Abstract
Metal pollution is a subject of growing concern as it affects the whole food chain of an ecosystem by bioaccumulation. Growing industrialization and anthropogenic intervention have put tremendous pressure on self-sustaining ecosystems worldwide. Sundarbans mangrove estuary, being a UNESCO World Heritage site, suffers severely from anthropogenic stress, urbanization, ecotourism, overexploitation of natural resources and discharges of industrial as well as municipal waste products. Our study unfolds the extent of metal pollution in the sediment of this estuarine mangrove ecosystem and also investigates the source and distribution of these metals. Extensive samplings were performed during three major seasons, namely pre-monsoon, monsoon, and post-monsoon for two consecutive years at ten sampling stations along the major river networks of the mangrove estuary. Seasonal variations of these metals, physicochemical properties, and soil texture studies were performed to explore the sediment quality of the study area. Positive correlation was observed between the pollutants and siltation. Several environmental indices were investigated to explore the degree of metal pollution which revealed contamination of Cd, Cr, and Pb to cross the permitted safe index in the study area. Pollution load index indicates the spatial as well as seasonal variation of eco toxic metal load along the course of the rivers. Statistical analyses such as principal component analysis and correlation matrix identified different sources for metal contamination. Almost 700 tannery industries are located in the upstream region of the rivers, and several small- and large-scale battery industries seem to be the main possible source for Cd, Cr, and Pb pollution. Analysis of the results indicates the alarming condition of this heritage site. The metal concentrations beyond toxicity thresholds are responsible for gradual deterioration of this estuarine mangrove which may only be protected by developing sustainable management planning.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mangrove forests are one of the most productive ecosystems of the earth, existing only in tropical and subtropical countries. Mangrove provides a number of ecological services including protection to the coast against natural calamities such as cyclones and tsunamis, as well as conservation of the shore and inland natural resources by preventing soil erosion. Apart from this, mangrove forest also reduces the harmful effects of greenhouse gases, slows down global warming, and reduces carbon footprint by absorbing a large amount of carbon monoxide and carbon dioxide. Anthropogenic pollution, land conversion, aquaculture, and deforestation are destroying mangrove forests regularly. Extensive pollution load is eradicating ecosensitive floral and faunal species leading to declining mangrove biodiversity. In recent decades, the mangrove wetland ecosystems are being converted into a large pollution sink globally. Metal ions such as cadmium and lead also accumulate in mangrove-associated shrimp, crab, and fishes (Krishnamurti and Nair 1999). The extent of pollution also stretches along the adjoining terrestrial habitat through the food chain.
Mangrove forests have been overexploited globally and rapid urbanization, aquaculture, deforestation and industrialization have adversely affected this important ecosystem. In the last 50 years, nearly one third of the mangrove forests have been lost (Alongi 2002). Mangroves fight against several natural disasters like storms, floods, cyclones, tsunami, soil erosion and diseases continuously. Earlier report revealed the extent of diseases when reportedly 20% of the ecologically vulnerable Heritiera fomes trees have been severely affected by “top dying” disease in the Sundarbans of Bangladesh (Spalding et al. 1997). Mangroves become more susceptible to diseases with the increase of contaminants in the soil. Metal accumulation in the sediment of mangrove forest can cause dead roots of mangrove flora as was observed in the mangroves of Western Australia (Alongi et al. 2004). A study on Futian National Nature Reserve, Shenzhen Bay of China, revealed toxic metal contamination ranging from medium to high with a remarkable concentration of Cd and As (Li et al. 2016). They found the metal input decreased in the order of natural mangrove > restored mangrove > mud flat, indicating the mangrove to be instrumental in the accumulation and storage of metals in the sediment layers. Metal pollution have also been found to affect the mangrove ecosystem in Gulf of Kachchh, Gujarat (Kumar et al. 2015) and the estuarine ecosystem of Dhamra estuary, Odisha, India (Satapathy and Panda 2015). Elevated concentrations of metals in soil and water have significant effect on food chain. Primary producers tend to accumulate metal ions which further passes on to the consumers via biomagnification process (Duruibe et al. 2007). Biotoxic effects of the metals in human population have also been reported. Although some common health effects like diarrhea, vomiting, convulsion, anxiety, sleeplessness and skin irritation are related to metals at toxic concentration, each metal ion has specific effects on human health. Cd can exhibit toxicity at a very low concentration and exposure to Cd causes renal disorders. Cr [VI] exhibit greater toxicity compared to Cr [III] and regular exposure to Cr causes irritation in respiratory tract, irritative dermatitis, and chronic ulcer of skin (Dayan and Paine 2001). Pb poisoning inhibits hemoglobin synthesis, causes acute damage to central and peripheral nervous system, and develops neuronal, abdominal, and cardiovascular diseases. The International Agency for Research on Cancer (IARC) has classified Cd as “human carcinogen,” Pb as “possible human carcinogen” and Cr to be the cause of rare sinonasal cancer (Jarup 2003; Dayan and Paine 2001).
Sundarban is the largest single chunk of mangrove which is also renowned for its enormous biodiversity. A huge number of channels and creeks are connected altogether and form a complex system of riverine network in this estuarine mangrove ecosystem. The high tidal activity allows influx of a huge amount of salt water in this region. Bidyadhari, Matla, Thakuran and Saptamukhi are some of the most notable rivers of the Indian Sundarbans. Upstream of its course, Bidyadhari runs through mangrove transition zone where it meets the waste canals of Calcutta Metropolitan Corporation (Saha et al. 2001). The river carries the tannery effluents through the mangrove, ultimately releasing in Bay of Bengal. We have attempted to investigate the level of metal pollution in Sundarbans; the sediment samples of the canal were collected and analyzed. Increased deposition of effluents in the sediment enhances the pollution level of the mangrove ecosystem. Due to the anthropogenic stress and release of industrial effluents in the upstream of these rivers, they carry significant amount of pollutants to the estuary every year. Till now, no in-depth study has been conducted relating the course of these important rivers and the waste material they carry through the Sundarban mangrove ecosystem. Previous studies on metal contamination have been performed in the inhabited part of Sundarbans (Saha et al. 2001; Akhand et al. 2016), but our study attempts an in-depth analysis of metal deposition from the interacting point of mangrove forests with the inhabited part to the mouth of the riverine network where it meets the Bay of Bengal. Seasonal variation of metal deposition along with siltation and source identification of the pollutants are the principal objectives of this study.
