkar et al. 1995). The HCl-butanol method was used for the quantitation of CTs and the data were expressed as leucocyanidin equivalent (Makkar 2003). The aluminum chloride colorimetric method was adopted for evaluating the total flavonoids content (Chang et al. 2002).
Animal donors and inoculum preparation
Four adult rumen-cannulated Barki sheep with an average body weight of 49.0 ± 2.3 kg were used as inoculum donors. The animals were fed on BH ad libitum and 750 g commercial concentrate mixture per day. All the animals had free access to a mineral premix and fresh water. The animals had never been in contact with guava leaves before. The proximate analysis of the concentrate mixture was 895, 145, 27, 382, and 226 g/kg for OM, CP, EE, NDF, and ADF, respectively. Before feeding in the morning, both solid and liquid rumen contents were collected separately via cannula using a stainless steel probe (2.5-mm screen) attached to a large-capacity syringe. Liquids and solids were transferred in pre-warmed (39 °C) insulated flasks and incubated under anaerobic conditions. The pooled rumen contents (50:50 v/v) were squeezed through a four-layered cheesecloth and further incubated in a water bath at 39 °C saturated with CO2 until further inoculation.
Experimental design and in vitro gas production assay
The GLs were tested as a substrate either alone or in combination of medium concentrate diet (50:50; concentrate roughage ratio) at different substitution levels of BH. The experimental diets were GL as a sole substrate, BH as a sole substrate, control group (GL0, 50% BH + 50% concentrate), GL12.5 group (12.5% GL + 37.5% BH + 50% concentrate), GL25 group (25% GL + 25% BH + 50% concentrate), GL37.5 group (37.5% GL + 12.5% BH + 50% concentrate), and GL50 group (50% GL + 50% concentrate). The concentrate mixture was comprised of (g/kg) yellow corn (200), wheat bran (125), soybean meal (50), crushed horsebean (107.5), limestone (10), salt (5), and minerals and vitamin mixture (2.5).
The in vitro gas production assay was executed via a semi-automated system (Mauricio et al. 1999) using a pressure transducer and data logger (GN200, Sao Paulo, Brazil) in 120-mL serum bottles incubated at 39 °C for 24 h. The ground samples (0.3 g as-fed) were incubated with 15 mL mixed rumen fluid and 30 mL MB9 incubation medium. The MB9 media contained NaCl 2.8 g, CaCl2 0.1 g, MgSO4·7H2O 0.1 g, KH2PO4 2.0 g, and Na2HPO4 6.0 g per liter. The pH was adjusted to 6.8 and CO2 was flushed for 30 min (Onodera and Henderson 1980). The bottles were closed with rubber stoppers, manually shaken, and incubated at 39 °C. The gas headspace pressure readings were taken at 6, 12, and 24 h of incubation using a pressure transducer. The amount of gas production (GP) at each interval was estimated according to the regression equation gas volume on pressure obtained and conditioned in our laboratory from 500 samples (unpublished data). The GP was calculated by the following equation:
where V is gas volume (mL) and p is measured pressure (psi). The GP was repeated thrice and bottles in each run were included as four blank bottles (only buffered rumen fluid without substrate) and six bottles for each sample. The gas values were expressed as milliliters per gram of incubated DM or TDOM.
The CH4 emission and analyses
For CH4 analysis, the corresponding gas samples were collected from the bottles by a 1.5-mL syringe and transferred in vacationer tubes (5 mL) at 6, 12, and 24 h incubation. The CH4 was determined by gas chromatography (Model 7890, Agilent Technologies, Inc., CO, USA) with three valve systems using 1/8-in. packed columns having early back flush of the C6 components and equipped with a thermal conductivity detector. The separation was accomplished using the micro packed column, with the helium as a carrier gas and at a flow rate of 28.0 mL/min. The detector and column temperatures were 250 and 60 °C, respectively. The test of linearity and calibration were achieved using a standard gas curve in the range of possible concentrations of the samples. The CH4 production at the end of incubation was calculated as follows:
Net GP and CH4 were corrected for the corresponding blank values.
