Abstract
The contamination of soil with heavy metals is a severe problem due to adverse impact of heavy metals on environmental safety and human health. It is essential to remediate soil contaminated with heavy metals. This study has evaluated the effects of pine biochar, kaolin, and triple super phosphate (TSP) on multiple heavy metals (Ni, Zn, Cu, and Cd) in contaminated soil and accumulation of heavy metals in plants. The amendments can reduce availability of heavy metals in soil by increasing pH, adsorption, complexation, or co-precipitation. Different amendments have variable effects on accumulation of heavy metals in plants and in soil due to its diverse mechanism of stability. The results showed that application of triple super phosphate (TSP) has significant reduced soil Cd exchangeable (EXC) fraction from 58.59 to 21.30%. Bound to carbonates (CAR) fraction decreased from 9.84 to 5.11%, and bound to Fe-Mn oxides (OX) fraction increased from 29.61 to 69.86%. The triple super phosphate (TSP) has the ability to stabilize Cu and especially Cd. However, triple super phosphate (TSP) has enhanced ecological risk of Zn and Ni. Application of pine biochar has significantly enhanced soil pH. The kaolin has significantly reduced EXC fraction of Cd and increased OX fraction of Cu. The amendments and heavy metals have not caused significant effect on SPAD value of Buxus microphylla Siebold & Zucc (B. microphylla). The triple super phosphate (TSP) has significant decreased biomass of B. microphylla and bamboo-williow (Salix sp.) by 24.91 and 57.43%, respectively. Pine biochar and kaolin have increased the accumulation of Zn and Cd in plants. It is concluded that triple super phosphate (TSP) was effective in remediation of Cd and kaolin was effective in remediation of Cd and Cu. Pine biochar was effective in remediation of Cd, Cu, and Zn.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pollution of soils by heavy metals has seriously threatened the ecological environment, food safety, and human health (Huang et al. 2016; Kumpiene et al. 2008; Shirvani et al. 2015). The improper management of metallurgical industrial wastes, mining wastes, pesticides, and fertilizers have caused contamination of soils with heavy metals, e.g., Pb, Cd, Cu, Zn, Ni, etc. (Boyd and Rajakaruna 2013; Niazi et al. 2015). The remediation technology of pollution due to heavy metals has reduced the amount of heavy metals in soil or has reduced biological availability of heavy metals in soil (Li et al. 2015; Liu et al. 2015). Electrokinetic extraction, chemical stabilization, and phytoremediation are at the development stage (Liu et al. 2018). The application of stabilization material in soil has adjusted or changed proportion of bioavailable fraction of heavy metals, which has reduced biological availability and mobility of heavy metals in soil environment (Cao and Dai 2011). Phytoremediation is green and cost-effective technology in organic and inorganic pollutants (Rizwan et al. 2018).
The phosphate materials (Seshadri et al. 2017), clay minerals (Xu et al. 2017), and biochar (Maroušek et al. 2017a; Wang et al. 2018) etc. were usual used as soil amendments to stabilized heavy metals in soils. The cadmium leachable concentration was reduced from 306 to 140, 34, and 12 mg kg−1 after 60-day stabilization of contaminated soils with the stabilization efficiency as triple super phosphate (TSP) > diammonium phosphate (DAP) > phosphate rock (PR) (Thawornchaisit and Polprasert 2009). Biochar is a solid organic carbon compound obtained from incomplete combustion of organic materials in an oxygen-limited environment (Wang et al. 2014). The preparation and cost of biochar are becoming friendly (Maroušek et al. 2015a, b). Biochar is widely advocated as a soil amendment (Liang et al. 2017; Maroušek et al. 2017b; Li et al. 2018a, b). The biochar has large number of tiny pores, negative charge, high charge density, and larger specific surface area and its surface contains rich oxygen which consist functional groups (Xu et al. 2014; Li et al. 2018a, b). Wu et al. (2016) reported that limestone + sepiolite is more suitable for long-term remediation of Cd-polluted soil than Pb-polluted soil. Usman et al. (2005) revealed that sodium base bentonite, calcium base bentonite, and zeolite contain Zn, Cd, Cu, and Ni in heavy metal-contaminated soil. Kaolin treatment increased stomatal conductance, photosynthesis, and transpiration rates in all species and water content was observed in treated plants than in control plants (Varela et al. 2015). These three kinds of clay minerals can reduce the contents of Zn, Cd, Cu, and Ni bioavailable fraction in soils contaminated with heavy metals.
The purpose of this study was to compare remediation effect of pine biochar, kaolin, and triple super phosphate on heavy metal-contaminated soil with from heavy metal fraction distribution. This study has investigated the effects of three amendments (i.e., pine biochar, kaolin, and triple super phosphate) on heavy metal accumulation in landscape and fast-growing plants.