Material and methods
Study area
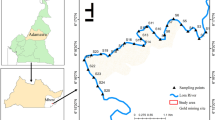
Sundarbans Mangrove Estuary is the largest tidal halophytic tropical estuarine ecosystem of the Indian subcontinent. It covers an area of 10,200 km2 spreading over India (4263 km2 of reserve forest) and Bangladesh (5937 km2 of reserve forest) (Manna et al. 2012). The Indian part is bounded by the Hooghly River in the west; the international border between India and Bangladesh over Ichhamati, Raimangal, and Harinbhanga rivers in the east; the Dampier-Hodges line, drawn in 1829–1830, in the north and the Bay of Bengal in the south (Danda and Sriskanthan 2011). The study area covers an area of 4200 km2 extending from 21° 35′ 00″ N to 22° 12′ 00″ N which covers a distance of 70 km and from 88° 15′ 00″ E to 88° 50′ 00″ E covering a distance of 60 km. Among the river systems of Indian Sundarbans, Matla and Bidyadhari run in south west direction and undoubtedly those are the most important channels of Indian Sundarbans. Due to siltation in upstream region, both of these rivers are deprived of sufficient fresh water connection (Manna et al. 2010). The confluence of these two rivers divides the core area of Sundarbans Project Tiger from the Buffer Region. Saptamukhi is located in the extreme western part of Sundarbans and flows towards the Bay of Bengal through the mangrove transition zone on the west bank and Lothian Island Wildlife Sanctuary on the other side. It is connected to the Hooghly River through Muriganga and is susceptible to the pollutants of Haldia, the largest port in this region and an industrial city having oil refineries and several heavy industries. Thakuran flows between Matla and Saptamukhi through the buffer region and is connected with Matla and Bidyadhari. A sum total of ten sampling stations were selected for the study. These stations are Gadkhali (S1), Bashirampur (S2), Jharkhali (S3), Shahidnagar (S4), Bonnie Camp (S5), Kalash (S6), Dhulibhasani (S7), Dhanchi (S8), Lothian (S9), and Shushnir Char (S10). Among those stations S1, S2, and S4 are positioned on the bank of Bidyadhari River, S3 and S5 are located on the riverside of Matla, S6 and S7 are influenced by Bidyadhari-Matla riverine network, S8 resides on the bank of Thakuran River, and the remaining S9 and S10 are located on Saptamukhi riverbank (Fig. 1).
To study the possible source area, sediment samples were also collected from Bantala (P1), Bhojerhat (P2), and Ghushighata (P3) during monsoon of 2016. These three sampling sites are positioned along the canal (Fig. 1) carrying metropolitan waste from Kolkata and the waste product from Calcutta Leather Complex situated at Bhojerhat. The waste is discharged in the river Bidyadhari at Ghushighata.
Sediment sample collection and analysis
Sediment samples were collected from all of the ten sampling stations during pre-monsoon (March–May), monsoon (June–August) and post-monsoon (December–January) in the year 2014–2015 in triplicate manner. For each sample, three random areas of 1 m2 at minimum distance of 10 m were marked and a layer of half inch sediment was collected and mixed well prior to the collection of final sediment sample in polypropylene bottles. These bottles were kept in iceboxes at 4 °C, transported to the laboratory, and stored in − 20 °C. In the laboratory, sediment samples were thawed, air dried, crushed and passed through 2 mm and 63 μm mesh sieve prior to analysis of sediment quality parameters and element analysis respectively.
Sediment texture analysis was done using Bouyoucos hydrometer. Air-dried sediment samples of 50 g were weighed and soaked with distilled water and 5 ml 1(N) sodium hexametaphophate for 15 min. Suspension was placed in a 1000-ml sedimentation cylinder and filled with distilled water to 1000 ml mark. Suspension was mixed well and Bouyoucos hydrometer was placed in the sedimentation cylinder. Hydrometer readings were noted at 40 s and 2 h. Blank reading and temperature were also noted for further calculation and temperature correction (Anderson and Ingram 1993; Day 1965).
To measure total organic carbon, 250 mg sediment sample was taken in a 2-ml centrifuge tube and mixed with 5% sulfuric acid and 5% phosphoric acid. It was then digested at 98 °C for 1 h and filtered through Whatman 1 filter paper. Modified version of Walkley-Black procedure (1934) was performed for the digestion procedure as described by Mebius (1960). Inorganic carbon was drained out with the filtrate and the remaining sediment containing only organic carbon was air dried and weighed. It was finally used to measure the total organic carbon content in a TOC analyzer. Total organic carbon was measured using Aurora 1030 TOC analyzer from O. I. Analytical. Sediment samples were mixed with distilled water in 1:2.5 ratio to determine pH and conductivity (mS cm−1) using HACH HQ40d portable multimeter.
For element analysis, 5 g of 63 μm sieved sediment was dissolved in 100 ml freshly prepared aqua regia and kept overnight at room temperature for pre-digestion; the suspension was digested at 130 °C for 2 h in a digestion chamber (Sastre et al. 2002) and filtered twice through Whatman 1 filter paper (pore size 11 μm) and stored in volumetric glass bottle. Final volume was corrected to 100 ml and stored at 4 °C. The amounts of metal ions present in the samples were measured in a Varian Atomic Absorption Spectrophotometer (Varian Spectra AA240). Standard solutions were prepared with 1000 ppm MERCK standard solutions. For quality control, CRM-SOIL-B Soil Solution B from High-Purity Standards was analyzed. Recovery rate of the elements were found within 80% to 110% which indicates satisfactory analytical accuracy for the analysis procedure.
Ecological indicators
We have selected and derived some relevant environmental indices to estimate the pollution risk of this heritage site which are explained in the following part:
Contamination factor
The level of contamination for a metal in a particular area is expressed by contamination factor (CF). It is the ratio of measured concentration and background concentration of a pollutant and calculated by the following formula:
where CmSample is the concentration of a metal in sediment and CmBackground is the background concentration of that metal in sediment (Hakanson 1980; Salah et al. 2012).
Enrichment factor
Enrichment factor (EF) indicates the status and degree of contamination in an area for a particular metal. The EF calculations compare each value with a given background level, either from the local site, using older deposits formed under similar conditions, but without anthropogenic impact, or from a regional or global average composition (Cato 1977). EF values were calculated using the following formula (Sinex and Helz 1981):
where Cm is the concentration of the metal of interest and Cref is the concentration of the reference metal. Iron was chosen as the reference metal due to its dominating presence.
Geo-accumulation index
Geo-accumulation index (Igeo) is a quantitative indicator proposed by Muller (1969). Enrichment of metal contamination above baseline concentration was calculated using the Igeo value. The equation of geo-accumulation index is:
where CmSample is the concentration of a metal in sediment and CmBackground is the background concentration of that metal in sediment.
Ecological risk factor
Ecological risk factor (Eri) was proposed by Hakanson (1980) as metal ions have different toxicity levels. Thus, a toxicity factor was important to access the risk factor associated with different metal ions. The formulae to calculate Eri is:
where Tri is toxic response factor of the substance i and CFi is the contamination factor of substance i. Tri values for Cd, Cr, Cu, Ni, Pb, and Zn are 30, 2, 5, 5, 5, and 1 respectively (Hakanson 1980; Jiang et al. 2017).
Pollution load index
Pollution load index (PLI) signifies the cumulative pollution load for all of the measured elements in a sampling station. As the sediment samples were collected during three seasons, PLI gives us the idea of seasonal difference in pollution load for each sampling station. It was proposed by (Tomlinson et al. 1980) and PLI values were calculated using the following formula:
where n is the number of metals and CF(1, 2, 3,….n) are the contamination factors for different metal ions.
Nemerow pollution index
Nemerow pollution index (PN) is used to evaluate the comprehensive pollution status of sediment (Chen 2010; Yan et al. 2015). Metal ions are found in different concentrations at a site; PN differs from the other pollution indices as it takes in account the concentration maxima as well as the average concentration. It is measured by the following formula:
where \( \overline{CF} \) is the average of contamination factors and CFmax is the maximum contamination factor found for an element in a sample.
Potential ecological risk index
Potential ecological risk index (RI) was proposed by Hakanson (1980) for the better understanding of the threat constituted by metal ions in surface sediment. Unlike other ecological factors, it takes account on the toxicology of different metals. The equation to calculate RI was proposed by Hakanson (1980).
where ERi is the potential ecological risk factor for a given substance i.
Statistical analysis
Each sample was analyzed in triplicates and comparisons were performed using the mean values. Pearson correlation matrix and principal component analysis were performed using IBM SPSS Statistics for Windows Version 20.0 (SPSS Inc. Chicago, IL, USA). Color matrix for Pearson correlation matrix result was created using MATLAB.