Determination of true nutrient degradation, partitioning factor, ammonia-N, protozoa count, and volatile fatty acids
At the end of incubation (24 h), the contents of three bottles were used for the establishment of true degradability of dry matter (DM) and OM (TDDM, TDOM) with 50 mL neutral detergent solution and refluxed for 3 h at 105 °C. The residual DM and ash were estimated according to Blümmel et al. (1997). The contents of another three bottles were used for determining the NH3-N concentration, volatile fatty acids (VFAs), and protozoa count. The protozoa were counted microscopically, after sample preparation according to Kamra et al. (1991). Ammonia concentration was colorimetrically analyzed using a commercial kit manufactured by Biodiagnostic Company, Egypt.
The VFAs were determined according to the method of Palmquist and Conrad (1971) by acidifying 1 mL of the rumen samples with 200 μL meta-phosphoric acid 25% (w/v) in microcentrifuge tubes and refrigerated for future analysis. The samples were later defrosted and centrifuged at 30,000×g (15,000 rpm, JA–17 rotor) for 20 min. The supernatant was transferred to GC vials for VFA analysis using a gas chromatograph (GC Thermo TRACE 1300) with an injection temperature at 220 °C and a capillary column (TR-FFAP 30 m × 0.53 mm ID × 0.5 μm film (thermo-part NO: 260N225P)). The column temperature was increased from 100 to 200 °C at a rate of 10 °C/min. The carrier gas nitrogen was set at a flow rate of 7 mL/min. The temperature of the flame ionization detector (FID) was set at 250 °C. Gas flow air was 450 mL/min, hydrogen 40 mL/min, and make-up gas 35 mL/min. The VFA of known concentrations was used as a standard for calibration, and no internal standard was used.
The partitioning factor (PF) was calculated as the ratio of TDOM (mg) to gas volume (milliliters in 24 h) (Blümmel et al. 1997). Microbial protein (MP) production was calculated as 19.3 g microbial nitrogen per kilogram OMD following Czerkawski et al. (1986). The volume of hexoses fermented (HF) was determined using the formula of individual VFA (Demeyer and Van Nevel 1975) as follows:
where C2, C3, C4, and C5 represent millimoles per liter of acetate, butyrate, propionate, and valerate, respectively.
Statistical analyses
The in vitro assay was statistically analyzed by the generalized linear model procedure, and the following model was proposed:
where μ is the overall mean, Ti is the treatment, and eij is the random error term. The contrast statement was used to determine the linear, quadratic, and cubic variable response to increasing levels of GL in the diet. The differences between the treatments were proclaimed to be significant (P < 0.05) by Tukey’s correction for multiple comparisons.
Results
Chemical composition, nutrient degradation, and gas production
The CP content was 158.9 and 186.8 g/kg DM for BH and GL, respectively. The GL had lower cellulose and hemicellulose contents (19.64 vs. 26.73; 5.80 vs. 20.11, respectively), but higher ADL (17.13 vs. 7.18) compared to the BH. The GL was rich in TP (138.50 eq-g tannic acid/kg DM), TT (54.20 eq-g tannic acid/kg DM), CT (8.60 eq-g leucocyanidin/kg DM), and flavonoids (8.40 mg/kg DM). Thus, replacement of BH by gradually increasing levels of GL was directly proportional to the proximate analysis and tannin content of experimental diets (Table 1).
By comparing GL alone to BH, the values of cumulative GP (expressed per unit of DM or TDOM), TDDM, and TDOM were significantly reduced with the presence of GL by 56, 70, 32, and 35%, respectively, whereas the partitioning factor value was significantly (P < 0.001) elevated by 238% compared to the respective values in the presence of BH (Table 2).
When GL was incubated with TMR, GP (mL/g TDOM or DM) was decreased in a linear manner (P < 0.001) (Table 2). The percent reduction recorded in the GP (mL/g DM) for GL12.5, GL25, GL37.5, and GL50 in comparison to control (GL0) was 13.9, 29.4, 37.6, and 49.1%, respectively. The inclusion of GL at 37.5 and 50% levels caused a significant linear (P < 0.05) drop in both TDDM and TDOM values against the control, whereas at 25 and 50% levels, no significant changes were observed. The partition factor (mg TDOM/mL GP) exhibited a substantial linear rise (P < 0.001) with the inclusion of 25, 37.5, and 50% of GL in a medium concentrate diet.