Materials and methods
Soil collection and preparation
The soil sampling was collected from a crop land in the vicinity of a galvanized factory in Fuyang district, Hangzhou city, Zhejiang province. The soil samples were collected from surface up to depth of 20 cm. The soil samples were air-dried, sieved to 5 mm, and packed in plastic sealed bag for physical, chemical characterization, and analysis of heavy metals. The physical and chemical properties of soil are presented in Table 1.
The amendment of pine biochar was acquired from Liaoning new energy technology Co. Ltd. Pine biochar was produced at approximately 550 °C with abatch pyrolysis facility. The kaolin was acquired from Shanxi hengyuan kaolin Co. Ltd. The triple super phosphate (TSP) was obtained from Yunnan three circles chemical Co. Ltd. The characteristics of amendments are presented in Table 2. The experiment was conducted in greenhouse. There were four treatments (i.e., control/CK (no amendment applied to compound contaminated soil), pine biochar, kaolin, and TSP treatment) in this experiment. Each stabilization test included 2 kg soil, 100 g amendment (5% weight of the soil) (Kang et al. 2015), having six replicates for each treatment, with stabilization period of 5 days. Each treatment had three pots planted with Buxus microphylla, and other three pots were planted with Slaix sp., with three plants in each pot.
Collection of soil and plant samples
The plant and soil samples were collected after 60 days of stabilization. The soil samples were air-dried sieved to 2 mm for analysis of chemical parameters and sieved to 0.149 mm (100 mesh sieve) for analysis of elements of heavy metals. The plant samples were soaked in 20 mmol L−1 EDTANa2 for 20 min, and heavy metals were removed from surface of underground part of plants. The plants were washed three times thoroughly with deionized water. Plants were separated in shoot and root parts and placed in kraft paper envelope, 105 °C for 30 min, then oven-dried to a constant weight at 65 °C, and its dry weight was recorded (Li et al. 2014). These tissues were passed through 0.149 mm sieve for analysis of heavy metals.
Soil analysis
Soil pH was determined in 1:2.5 soil/water suspensions using pH electrode. The soil sample was performed by soil heavy metal speciation analysis according to method of Table 3 (Tessier et al. 1979).
Plant elemental analysis
The heavy metal concentrations in plants were measured according to methods of Liu et al. (2014). The 0.3 g dried plant samples (sieved to 0.1 mm) were collected in glass tube and extracted with HNO3/HClO4 solution. The constant volume was extracted 2 h at 145 °C. The heavy metal content was analyzed by ICP-MS (Agilent 7500a, Japan).
Determination of plant SPAD value
The SPAD value of plants was determined by SPAD 502 relative chlorophyll content detector (KONICA MINOLTA. Inc., Tokyo). The relative chlorophyll content was measured at two-third of fresh leaves from leaf margin and three leaves were randomly determined for each plant. The SPAD parameters were measured in vitro leaves of plants. Each leaf was repeated for three readings, and their average was calculated. The determination of SPAD value was determined according to method of Jia et al. (2007).
Determination of plant MDA value
The plant leaf sample of 1 g was grinded with quartz sand and tri-chloroacetic acid until homogenized. The homogenate was centrifuged for 20 min at 4000 revolutions per minute (r/min) and supernatant was used for next chromogenic reaction with thiobarbituric acid (Liu et al. 2008).
Statistical analysis
The statistical analysis was conducted with SPSS statistical package (version 21.0). All values reported are means of at least three independent replications. Data were tested at significant levels of p < 0.05 by one-way ANOVA. Graphical work was carried out using Origin software pro v.9.0.
Results
Effect of amendments on soil pH
Figure 1 reveals that different amendments have variable affect on soil pH. Application of pine biochar has significantly enhanced soil pH (8.28) than soil pH of control (7.90). The treatment of kaolin has reduced soil pH by 1.01% compared with control (CK). Application of TSP has significantly decreased soil pH to 6.21 than control (7.90) which indicated 21.39% reduction in soil pH.
Effect of different amendments on distribution fraction of heavy metals
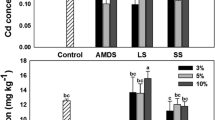
The distribution of Zn, Ni, Cu, and Cd fraction in soil is presented in Fig. 2. The Zn, Ni, and Cu in soil were predominantly distributed in OX fraction. The OX fraction comprised 67.73 to 92.19% of all treatments. The Cd was predominantly distributed in EXC fraction of soil. The EXC fraction was ranged from 21.30 to 58.59%.
Application of TSP has significantly increased EXC fraction of Ni in soil, and proportion of Ni EXC fraction was enhanced by 13.60%. Figure 2a revealed that application of TSP has transformed OX fraction to EXC fraction. Pine biochar has decreased OX fraction and has increased OM fraction of Ni compared with control (CK). The kaolin has reduced total EXC and CAR fraction of Ni. However, there was non-significant different with control (CK).
Application of TSP has changed the distribution of soil Zn fraction which has significantly increased EXC fraction, and transformed CAR to EXC fraction. The EXC fraction was increased from 0.54 to 2.0% compared with control (CK). The pine biochar has significantly decreased OX fraction and increased OM fraction. The kaolin has non-significant different compared with control (CK) (Fig. 2b).