Geostatistical analysis
Map of the study area and possible source area were created using ArcMap 10.2 software. Basemaps from ArcGIS and Google Earth have been used. Soil texture values were analyzed to identify the texture type in the soil texture triangle proposed by United States Department of Agriculture (USDA 1987) (Fig. 2). The TAL software was used to create the sediment texture triangle. It is imperative to study the geospatial methods to understand the location of contaminants and to trace the spatial distribution.
Result and discussion
Physicochemical parameters
The sediment of this area was found to be mildly alkaline. The pH values were found to reach lowest during monsoon. Average pH values of S1, S2, S3, S4, S5, S6, S7, S8, S9, and S10 were 7.81 ± 0.17, 7.81 ± 0.16, 7.81 ± 0.21, 7.81 ± 0.18, 7.95 ± 0.24, 7.82 ± 0.19, 7.96 ± 0.21, 7.92 ± 0.22, 7.96 ± 0.15, and 7.93 ± 0.15 respectively for three seasons (Table 1). Sundarban mangrove estuary is a brackish water ecosystem and the salinity of the sediment was found to be in the range of 15.1–23.23 PSU during all the seasons. During pre-monsoon, the salinity reaches its maximum and during the other two seasons it remains comparatively lower. Station S4 shows the overall lowest salinity throughout all seasons and station S10 shows the highest. Huge variation was found in organic carbon concentrations of the sediments. The overall TOC content ranges from 3.89 to 11.375%.
Sediment texture analysis
Sediment texture studies revealed higher percentage of sand (particle diameter is between 0.06 and 2 mm) followed by silt (particle diameter is between 0.002 and 0.06 mm), and clay (particle diameter is < 0.002 mm) in the sediments. The presence of clay found to be similar in all three seasons with a very small increase during monsoon. The changes were found mainly in the sand and silt percentage. Silt percentage was highest during post-monsoon followed by pre-monsoon (Fig. 3). During monsoon, the presence of silt in the sediment was found to be the lowest when sand was found to be the highest. This phenomenon could be the effect of high water level and tidal activity in this area. Sand was found to be the major component of sediment samples but the smaller particles (silt and clay) were present in a considerably high percentage. Although the sediment components were found similar during the 2 years of our study period, siltation was found to be a little higher in 2015 than the previous year (Fig. 3). No significant variation was observed in the sediment type throughout the study area. Out of the 12 soil texture categories proposed by USDA, all of the collected sediment samples (S1–S10) were clustered in two categories, sandy loam and loam (Fig. 2).
Spatial distribution of metals, contamination factor, and enrichment factor
This study was conducted on seven different metal ions during three seasons. As background values of metal ions were not available for this area, world surface rock average (Martin and Meybeck 1979) values were used as background values (Table 2). Fe concentration was found maximum as is expected and used to calculate enrichment factors. Among the other metal ions, Cd, Cr, and Pb were found to occur at higher concentration in the sediment when compared to background values. To compare the metal concentration, we used the Sediment Quality Guidelines described by United States Environment Protection Agency (USEPA 1999) and Canadian Council of Ministers of the Environment (CCME 1999). We have also referred and used the Guidelines for Drinking Water Quality by World Health Organization (2011) and mean shell concentration described by Raju et al. (2012). The comparative study revealed extreme pollution level of the possible source area. The study area contains higher concentrations of Cd, Cr, and Ni compared to the Sediment Quality Guideline described by USEPA (1999) and CCME (1999). However, report by WHO (2011) explains the potential threat of metal contamination in the drinking water in the study area (Table 2) and the threshold level.
Maximum Cd concentration (1.977 mg/kg) was measured at station S1 during pre-monsoon and minimum Cd concentration (0.513 mg/kg) was found at station S5 during monsoon (Table 3). During our study period, the highest Cd accumulation was observed in the pre-monsoon whereas the lowest accumulation was noted at monsoon (Table 3). CF values show moderate to very high Cd contamination in the study area and EF values mark moderate to significant enrichment (Table 4, Suppl. Figs. S1 and S2).
The highest Cr concentration (491.73 mg/kg) was found at station S1 during pre-monsoon, whereas the minimum Cr concentration (84.67 mg/kg) was observed at S10 during monsoon (Table 3). Pre-monsoon appeared to be the season with the highest Cr accumulation and monsoon was the season with the lowest. CF values of the sampling stations reveal moderate to very high contamination (Suppl. Fig. S1). EF values suggest minimal to significant enrichment in the sediments (Suppl. Fig. S2).
Pb concentration was found maximum (52.9 mg/kg) at station S2 during post-monsoon and minimum (11.67 mg/kg) at station S8 during pre-monsoon (Table 3). Station S2 shows the highest average Pb concentration during the study period. Monsoon appeared to be the season with lowest Pb accumulation and post-monsoon was the season with highest. The EF values for Pb ranges from 0.919 to 3.933 indicating the sediment samples lies from minimum to moderate enrichment category (Table 4). CF values indicate that the sampling stations lie in low, moderate, and considerable contamination category (Suppl. Fig. S1).
Zn concentrations were found lower in all of the stations than background values and the remaining metals show similar concentrations to the background values (Tables 2 and 3). EF values show deficiency to minimal enrichment for Cu, Ni, and Zn in all of the stations (Suppl. Fig. S2). CF values refer overall low contamination class for Cu, Ni, Zn, and Fe in the study area (Suppl. Fig. S1). Igeo values reveal unpolluted nature of the study site for Cu, Ni, Zn, and Fe (Fig. 4).
Among all the stations, Cr concentration was high in S1 and S2 throughout the study irrespective of seasons. The stations showing the highest Cr contamination were positioned at the upper part of Bidyadhari River. Several tanneries and fisheries are positioned upstream of this region and their waste materials along with the municipal waste of Kolkata metropolitan area is released in the Bidyadhari River through different canals (Saha et al. 2001).Various studies suggested that Cr contamination is positively correlated with presence of tannery industries as Cr-contaminated sludge is produced as a byproduct of wastewater treatment (Apte et al. 2005).
EF and CF values suggest that among all pollutants, Cd is the most significant one for the whole study area. Out of the ten sampling stations, six stations were found to be highly contaminated with Cd. Among these six stations, two are situated in the upstream of Bidyadhari, another two are influenced by Matla and the remaining two are positioned on the bank of Saptamukhi (Suppl. Fig. S1, Suppl. Fig. S2). The less contaminated stations were found in the lower part of Bidyadhari-Matla confluence and on the bank of Thakuran. Previous studies suggest that Cd and Pb are released from the industries like battery, electroplating, and tannery. Kolkata is stationed in the upstream of Bidyadhari-Matla riverine network and the city and its suburbs are hub to numerous small-scale battery and electroplating industries (Roy et al. 2013; Samanta et al. 2005) as well as a huge number of tanneries.
Pb contamination is found more or less same in the whole study area and the reason might be various sources of the contaminant. Pb contamination is caused by both municipal waste and agricultural waste. Municipal and industrial waste products were restricted to some specific area but agriculture is done throughout the study area. Large extent of ecotourism in boats and launch which use fossil fuel is also a governing factor for Pb pollution (Jarup 2003; Duruibe et al. 2007). Local people use motor boats for transportation and the burning of fossil fuel creates air pollution containing Pb which in due course settled down in the sediment.