CH4 emission and fermentation products
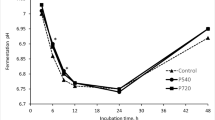
The GL as a sole substrate was able to reduce the CH4 emission significantly (P < 0.05) on TDOM and DM basis by 58 and 35%, respectively, compared to BH; however, when the reduction was expressed per 100 mL volume of the mixed GP, it was non-significant (P = 0.132) (Table 3). Likewise, the NH3-N concentration also declined linearly (P < 0.05) by 33% relative to the respective values with BH. Conversely, the pH values were significantly (P < 0.05) higher in GL than BH. Nevertheless, the protozoal count did not show any significant difference between the two substrates (P = 0.155).
Supplementation of GL in a medium concentrate diet at 25, 37.5, and 50% levels led to a significant linear (P < 0.05) decline in CH4 production by 26.8, 46.3, and 57.7% on TDOM basis and by 20.8, 40.3, and 53.7% on DM basis compared to the GL0 group (Table 3). Nonetheless, a notable rise in the pH was recorded with the GL37.5 and GL50 groups compared to the GL0 group (P < 0.05). However, no linear, quadratic, or cubic differences (P > 0.05) were recorded in protozoal count irrespective of the levels of GL included (Table 3).
The GL as a sole substrate led to a significant (P < 0.05) decline in the total VFA content along with the individual concentration of acetate, propionate, butyrate, and valerate by 45.5, 43.4, 39.2, and 62.563% compared to BH. No significant difference was recorded in the acetate to propionate ratio (P = 0.419; Table 4). In the same line, the incubation of GL with TMR at 37.5 and 50% levels showed a significant decrease in total VFA, acetate, and valerate concentration (Table 4).
Estimated parameters
The sole presence of GL resulted in the lowering of the overall predictive MP and HF values (P < 0.05) by 24 and 64%, respectively, compared to BH. Similarly, the linear decline (P < 0.001) in MP was also evident with inclusion of GL at 37.5 and 50% levels. However, the predicted HF value did not alter at any of the GL substitution levels (Table 5).
Discussion
Several studies have scrutinized various tannin-containing plants for inhibiting the CH4 production (Bhatta et al. 2012; Soltan et al. 2012). However, little information is available about the anti-methanogenic action of GL when combined as a part of the complete diet. There is a need to determine appropriate levels of GL to achieve an optimal effect on preventing enteric CH4 production with a trivial adverse effect on rumen fermentation.
The GL had a higher content of CP, TP, TT, and CT but lower cellulose and hemicellulose contents compared to BH, which are consistent with the previous report (Ngamsaeng et al. 2006). In the current study, it was also reported that the replacement of BH in a medium concentrate ration, by increasing levels of GL, exhibited similar variations in the proximate analysis and tannin content of experimental diets.
In the present investigation, a significant dwindling effect on GP was shown by the presence of GL alone or in combination (37.5 and 50% GL) in TMR diets as compared to BH. This was largely attributed to the high level of TP, CT, and flavonoids in GL. Tiemann et al. (2008) reported that the magnitude of the effect of tannin-containing plant species on rumen fermentation varies according to their chemical composition. Furthermore, Hassen et al. (2016) reported that in vitro gas production was reduced up to 20, 15.5, and 7.3% when Acacia luederitzii (high tannin), A. haematoxylin (moderate tannin), and A. mellifera (low tannin) were used as the substrate.
Here, a notable decline in both TDDM and TDOM was recorded with the presence of GL alone or at 37.5 and 50% level in TMR diets, whereas the PF value was significantly elevated with an increasing GL level. This effect could also be associated with the capability of CT of GL to interfere with microbial attachment to feed particles that subsequently caused a deleterious impact on the microbial population inhibiting ruminal fermentation to a certain extent (McSweeney et al. 2001). Jayanegara et al. (2011) found that the degradability of OM was negatively correlated with TT, CT, and hydrolyzable tannin concentration. This was more evident when the effect of different levels of GL was evaluated (37.5 and 50% GL) for TDDM and TDOM in the mixed ration.
PF is often used as an indicator of microbial protein synthesis efficiency (Sallam et al. 2010). In the current study, the PF value was significantly higher in GL (8.41) than AF (2.49). The previous reports stated that the PF of feedstuffs can supposedly vary from 2.75 to 4.41, reflecting the ATP yield of 10 to 40 (Blümmel et al. 1997). The sharp increase in PF of GL might be because the tannin compounds formed complexes with proteins, which were largely insoluble in neutral detergent solution and may contribute to the non-degradable fraction (Makkar et al. 1997). However, the PF values ranged from 2.40 to 4.60 with the rising substitution levels of GL for BH in a complete diet, which is similar to natural levels of feedstuffs largely.
A significant (P < 0.05) drop was observed in CH4 production with GL alone or at 25 to 50% level in TMR diets. Similarly, it was demonstrated that ethanol extract of GL strongly inhibited in vitro methanogenesis (Kamra et al. 2006). It was also reported that GL possesses low methanogenic potential in vitro (Chatterjee et al. 2014). This could be attributed to the high TP, CT, and flavonoids of GL, which is potentially important in limiting in vitro fermentation (Huang et al. 2010; Hassen et al. 2016). The inhibition of methanogenesis has also been reported to be linked with high concentrations of TT, TP, and CT of GL (Pal et al. 2015).
It was supposed that rumen methanogenesis was probably reduced due to the direct effect of tannins on methanogenic Archaea activity and an indirect effect via fiber digestion (Tavendale et al. 2005). In this study, GL showed low degradation of organic and dry matter, which could be due to the reduced population of cellulolytic bacteria (McSweeney et al. 2001), and/or corrupted bacterial sticking to the substrate and fibrolytic action of rumen microbes (Bento et al. 2005). Jones et al. (1994) observed an inhibition in the growth of Butyrivibrio fibrisolvens bacteria, which also contribute to fiber degradability, as affected by CT.
In the present study, the reduction in NH3-N concentration caused by the presence of GL alone or at 37.5 and 50% level in the diet is consistent with the earlier findings (Bhatta et al. 2009; Pellikaan et al. 2011). During rumen fermentation, ammonia is produced from amino acid deamination (Van Soest 1994). The protein binding activity of CT from GL with the subsequent formation of CT-protein complexes could possibly be accountable for the reduced proteolysis to yield ammonia (Pal et al. 2015). The pH values were significantly higher in GL than BH, and this could be associated with the decline of GP, TDDM, TDOM, and VFA (Meng et al. 2016).
No significant changes were detected in the protozoal count either by GL alone or with different levels in the complete diet. It is a well-known fact that rumen ciliate protozoa are strong hydrogen-producing microorganisms (Jouany and Lassalas 2000), and therefore, more CH4 is generated when protozoa are found in the rumen. In the current report, though protozoal count did not change (P > 0.05), CH4 emission was suppressed. The absence of any correlation between protozoal population and CH4 production has also been reported earlier (Goel et al. 2007). The explanation for this lack of correlation is yet to be elucidated, although it has earlier been reported (Kamra 2005) that plant secondary metabolites may differ in their anti-protozoal and anti-methanogenic activities. Hence, the relationship between protozoal count and rumen fermentation pattern may probably be attributed to both type of diet and protozoal species (Krzywiecki et al. 2006).
In the current study, the presence of GL alone or at 37.5 and 50% level in the diet significantly reduced the total VFA content including the individual fraction of acetate and valerate. Similarly, Martınez et al. (2006) and Getachew et al. (2008) demonstrated that total VFA concentration, when quebracho tannins were supplied, was at 50 g/kg DM in batch culture fermentation relative to the control. The total VFA response is possibly related to the reduction in GP as various studies confirmed a close association between VFA and GP in vitro (Wu et al. 2016; Russo et al. 2017).
In the current research, the presence of free GL had a significant dwindling effect on the MP production and the amount of theoretically calculated HF relative to BH. The decline in HF is possibly linked to the coating of CT to the carbohydrate molecules (Barahona et al. 2003). In line with these findings, Pal et al. (2015) elucidated that microbial biomass production was lowest for GL among other tropical tree leaves. In several reports, tannins have been well documented for their inhibitory activity on feed digestion, microbial population, and enzyme activity (Patra and Saxena 2011); this could also be a possible explanation to the depletion of MP.