Comparison of sediment quality with other UNESCO World Heritage sites
Metal content in the sediment of our study area indicates significantly high pollution in Sundarbans and East Kolkata Wetland (Ramsar Site and a possible source area of metal contamination in Sundarbans) compared to other UNESCO World Heritage sites (Table 5). Among these areas, Donana National Park of Spain reveals the highest level of metal pollution. In 1998, Anzacollar mine released 4 million cubic meter of acidic water and 2 million cubic meter of toxic mud containing huge amount of metals in Agrio River. The contaminants finally found its way to the Donana National Park through the Guadiamar River (Grimalt et al. 1999), which is the probable source of huge amount of Cd, Cu, Pb, Zn, and Fe. The Great Barrier Reef and Mackay region estuary of Australia and Saloum delta of Senegal were found to be in overall pristine condition (Haynes and Johnson 2000; Duke et al. 2005; Bodin et al. 2013), although the concentration of Fe in Saloum delta was found to be surprisingly low. All of the remaining UNESCO World Heritage sites indicate metal contamination. Banc d’Arguin of Mauritania, Belize Barrier Reef of Belize, and Greater Blue Mountain of Australia also reported to have high cadmium contamination (Nolting et al. 1999; Gibbs and Guerra 1997; Harrison et al. 2003). High chromium contamination was recorded in mangroves of New Caledonia, France (Marchand et al. 2010). The Belize Barrier Reef, Everglades National Park, and Greater Blue Mountain have elevated amount of Cu in their sediments. Ni concentration was found higher than the toxic limit in mangroves of New Caledonia and the Great Barrier Reef. The Belize Barrier Reef, Greater Blue Mountain, and mangroves of New Caledonia reveal significant Zn pollution, whereas lead contamination was noted in the Belize Barrier Reef, Everglades National Park of the USA, Greater Blue Mountain of Australia, and Ha Long Bay of Vietnam (Gibbs and Guerra 1997; Duan 2012; Harrison et al. 2003; Ho et al. 2010). Worldwide comparison with other World Heritage sites clarifies the dire scenario of our study area, Sundarbans.
Seasonal variation
The hydrology of Sundarban estuarine system is characterized by high monsoonal precipitation and tidal interplay (Bhattacharya 1988; Sarkar et al. 1985). The total estuary system is governed by a dense network of intersecting creeks, estuaries, inlets, and tidal water ways. During monsoon, run off from a wide area including the cities Kolkata and Salt Lake along with North 24 Parganas district creates a huge quantity of metropolitan waste arising from industrial and domestic sources. It is then carried through canals and discharged into the outfall of the Bidyadhari River. During monsoon, the Cr concentration remarkably decreases along the whole stretch of the Bidyadhari River. Pb, Cd, and Cr concentrations get higher in post-monsoon and follows till the pre-monsoon of the following year (Table 3). Local pollution resulting from rampant use of boats and vessels and agricultural and domestic wastes throughout the year makes the situation even worse. Monsoonal washout might reduce contamination but uninterrupted deposition of waste again increases the concentration level of the metals. High rate of siltation also enhances the probability of metal deposition in the sediment. In recent decades, rapid siltation has been taking place in the small khals and kharis of Sundarban estuarine system (Gour 2012) raising the ecotoxicity of the environment.
Geo-accumulation index
The estuarine sediments show positive Igeo values for Cd, Cu, and Pb (Fig. 4). Other studied elements reveal negative Igeo values. The study site is moderately to strongly polluted with Cd during pre-monsoon and moderately polluted during monsoon. In post-monsoon S1, S2, S3, S4, and S10 show class 3 Igeo values and the other sites reveal class 2 Igeo values for Cd (Table 4). In the case of Cr, S1 and S2 are moderately to strongly polluted during pre-monsoon and moderately polluted during monsoon and post-monsoon. Most of the other stations have class 2 Igeo values during pre-monsoon and post-monsoon and class 1 Igeo values during monsoon. Stations S8, S9, and S10 have negative Igeo values during monsoon. Igeo values for Pb shows the study site to be unpolluted to moderately polluted.
Pollution load index
From the PLI values, it was evident that stations S1 and S2 are under severe pollution load (Fig. 5). Both of these two stations reside along the Bidyadhari River and situated in the northern (upstream) part of our study area. Station S9, positioned on the bank of the Saptamukhi River, also reveal considerable pollution load (Table 4). The sampling stations S3, S4, S5, and S6, situated on the bank of the Bidyadhari-Matla River network, have high pollution load. A tendency of gradual decrease in PLI towards the mouth of sea is noticeable as S7, S8, and S10 have minimum pollution load (Fig. 5). All of these stations are very close to the Bay of Bengal and subjected to high tidal activity throughout the year. Seasonal variation of pollution load was similar in all of the stations. Most of the stations reveal maximum pollution load during pre-monsoon. During monsoon, pollution load was minimum in all of our study area. Comparative analysis of PLI values (Table 6) with other wetland system in the world depicts alarming condition of the mangrove ecosystem.
The PLI and the spatial distribution of metals show higher concentration of Cr, Pb, and Cd in the sampling stations situated at the upstream of the rivers. The studies also reveal that station S7 which lies near the sea shows the lowest contamination level among all the sampling sites. Situated along the Saptamukhi River, stations S9 and S10 are significantly contaminated. In this study, the riverine network dominates the source of pollution in every sampling station. Saha et al. (2001) evidenced metal pollution in the sediment of Jagannath canal which further converge to the Bidyadhari River. Bhattacharya et al. (2014) estimated mercury concentration in the Bidyadhari River at four different stations. Alarming concentration of mercury was reported in the water, which was contributed by the agricultural runoff, sewage, and effluents from various industries, and Kolkata metropolitan, Salt Lake City, and adjacent areas of North 24 Parganas. Chowdhury and Maiti (2016) reported gradual increase of Pb and Cd in the rivers of Sundarban due to fossil fuel-operated boats for transport and rechargeable batteries. Thus, it is evident that the city wastes carried by the rivers are the major source of pollution whereas local discharges also contribute place to place. These metal contaminations beyond toxic threshold are the real threats to this vulnerable ecosystem of Sundarbans.
Nemerow pollution index
According to Nemerow pollution index (PN), maximum pollution in the estuarine ecosystem was found during pre-monsoon followed by post-monsoon and monsoon (Fig. 5). Though average PN values reveal moderate pollution in the estuary; Stations S1, S2, and S4 reveal PN values to reach over 7 during pre-monsoon and post-monsoon. Out of the ten sampling stations, only S5 and S8 indicate low pollution during monsoon. The minimum and maximum PN values calculated in the mangrove estuary are 2.01 and 7.34 respectively indicating low to high pollution (Table 4). PN studies exhibit high pollution in Bidyadhari, Matla and Saptamukhi riverbanks. Gradual increase of PN indicates that the ecosystem is approaching to be a highly polluted zone in near future.
Potential ecological risk
Pollution level in mangrove forests all over the world has exceeded the regulation limit for decades. Negligence and lack of continuous monitoring in Sundarbans mangrove estuary have pushed this ecosystem to a pollution sink. Ecological risk factor (Eri) for Cd was found maximum among the metals followed by Cr and Pb. Eri values for Cd ranges from 77 (monsoon, S5) to 296.5 (pre-monsoon, S1) indicating high ecological risk (Table 4). Minimum and maximum Eri values for Cr and Pb range from 2.385 to 13.85 and 3.646 to 16.531 respectively (Suppl. Fig. S3). Eri values for Cr and Pb describe low ecological risk due to low toxic response factor (Tri). Eri values describe ecological risk for a single element but in reality all of the pollutants act together in an ecosystem. Potential ecological risk index (RI) takes into account all pollutants and hence a better tool to discuss potential ecological risk in any ecosystem. RI values of our study area notify higher ecological risk during pre-monsoon and post-monsoon (Fig. 5). The study area exhibits moderate level of potential ecological risk (Table 4). When compared to other wetland system (Gati et al. 2016), our study area illustrates very high potential ecological risk (Table 6). Stations S1, S2, and S4 demonstrated high potential ecological risk at some point during pre-monsoon and post-monsoon. The downstream (southern) part of our study area reports low potential ecological risk during monsoon evidencing better mangrove health in the downstream region than the upstream region.