Conclusions
In the present study, attempts were made to explore the anti-methanogenic potential of guava leaves in the rumen along with nutrient digestibility and other vital parameters. Despite their ability to dwindle CH4 production, use of guava leaves as such cannot be recommended as a sole feed resource for ruminants due to its negative effects on rumen fermentation, nutrient degradability, and microbial protein synthesis. Besides, it was unequivocally established that the optimal proportion of GL that could limit CH4 production without hindering rumen fermentation and nutrient digestibility is 25% GL and 25% BH in a medium concentrate diet, which is economic as well as safe to the environment. Furthermore, the proposed level of GL supplementation is within the reasonable range that could be suitable in vivo. Nevertheless, further confirmatory and feasibility studies need to be carried out in order to promote GL at a commercial level.
References
Adjorlolo L, Timpong-Jones E, Boadu S, Adogla-Bessa T (2016) Potential contribution of neem (Azadirachta indica) leaves to dry season feeding of ruminants in West Africa. Livest Res Rural Dev 28, Accessed from http://www.lrrd.org/lrrd28/5/adjo28075.htm
AOAC (2006) Official methods of analysis, 18th edn. USA, Washington, DC
Badan P (2004) Monografi ekstrak tumbuhan obat Indonesia (volume 1). Badan POM, Jakarta, p 159
Barahona R, Lascano CE, Narvaez N, Owen E, Morris P, Theodorou MK (2003) In vitro degradability of mature and immature leaves of tropical forage legumes differing in condensed tannin and non-starch polysaccharide content and composition. J Sci Food Agric 83:1256–1266
Bento M, Acamovic T, Makkar H (2005) The influence of tannin, pectin and polyethylene glycol on attachment of 15 N-labelled rumen microorganisms to cellulose. Anim Feed Sci Technol 122:41–57
Bhatta R, Uyeno Y, Tajima K, Takenaka A, Yabumoto Y, Nonaka I, Enishi O, Kurihara M (2009) Difference in the nature of tannins on in vitro ruminal methane and volatile fatty acid production and on methanogenic archaea and protozoal populations. J Dairy Sci 92:5512–5522
Bhatta R, Saravanan M, Baruah L, Sampath KT (2012) Nutrient content, in vitro ruminal fermentation characteristics and methane reduction potential of tropical tannin-containing leaves. J Sci Food Agric 92:2929–2935
Blümmel M, Steingaβ H, Becker K (1997) The relationship between in vitro gas production, in vitro microbial biomass yield and N incorporation and its implications for the prediction of voluntary feed intake of roughages. Br J Nutr 77:911–921
Chang C-C, Yang M-H, Wen H-M, Chern J-C (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10:178–182
Chatterjee P, Kamra D, Agarwal N, Patra A (2014) Influence of supplementation of tropical plant feed additives on in vitro rumen fermentation and methanogenesis. Anim Prod Sci 54:1770–1774
Czerkawski J, Milligan LP, G WL, aD A (eds) (1986) Degradation of solid feeds in the rumen: spatial distribution of microbial activity and its consequences, proceedings of 6th international symposium on ruminant physiology. Banff (Canada). Prentice-Hall, Englewood Cliffs
Demeyer D, Van Nevel C (1975) Methanogenesis, an integrated part of carbohydrate fermentation and its control. In: IW M, ACI W (eds) Digestion and metabolism in the ruminant. University of New England Publishing Unit, Armidale, pp 366–382
Eckard R, Grainger C, De Klein C (2010) Options for the abatement of methane and nitrous oxide from ruminant production: a review. Livest Sci 130:47–56
FAO (2006) Livestock’s long shadow. Environmental issues and options. Food and Agriculture Organization of the United Nations, Rome
Getachew G, Pittroff W, Putnam DH, Dandekar A, Goyal S, DePeters EJ (2008) The influence of addition of gallic acid, tannic acid, or quebracho tannins to alfalfa hay on in vitro rumen fermentation and microbial protein synthesis. Anim Feed Sci Technol 140:444–461
Goel, G, Makkar, H, Becker, K (2007) Effect of saponin-rich fractions of different plants on rumen fermentation with particular reference to methane emission in vitro. Proceedings of the Society of Nutrition Physiology