Mangrove plants and associated bacterial community showed reduced growth rate when the metal concentration was five times greater than pristine mangrove ecosystem (Yim and Tam 1999). Cr, Pb, and Cd are known to have very harsh effect on flora and fauna. The conserved floral and faunal diversity of Sundarbans mangrove ecosystem may also be affected by anthropogenic activity further leading to destruction of the habitat. In the last two decades, four islands (Bedford, Lohachara, Kabasgadi, and Suparibhanga) of Sundarbans mangrove estuary have been completely destroyed due to sea level rising and soil erosion. Another two islands (Ghoramara and Mousuni) are reported to be on the verge of extinction (Danda 2010) only due to indifferent destruction of this estuarine ecosystem. Mangroves act as anchor to the soil against the tidal activity in coastal areas. Thus, the loss of mangrove could cost the whole region from its existence. Elevated metal concentration affects the food chain also and several studies report the accumulation of metals in amphibians and reptiles of Sundarbans. As studies suggests that fiddler crab shows bioaccumulation potential in respect to different levels of salt stress (O’Hara 1973). Akhand et al. (2012) reported that Uca sp. and the amphibious detretivour, mudskipper (Boleopthalmus sp.) can also accumulate elevated concentrations of Cd, Cr, Pb, and Zn in their body if exposed to tannery and municipal effluents at Indian Sundarbans. The floral diversity of Sundarbans has already been transformed in the last decades. Avicinnea sp. is reported as one of the most tolerant mangrove species and their number have been increased significantly in different areas of Sundarbans (Maiti and Chowdhury 2013). On the other hand, Heritorria litoralis which is locally called Sundari is becoming much rare in recent time (Awal 2014). Various studies suggests that marine turtles are seriously affected by metal pollution mainly Cd, Pb, Ni, Mn, As, Se, and Al that affects their growth and the central nervous system (Komoroske et al. 2011; Wennberg 1994). Fishing is one of the important employments in Sundarbans and fishes also accumulate a larger extent of metals like Cd, Fe, Pb, and Zn in their tissues (Banerjee et al. 2016). Another study has shown that tiger shrimp (Penaeus mondon) and mullet (Liza parsica) have higher potential to accumulate most of the metals at elevated concentrations (Guhathakurta and Kaviraj 2000). Thus, there exists a number of direct and indirect ways of human exposure to metal contamination. Thus, elevated metal contamination is a significant risk factor for this heritage ecosystem.
Pearson correlation study
Correlation study was performed between metal concentrations, physicochemical parameters, and sediment texture components. This study explains significant positive correlation between siltation and metal ions (Fig. 6). Cd, Cr, Cu, Ni, and Pb increase with siltation. Although no significant correlation was found between clay and metals, salinity, pH, and TOC values are found to be negatively correlated with metals. Though a positive correlation was noted, our observations illustrate the decreasing concentration of metals towards the sea. Huge amount of litter fall in this area contributes to a detritus ecosystem which increases the organic carbon content in the water and the sediment. The mangrove forest is also dense towards the sea and it justifies the distinct behavior of TOC. Significant negative correlation was found between salinity and Cr. pH has significant negative correlation with Cd, Cr, and Cu. TOC bears significant negative correlation only with Pb. Negative correlation was significant in the case of sand with silt and clay. Hence, Cd, Cr, Cu, Ni, and Pb reveal significant negative correlation with sand. Salinity has significant positive correlation with pH which describes increasing salinity and pH along the rivers towards mouth of the sea. pH and salinity have positive correlation with sand and negative correlation with clay. In the case of the metal ions, significant positive correlation was found between Cd, Cr, Cu, Ni, and Pb.
Multivariate analysis
To understand the source and the distribution of the metals, principal component analysis (PCA) has been done with the metal ions, pH, salinity, TOC, and sediment components. PCA was done separately for three seasons to compare the seasonal variation (Fig. 7). In all the three seasons, pH and salinity was found close to each other. The main factor affecting these two components is the influx of sea water. Both of these components show stability with minor changes during monsoon as rainwater plays a significant role. TOC was found closer to pH and salinity during pre-monsoon and monsoon, although a distance is found during post-monsoon. The negative correlation between sand with smaller particles and metals is evident in this analysis. Fe has a continuous high concentration in the sediment and it positions itself close to other metals in all the three seasons. Zn displays a completely different characteristic than the other metal ions. It is closer to other pollutants during post-monsoon but has a great distance during the other seasons. It might imply a different source of Zn in our study area. The major pollutants (Cd, Cr, and Pb) along with Ni and Cu are closely related with silt and clay. Previous studies (Wahba and Zaghloul 2007; Zhuang and Gao 2014) reveal that the smaller particles in sediment attract much more metal ions than the larger particles. Hence, the close proximity of silt and clay with metal ions can explain the situation. Previously, we found the metal concentrations to be increased along with siltation. Silt, clay, and metals have the same source in this region and that is the upstream part of the rivers. The PCA analysis overall supports our hypothesis about the source of metal pollution.
Identification of possible source area
Indian Sundarbans has the adjacent city of Kolkata as a possible source of pollution. Kolkata is the third most populous city in India and plays a critical role in the economic activities in eastern India. This area is a source of huge amount of metropolitan and industrial waste. The upstream region of the Bidyadhari River is well connected to the canals of East Kolkata Wetland. Despite being a ramsar site, this area is currently being used as a dumping ground of the municipal waste. This region also has several tanneries and battery industries. There are nearly 350 tanneries in The Calcutta Leather Complex (CLC) near Bantala. Although most of the tanneries have been shifted to CLC, still there are nearly 350 legal and illegal tanneries outside CLC (Dawn and Basu 2016; Vicziany et al. 2017). The canals in this area collect municipal and industrial (mainly tannery and battery) wastes at stations P1 and P2 and carry it to the Bidyadhari River at station P3. Stations P1 and P2 reveal Cd concentration of 16.76 mg/kg and 15.41 mg/kg which are very high compared to the background value (Table 7). CF, EF, Igeo, and ERi values show optimum level of contamination for Cd in these two stations (Suppl. Table S2). However, P3 shows Cd concentration of 1.84 mg/kg and further reveals very high CF value, significant EF category, class 3 Igeo value and high potential ecological risk factor. Cr concentration in P1 and P2 were found to be 1040.91 mg/kg and 1536.21 mg/kg respectively (Table 7). P2 reveal highest concentration of Cr in our entire study area and it also coincides with the position of CLC. These two stations reveal very high CF and EF values and class 4 Igeo values for Cr (Suppl. Table S2). Station P3 reveals Cr concentration of 168.87 mg/kg which is comparable to the amount of Cr found in the mangrove estuary. P3 shows moderate CF and EF values and class 1 Igeo value. Pb concentration in P1 and P2 were found 203.27 mg/kg and 142.76 mg/kg respectively (Table 7). These two stations have very high CF and EF values, moderate ERi values, and class 4 and class 3 Igeo values respectively for Pb (Suppl. Table S2). Station P3 has Pb concentration of 29.84 mg/kg, moderate CF and EF values, class 0 Igeo value, and low ERi value. Stations P1 and P2 also show high Cu and Zn concentration, moderate CF values, significant EF values, and class 2 Igeo values (Suppl. Table S2), whereas P3 shows concentrations of these metals near background level. PLI values at P1 and P2 demonstrate nearly five times pollution load compared to the mangrove estuary (Table 4, Table 7). This area reveals the highest PLI and RI values (Table 6) in comparison to the other wetland systems in the world. Massive PN and RI values of the whole possible source area suggest very high potential risk to Sundarbans situated in the downstream region. The waste canals have metal gates which can separate solid wastes from the canal (Vicziany et al. 2017), but prior joining to the Bidyadhari River, the waste materials of the canal do not receive further treatment. There are lock gates present at station P3 to control the flow of the canal (Maiti and Chowdhury 2013). These gates are kept closed during high tide so that the water from the river does not overflow the waste canals. It is also reported (Dawn and Basu 2016) that due to power cut or mechanical issues, sometimes untreated wastes are also dumped in the open canal from the CLC. Local people, mainly workers in different tanneries of this area are prone to a number of diseases like skin infection, lung disorder, eye irritation, and breathing discomfort (Dawn and Basu 2016). Increasing metal concentration in the sediment of Sundarbans poses a threat to the population living in the area. We have previously discussed that the waste canals dump the pollutants at station P3, from where the Bidyadhari River carries it to the mangrove estuary. Thus, the striking dissimilarity of metal pollution at P3 than P1 and P2 gives us enough reason to believe that the municipal and industrial wastes of Kolkata is a major source of toxic metal pollution of Sundarbans mangrove estuary.
Sundarbans attract a large number of visitors from India and abroad each year because of its unique biodiversity. As a result, the numbers of vessels, hotel, and lodges have increased significantly in the last decade. Increased anthropogenic intervention is also an additional component to the pollution status of Sundarbans. As it is hard to impede the effect of industrial waste or water transportation, only regular monitoring can be a significant way out. The people of Sundarbans live an economical marginalized life so it is not possible to implement stringent law like restriction on fishing in conserved water, forcing them not to be exposed to hazardous materials (Jalais 2010; Chowdhury and Maiti 2014). Only public awareness and campaigning can convince the community regarding the sustainable development and conservation of this ecosystem.
Socioeconomic status and health issues of local population
Indian Sundarbans is home of nearly 4.5 million people and 43.32% of them live under the poverty level. The local population is the worst sufferer of sea level rise and climate change. Apart from their harsh life struggle and nutritional deficiency, inadequate healthcare facilities made the situation even worse (Danda and Sriskanthan 2011). The main source of income of this region is agriculture followed by fishing, collection of honey and other goods from the mangrove forest, and ecotourism. Metal pollution in soil and water is causing a serious threat to the local population as agricultural products, fish, prawns, and crabs have been reported to contain significant amount of metal ions (Dutta et al. 2017; Mitra et al. 2012). Metal exposure in the upstream region has resulted into serious health problems. Tannery workers in East Kolkata Wetland have been found susceptible to skin infection, breathing trouble, eye irritation, and lung disorders (Dawn and Basu 2016). Ill-treated tannery and municipal waste combined with anthropogenic stress, ecotourism, and common practice of chemical fertilizer and pesticides are driving this UNESCO World Heritage Site towards destruction.
Conclusion
The fragility of Sundarbans originates from its uniqueness. Thus, to prevent its diversity from extinction, conservation of this mangrove ecosystem is vital. In 2011, WWF published an article and discussed on the management plans to restore the Indian Sundarban Delta. It suggests creating a biosphere district and laid a five step planning which can possibly restore and regenerate the mangrove ecosystem by 2050 (Danda and Sriskanthan 2011). In the year 2011, Government of India promulgated a new Coastal Regulation Zone (CRZ) notification titled 2011 and implemented that with immediate effect. One of the important parts of it was to consider Sundarbans as a Critically Vulnerable Coastal Area (CVCA). A participatory management action plan for individual CVCAs is supposed to be formulated under this notification. That work is going on. The India Integrated Coastal Zone Management (ICZM) funded by the World Bank is actually a follower of that notification. Incidentally, this work is a component of the India ICZM project. This study ascertains the relation between seasonal variation of metal concentration with siltation and hazardous waste materials from industrial, municipal, and agricultural fields. The untreated wastes coming from the upstream in addition with local wastes has put together a pollution load on the sediment of Sundarbans. It is not possible to close down all the tanneries and battery industries. Thus, tannery managers and owners need to be encouraged to explore green chemistry for treatment of the hydes rather than toxic chemicals. The Central Effluent Treatment Plant (CETP) of Bantala leather complex needs to be monitored by the regulatory authorities like the West Bengal Pollution Control Board (WBPCB) and Central Pollution Control Board (CPCB). There should be proper vigilance so that no untreated effluent and tannery waste can be disposed directly to the municipal sewage carrying canal (storm weather flow canal or SWF canal) which is ultimately discharging to the Bay of Bengal affecting the biodiversity of this estuarine mangrove forest.
References
Akhand A, Chanda A, Sanyal P, Hazra S (2012) Pollution load of four heavy metals in water, sediment and benthic organisms in the Kulti River of Sundarban fed by metropolitan sewage. Nat Environ Pollut Technol 11(1):153–156
Akhand A, Chanda A, Das S, Sanyal P, Hazra S (2016) High cadmium contamination at the gateway to Sundarban ecosystem driven by Kolkata metropolitan sewage in India. Curr Sci 110:386. https://doi.org/10.18520/cs/v110/i3/386-391
Alongi DM (2002) Present state and future of the world’s mangrove forests. Environ Conserv 29. https://doi.org/10.1017/S0376892902000231
Alongi DM, Wattayakorn G, Boyle S, Tirendi F, Payn C, Dixon P (2004) Influence of roots and climate on mineral and trace element storage and flux in tropical mangrove soils. Biogeochemistry 69:105–123. https://doi.org/10.1023/B:BIOG.0000031043.06245.af
Anderson JM, Ingram JS (1993) Tropical soil biology and fertility: a handbook of methods, 2nd edn. CAB International, Wallingford, pp 93–94
Apte A, Verma S, Tare V, Bose P (2005) Oxidation of Cr(III) in tannery sludge to Cr(VI): field observations and theoretical assessment. J Hazard Mater 121:215–222. https://doi.org/10.1016/j.jhazmat.2005.02.010
Awal MA (2014) Invention on correlation between the chemical composition of the surface sediment and water in the mangrove forest of the Sundarbans, Bangladesh, and the regeneration, growth and dieback of the forest trees and people health. Sci Innovation 2(2):11–21. https://doi.org/10.11648/j.si.20140202.11
Banerjee S, Kumar A, Maiti SK, Chowdhury A (2016) Seasonal variation in heavy metal contaminations in water and sediments of Jamshedpur stretch of Subarnarekha river, India. Environ Earth Sci 75. https://doi.org/10.1007/s12665-015-4990-6
Bhattacharya A (1988) Intertidal depositional characters in the Hoogly tidal islands, West Bengal. Indian J Geol 60:153–164
Bhattacharya S, Dash JR, Patra PH, Dubey SK, Das AK, Mandal TK, Bandyopadhyay SK (2014) Spatio-temporal variation of mercury in Bidyadhari River of Sundarban delta, India. Explor Anim Med Res 4(1):19–32
Bodin N, N’Gom-Kâ R, Kâc S, Thiaw OT, Tito de Morais L, Le Loc’h F, Rozuel-Chartier E, Auger D, Chiffoleau JF (2013) Assessment of trace metal contamination in mangrove ecosystems from Senegal, West Africa. Chemosphere 90:150–157. https://doi.org/10.1016/j.chemosphere.2012.06.019
Cato I (1977) Recent sedimentological and geochemical conditions and pollution problems in two marine areas in southwestern Sweden. Striae 6:1–150