Gutiérrez RMP, Mitchell S, Solis RV (2008) Psidium guajava: a review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol 117:1–27
Hariadi BT, Santoso B (2010) Evaluation of tropical plants containing tannin on in vitro methanogenesis and fermentation parameters using rumen fluid. J Sci Food Agric 90:456–461
Hassen A, Theart JJF, Adriaan van Niekerk W, Adejoro FA, Gemeda BS (2016) In vitro methane and gas production characteristics of Eragrostis trichopophora substrate supplemented with different browse foliage. Anim Prod Sci 56:634–640
Hernández D, Solana M, Riaño B, García-González M, Bertucco A (2014) Biofuels from microalgae: lipid extraction and methane production from the residual biomass in a biorefinery approach. Bioresour Technol 170:370–378
Huang X, Liang J, Tan H, Yahya R, Khamseekhiew B, Ho Y (2010) Molecular weight and protein binding affinity of Leucaena condensed tannins and their effects on in vitro fermentation parameters. Anim Feed Sci Technol 159:81–87
Jayanegara A, Wina E, Soliva CR, Marquardt S, Kreuzer M, Leiber F (2011) Dependence of forage quality and methanogenic potential of tropical plants on their phenolic fractions as determined by principal component analysis. Anim Feed Sci Technol 163:231–243
Jones G, McAllister T, Cheng K, Muir A (1994) Effect of sainfoin (Onobrychis viciifolia Scop) on growth and proteolysis by 4 strains of rumen bacteria: resistance of Prevotella (Bacteroides) ruminicola B14. Appl Environ Microbiol 60:1374–1378
Jouany J, Lassalas B (2000) Effect of protozoa on methane production in the rumen; consequences on carbon and hydrogen distribution among the other end products of fermentation, methane mitigation. US Environ Protection Agency and Siberian Branch of Russian Academy of Sciences, Novosibirsk
Kamra D (2005) Rumen microbial ecosystem. Curr Sci 89:124–135
Kamra D, Sawal R, Pathak N, Kewalramani N, Agarwal N (1991) Diurnal variation in ciliate protozoa in the rumen of black buck (Antilope cervicapra) fed green forage. Lett Appl Microbiol 13:165–167
Kamra D, Agarwal N, Chaudhary L (2006) Inhibition of ruminal methanogenesis by tropical plants containing secondary compounds. International Congress Series. Elsevier
Krzywiecki S, Szyrner A, Pasternak A, Czarna Z (2006) Influence of fodder silage type on fermentation processes and rumen microorganisms. Electron J Pol Agric Univ 9:e26
Makkar H (2003) Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rumin Res 49:241–256
Makkar HP, Blümmel M, Borowy NK, Becker K (1993) Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J Sci Food Agric 61:161–165
Makkar HP, Blümmel M, Becker K (1995) In vitro effects of and interactions between tannins and saponins and fate of tannins in the rumen. J Sci Food Agric 69:481–493
Makkar H, Blümmel M, Becker K (1997) In vitro rumen apparent and true digestibilities of tannin-rich forages. Anim Feed Sci Technol 67:245–251
Martınez TF, McAllister TA, Wang Y, Reuter T (2006) Effects of tannic acid and quebracho tannins on in vitro ruminal fermentation of wheat and corn grain. J Sci Food Agric 86:1244–1256
Mauricio RM, Mould FL, Dhanoa MS, Owen E, Channa KS, Theodorou MK (1999) A semi-automated in vitro gas production technique for ruminant feedstuff evaluation. Anim Feed Sci Technol 79:321–330
McSweeney C, Palmer B, Bunch R, Krause D (2001) Effect of the tropical forage calliandra on microbial protein synthesis and ecology in the rumen. J Appl Microbiol 90:78–88
Meng Y, Mumme J, Xu H, Wang K (2016) A biologically inspired variable-pH strategy for enhancing short-chain fatty acids (SCFAs) accumulation in maize straw fermentation. Bioresour Technol 201:329–336
Ngamsaeng A, Wanapat M, Khampa S (2006) Evaluation of local tropical plants by in vitro rumen fermentation and their effects on fermentation end-products. Pak J Nutr 5:414–818
Onodera R, Henderson C (1980) Growth factors of bacterial origin for the culture of the rumen oligotrich protozoon Entodinium caudatum. J Appl Bacteriol 48:125–134
Pal K, Patra A, Sahoo A, Kumawat P (2015) Evaluation of several tropical tree leaves for methane production potential, degradability and rumen fermentation in vitro. Livest Sci 180:98–105
Palmquist D, Conrad H (1971) Origin of plasma fatty acids in lactating cows fed high grain or high fat diets. J Dairy Sci 54:1025–1033
Patra AK, Saxena J (2011) Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J Sci Food Agric 91:24–37
Patra AK, Yu Z (2014) Combinations of nitrate, saponin, and sulfate additively reduce methane production by rumen cultures in vitro while not adversely affecting feed digestion, fermentation or microbial communities. Bioresour Technol 155:129–135
Pellikaan WF, Stringano E, Leenaars J, Bongers DJ, van Laar-van Schuppen S, Plant J, Mueller-Harvey I (2011) Evaluating effects of tannins on extent and rate of in vitro gas and CH4 production using an automated pressure evaluation system (APES). Anim Feed Sci Technol 166-67:377–390
Ramírez-Restrepo C, Barry T, López-Villalobos N, Kemp P, McNabb W (2004) Use of Lotus corniculatus containing condensed tannins to increase lamb and wool production under commercial dryland farming conditions without the use of anthelmintics. Anim Feed Sci Technol 117:85–105
Russo VM, Jacobs JL, Hannah MC, Moate PJ, Dunshea FR, Leury BJ (2017) In vitro evaluation of the methane mitigation potential of a range of grape marc products. Anim Prod Sci 57:1437. https://doi.org/10.1071/AN16495
Sallam SMA (2005) Nutritive value assessment of the alternative feed resources by gas production and rumen fermentation in vitro. Res J Agric Biol Sci 1(2):200–209
Sallam SM, Bueno IC, Nasser ME, Abdalla AL (2010) Effect of eucalyptus (Eucalyptus citriodora) fresh or residue leaves on methane emission in vitro. Ital J Anim Sci 9:299–303
Soltan Y, Morsy A, Sallam S, Louvandini H, Abdalla A (2012) Comparative in vitro evaluation of forage legumes (prosopis, acacia, atriplex, and leucaena) on ruminal fermentation and methanogenesis. J Anim Feed Sci 21:759–772
Soltan YA, Morsy AS, Sallam SM, Lucas RC, Louvandini H, Kreuzer M, Abdalla AL (2013) Contribution of condensed tannins and mimosine to the methane mitigation caused by feeding Leucaena leucocephala. Arch Anim Nutr 67:169–184
Tavendale MH, Meagher LP, Pacheco D, Walker N, Attwood GT, Sivakumaran S (2005) Methane production from in vitro rumen incubations with Lotus pedunculatus and Medicago sativa, and effects of extractable condensed tannin fractions on methanogenesis. Anim Feed Sci Technol 123:403–419
Tiemann TT, Lascano CE, Wettstein H-R, Mayer AC, Kreuzer M, Hess HD (2008) Effect of the tropical tannin-rich shrub legumes Calliandra calothyrsus and Flemingia macrophylla on methane emission and nitrogen and energy balance in growing lambs. Animal 2:790–799
Van Soest PJ (1994) Nutritional ecology of the ruminant. Cornell University Press, Ithaca
Van Soest PV, Robertson J, Lewis B (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74:3583–3597
Waghorn G (2008) Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production—progress and challenges. Anim Feed Sci Technol 147:116–139
Wu H, Meng Q, Yu Z (2015) Effect of pH buffering capacity and sources of dietary sulfur on rumen fermentation, sulfide production, methane production, sulfate reducing bacteria, and total Archaea in in vitro rumen cultures. Bioresour Technol 186:25–33
Wu H, Meng Q, Yu Z (2016) Evaluation of ferric oxide and ferric citrate for their effects on fermentation, production of sulfide and methane, and abundance of select microbial populations using in vitro rumen cultures. Bioresour Technol 211:603–609
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Al-Sagheer, A.A., Elwakeel, E.A., Ahmed, M.G. et al. Potential of guava leaves for mitigating methane emissions and modulating ruminal fermentation characteristics and nutrient degradability. Environ Sci Pollut Res 25, 31450–31458 (2018). https://doi.org/10.1007/s11356-018-3152-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3152-2