CCME (1999) Canadian water quality guidelines for protection of aquatic life. Technical Report, Canadian Environmental Quality Guidelines, Canadian Water Quality Index 1.0
Chen HM (2010) Environmental soil science, 2nd edn. Science Press, Beijing
Chowdhury A, Maiti SK (2014) Mangrove reforestation through participation of vulnerable population: engineering a sustainable management solution for resource conservation. Int J Environ Res Develop 4(1):1–8
Chowdhury A, Maiti SK (2016) Identifying the source and accessing the spatial variations, contamination status, conservation threats of heavy metal pollution in the river waters of Sunderban biosphere reserve, India. J Coast Conserv 20:257–269. https://doi.org/10.1007/s11852-016-0436-x
Danda A (2010) Sundarbans: future imperfect: climate adaptation report. WWF-India
Danda AA, Sriskanthan G (2011) Indian sundarbans delta: a vision. WWF-India
Dawn A, Basu R (2016) A profile of industrial pollution in Kolkata municipal corporation area: the case of tanneries. Trans Inst Indian Geographers 38(1):79–88
Day PR (1965) Particle fractionation and particle size analysis. Methods of soil analysis, Part 1 Chapter 43, American Society of Agronomy 545–567
Dayan AD, Paine AJ (2001) Mechanisms of chromium toxicity, carcinogenicity and allergenicity: review of the literature from 1985 to 2000. Hum Exp Toxicol 20:439–451. https://doi.org/10.1191/096032701682693062
Duan Z (2012) The distribution of toxic and essential metals in the Florida Everglades. FIU digital commons FIU electronic theses and dissertations Florida International University. https://doi.org/10.25148/etd.FI12080608
Duke NC, Bell AM, Pederson DK, Roelfsema CM, Nash SB (2005) Herbicides implicated as the cause of severe mangrove dieback in the Mackay region, NE Australia: consequences for marine plant habitats of the GBR World Heritage area. Mar Pollut Bull 51:308–324. https://doi.org/10.1016/j.marpolbul.2004.10.040
Duruibe JO, Ogwuegbu MOC, Egwurugwu JN (2007) Heavy metal pollution and human biotoxic effects. Int J Phys Sc 2(5):112–118
Dutta A, Ghosh S, Choudhury JD, Mahansaria R, Roy M, Ghosh AK, Roychowdhury T, Mukherjee J (2017) Isolation of indigenous Staphylococcus sciuri from chromium-contaminated paddy field and its application for reduction of Cr(VI) in rice plants cultivated in pots. Bioremediat J 21:30–37. https://doi.org/10.1080/10889868.2017.1282935
Franco C, Soares A, Delgado J (2006) Geostatistical modelling of heavy metal contamination in the topsoil of Guadiamar river margins (S Spain) using a stochastic simulation technique. Geoderma 136:852–864. https://doi.org/10.1016/j.geoderma.2006.06.012
Gati G, Pop C, Brudaşcă F, Gurzău AE, Spînu M (2016) The ecological risk of heavy metals in sediment from the Danube Delta. Ecotoxicology 25(4):688–696. https://doi.org/10.1007/s10646-016-1627-9
Gibbs RJ, Guerra C (1997) Metals of the bottom muds in Belize City Harbor, Belize. Environ Pollut 98(1):135–138
Gour J (2012) Changing fluvio-geomorphological environment in the Matla-Bidyadhari interfluve—a model unit of active & mature Indian Sundarbans. Geo-Analyst 2(1)
Grimalt JO, Ferrer M, Macpherson E (1999) The mine tailing accident in Aznalcollar. Sci Total Environ 242:3–11
Guhathakurta H, Kaviraj A (2000) Heavy metal concentration in water, sediment, shrimp (Penaeus monodon) and mullet (Liza parsia) in some brackish water ponds of Sundarban, India. Mar Pollut Bull 40:914–920. https://doi.org/10.1016/S0025-326X(00)00028-X
Hakanson L (1980) An ecological risk index for aquatic pollution control, a sedimentological approach. Water Res 14(8):975–1001. https://doi.org/10.1016/0043-1354(80)90143-8
Harrison J, Heijnis H, Caprarelli G (2003) Historical pollution variability from abandoned mine sites, Greater Blue Mountains World Heritage area, New South Wales, Australia. Environ Geol 43:680–687. https://doi.org/10.1007/s00254-002-0687-8
Haynes D, Johnson JE (2000) Organochlorine, heavy metal and polyaromatic hydrocarbon pollutant concentrations in the Great Barrier Reef (Australia) environment: a review. Mar Pollut Bull 41(7.12):267–278
Ho HH, Swennen R, VanDamme A (2010) Distribution and contamination status of heavy metals in estuarine sediments near Cua Ong Harbor, Ha Long Bay, Vietnam. Geol Belg 13(1.2):37–47
Jalais A (2010) Forest of tigers: people, politics & environment in the sundarbans. Routledge, New Delhi
Jarup L (2003) Hazards of heavy metal contamination. Br Med Bull 68:167–182. https://doi.org/10.1093/bmb/ldg032
Jiang X, Xiong Z, Liu H, Liu G, Liu W (2017) Distribution, source identification, and ecological risk assessment of heavy metals in wetland soils of a river–reservoir system. Environ Sci Pollut Res 24:436–444. https://doi.org/10.1007/s11356-016-7775-x
Komoroske LM, Lewison RL, Seminoff JA, Deheyn DD, Dutton PH (2011) Pollutants and the health of green sea turtles resident to an urbanized estuary in San Diego, CA. Chemosphere 84:544–552. https://doi.org/10.1016/j.chemosphere.2011.04.023
Krishnamurti AJ, Nair VR (1999) Concentration of metals in shrimps and crabs from Thane and Basse in creek systems, Maharashtra. Indian J Mar Sci 28:92–95
Kumar G, Kumar M, Ramanathan AL (2015) Assessment of heavy metal contamination in the surface sediments in the mangrove ecosystem of Gulf of Kachchh, west coast of India. Environ Earth Sci 74:545–556. https://doi.org/10.1007/s12665-015-4062-y
Li R, Chai M, Qiu GY (2016) Distribution, fraction, and ecological assessment of heavy metals in sediment-plant system in mangrove forest, South China Sea. PLoS One 11(1):e0147308. https://doi.org/10.1371/journal.pone.0147308
Maiti SK, Chowdhury A (2013) Effects of anthropogenic pollution on mangrove biodiversity: a review. J Environ Prot 04:1428–1434. https://doi.org/10.4236/jep.2013.412163
Manna S, Chaudhuri K, Bhattacharyya S, Bhattacharyya M (2010) Dynamics of Sundarban estuarine ecosystem: eutrophication induced threat to mangroves. Saline Systems 6:8. https://doi.org/10.1186/1746-1448-6-8
Manna S, Chaudhuri K, SenSharma K, Naskar P, Bhattacharyya S, Bhattacharyya M (2012) Interplay of physical, chemical and biological components in estuarine ecosystem with special reference to Sundarbans, India. Ecological Water Quality-Water Treatment and Reuse, In Tech 10:205–238
Marchand C, Allenbach M, Lallier-Vergès E (2010) Relationships between heavy metals distribution and organic matter cycling in mangrove sediments (Conception Bay, New Caledonia). Geoderma 160:444–456. https://doi.org/10.1016/j.geoderma.2010.10.015
Martin JM, Meybeck M (1979) Elemental mass-balance of material carried by major world rivers. Mar Chem 7:173–206. https://doi.org/10.1016/0304-4203(79)90039-2
Mebius LJ (1960) A rapid method for the determination of organic carbon in soil. Anal Chim Acta 22:120–124
Mitra A, Chowdhury R, Banerjee K (2012) Concentrations of some heavy metals in commerciallyimportant finfish and shellfish of the river Ganga. Environ Monit Assess 184:2219–2230. https://doi.org/10.1007/s10661-011-2111-x
Mmolawa KB, Likuku AS, Gaboutloeloe GK (2011) Assessment of heavy metal pollution in soils along major roadside areas in Botswana. Afr J Environ Sci Technol 5(3):186–196 ISSN 1996-0786
Muller G (1969) Index of geoaccumulation in sediments of the Rhine River. GeoJournal 2(3):108–118
Nolting RF, Ramkema A, Everaarts JM (1999) The geochemistry of Cu, Cd, Zn, Ni and Pb in sediment cores from the continental slope of the Banc dÕArguin (Mauritania). Cont Shelf Res 19:665–691
O’Hara J (1973) The influence of temperature and salinity on the toxicity of cadmium to the fiddler crab, Ucapugilator. Fish Bull 71(1):149–153
Pain DJ, Sánchez A, Meharg AA (1998) The Doñana ecological disaster: contamination of a world heritage estuarine marsh ecosystem with acidified pyrite mine waste. Sci Total Environ 222:45–54
Raju KV, Somashekar RK, Prakash KL (2012) Heavy metal status of sediment in river Cauvery, Karnataka. Environ Monit Assess 184:361–373. https://doi.org/10.1007/s10661-011-1973-2
Roy US, Goswami AR, Aich A, Chattopadhyay B, Datta S, Mukhopadhyay SK (2013) Changes in physicochemical characteristics of wastewater carrying canals after relocation of Calcutta tannery agglomerates within the East Calcutta wetland ecosystem (a Ramsar site). Int J Environ Stud 70:203–221. https://doi.org/10.1080/00207233.2013.774810
Saha SB, Mitra A, Bhattacharyya SB, Choudhury A (2001) Status of sediment with special reference to heavy metal pollution of a brackishwater tidal ecosystem in northern Sundarbans of West Bengal. Trop Ecol 42(1):127–132
Salah EAM, Zaidan TA, Al-Rawi AS (2012) Assessment of heavy metals pollution in the sediments of Euphrates River, Iraq. J Water Resource Prot 04:1009–1023. https://doi.org/10.4236/jwarp.2012.412117
Samanta S, Mitra K, Chandra K, Saha K, Bandopadhyay S, Ghosh A (2005) Heavy metals in water of the rivers Hooghly and Haldi at Haldia and their impact on fish. J Environ Biol 26:517–523
Sarkar SK, Singh BN, Choudhury A (1985) The ecology of chaetognaths in the Hugli Estuary, West Bengal, India. Indian J Mar Sci 14:98–101
Sastre J, Sahuquillo A, Vidal M, Rauret G (2002) Determination of Cd, Cu, Pb and Zn in environmental samples: microwave-assisted total digestion versus aqua regia and nitric acid extraction. Anal Chim Acta 462:59–72. https://doi.org/10.1016/S0003-2670(02)00307-0
Satapathy DR, Panda CR (2015) Spatio-temporal distribution of major and trace metals in estuarine sediments of Dhamra, Bay of Bengal, India-its environmental significance. Environ Monit Assess 187:4133. https://doi.org/10.1007/s10661-014-4133-7
Sinex SA, Helz GR (1981) Regional geochemistry of trace elements in Chesapeake Bay sediments. Environ Geol 3:315–323. https://doi.org/10.1007/BF02473521
Spalding M, Blasco F, Field C (1997) World mangrove atlas. Okinawa, Japan: the International Society for Mangrove Ecosystems, 178 pp
Tomlinson DL, Wilson JG, Harris CR, Jeffrey DW (1980) Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresun 33:566–575. https://doi.org/10.1007/BF02414780
USDA (1987) USDA textural soil classification. Module 3, National Employee Development Staff, Soil Conservation Service, United States Department of Agriculture
USEPA (1999) US Environmental Protection Agency: screening level ecological risk assessment protocol for hazardous waste combustion facilities. Appendix E: Toxicity Reference Values, Vol 3
Vicziany M, Chattopadhyay D, Bhattacharyya S (2017) Food from sewage: fish from the east Kolkata wetlands and the limits of traditional knowledge. South Asia 40:619–644. https://doi.org/10.1080/00856401.2017.1341038
Wahba MH, Zaghloul AM (2007) Adsorption characteristics of some heavy metals by some soil minerals. J Appl Sci Res 3(6):421–426
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining organic carbon in soils: effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci 63:251–263. https://doi.org/10.1097/00010694-194704000-00001
Wennberg A (1994) Neurotoxic effects of selected metals. Scand J Work Environ Health 20:65–71
WHO (2011) Guidelines for drinking water quality, 4rth edn. World Health Organization
Yan N, Liu W, Xie H, Gao L, Han Y, Wang M, Li H (2015) Distribution and assessment of heavy metals in the surface sediment of Yellow River, China. J Environ Sci (China) 39:45–51. https://doi.org/10.1016/j.jes.2015.10.017
Yim MW, Tam NFY (1999) Effects of wastewater borne heavy metals on mangrove plants and soil microbial activities. Mar Pollut Bull 39:179–186
Zhuang W, Gao X (2014) Integrated assessment of heavy metal pollution in the surface sediments of the Laizhou Bay and the coastal waters of the Zhangzi Island, China: comparison among typical marine sediment quality indices. PLoS One 9:e94145. https://doi.org/10.1371/journal.pone.0094145
Acknowledgements
We acknowledge the World Bank for all the necessary support for the execution of the ICZM project (54-ICZMP/3P), West Bengal. Debojyoti Roy likes to acknowledge the University Grants Commission (RGNF-2015-17-SC-WES-16366) for providing his fellowship. Dr. Arnab Pramanik is supported by the Research Associateship from World Bank ICZM project. We would like to acknowledge the continuous encouragement and enthusiasm expressed by Mr. Tapas Paul and Dr. Herbert K. Acquay from The World Bank in our venture to explore the world heritage site, Sundarbans. We express our sincere gratitude to SPMU, NPMU, and IESWM for their continuous support. We are thankful to Dr. Somnath Bhattacharya and Professor Marika Vicziany for providing the necessary information about this ecologically important area. We like to acknowledge UGC-CAS, DST–FIST, DBT-IPLS, UGC-UPE in the Department of Biochemistry, University of Calcutta. It would not be possible to carry out this work without the help and support of the local people of the Sundarbans. We express our inability to acknowledge them individually.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
The article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Responsible editor: Severine Le Faucheur
Electronic supplementary material
ESM 1
(DOCX 3406 kb)
Rights and permissions
About this article
Cite this article
Roy, D., Pramanik, A., Banerjee, S. et al. Spatio-temporal variability and source identification for metal contamination in the river sediment of Indian Sundarbans, a world heritage site. Environ Sci Pollut Res 25, 31326–31345 (2018). https://doi.org/10.1007/s11356-018-3092-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3092-x