The EXC fraction of Cu was not detected after application of amendments (Fig. 2c). Application of TSP has significantly reduced the soil CAR fraction of Cu than control (CK). The CAR fraction was decreased from 17.68 to 7.00%. Application of pine biochar has significantly increased Cu RES and OM fraction than control (CK). The RES and OM fraction was primarily transformed by OX fraction, which increased from 4.63 to 7.32%, and 4.92 to 7.29%, respectively. Application of kaolin has significantly decreased EXC fraction of Cu by 2.7% and has significantly decreased OX fraction of Cu by 4.71%.
Figure 2d indicates that application of TSP and kaolin has significant effect on EXC, CAR, and OX fraction of Cd compared with control (CK). Application of TSP has significantly decreased soil ECX fraction of Cd from 58.59 to 21.30%. The CAR fraction of Cd was significantly decreased from 9.84 to 5.11%. The OX fraction was significantly increased from 29.61 to 69.86%. Application of kaolin has significantly decreased EXC fraction of Cd by 7.79%, CAR fraction was significantly increased by 2.19%, and OX fraction was significantly increased from 29.61 to 35.32%. The pine biochar has significantly decreased EXC fraction of Cd compared with control (CK).
Effect of amendments on plant biomass
Figure 3 shows that application of amendments had an adverse effect on plant growth. Application of pine biochar and kaolin has decreased biomass of B.microphylla and Sliax sp. compared with control (CK). However, reduction in biomass was non-significant compared with control (CK). Application of TSP has significantly decreased biomass of B. microphylla from 8.08 g to 6.15 g and biomass of Sliax sp. was reduced from 6.79 to 2.89 g, respectively. Application of TSP has significantly affected biomass of Sliax sp. and B. microphylla.
Effect of amendments on MDA value of plants
The malondialdehyde (MDA) is the final decomposition product of cell membrane lipid peroxidation. MDA content reflects the extent of plant damage caused by adversity (Asai et al. 2009). Figure 4 exhibits that effects of pine biochar and kaolin on MDA value of B. microphylla were not significant compared with control (CK). Our results indicated that pine biochar and kaolin have caused less damage to cell membrane in leaves of B. microphylla. The MDA content of B. microphylla was significantly higher than control (CK) which was increased by 39.57% with application of TSP. All treatments have significantly increased MDA content of Salix sp. MDA content was significantly higher in treatments of TSP than pine biochar and kaolin compared with control (CK) which was increased by 63.46%. It was observed that TSP amendment has caused maximum damage to cell membrane in leaves of Salix sp. and B. microphalla.
Effect of amendments on plant SPAD value
The chlorophyll fluorescence kinetics parameters can be used to describe optical system in the process of photosynthesis to light energy absorption, transfer, dissipation, and distribution. The chlorophyll reflects the “inner” characteristics, which is of great significance in the study of plant resistance (Shao et al. 2017). Pine biochar, kaolin, and TSP treatments have non-significant different SPAD value compared with control (CK). However, all amendments have non-significantly decreased SPAD value of Salix sp. (Fig. 5).
Effect of amendments on accumulation of heavy metal in plants
The effects of amendments on heavy metal accumulation and uptake in plants of B. microphylla and Salix sp. are presented in Figs. 6 and 7, respectively. Application of pine biochar and kaolin shows non-significantly increased in Ni content of shoot and roots of B. microphylla compared with control (CK). The treatment of TSP has significantly decreased Ni content of shoot in plants of B. microphylla (Fig. 6a). Application of pine biochar and kaolin has significantly increased Zn content in shoot and root of B. microphylla (Fig. 6b). The application of TSP has significantly reduced content of Cu in roots of B. microphylla (Fig. 6c). The pine biochar and kaolin have significantly increased content of Cd in roots of B. microphylla. Application of TSP has significantly decreased Cd content of roots in B. microphylla (Fig. 6d).
The pine biochar has significantly increased Cd and Zn content in shoot of Salix sp. compared with control (CK). The application of pine biochar has significantly increased Zn, Cu, and Cd content in roots of Salix sp. The application of kaolin has highly enhanced Zn and Cd content in shoot of Salix sp. The Zn and Cd content in shoot was significantly increased from 358.89 to 606.74 mg kg−1 and 6.425 to 15.16 mg kg−1, respectively. The kaolin has significantly increased Ni, Zn, Cu, and Cd content in roots of Salix sp. Application of TSP has significantly increased Zn and Ni content in shoot of Salix sp. by 62.18 and 240.10%, respectively (Fig. 7).
Discussion
Different kinds of amendments have variable reaction mechanism in soil polluted with heavy metals. Biochar has high carbon content (Zhang et al. 2017), large specific surface area, stable physical and chemical properties, etc. with good adsorption capacity for ions of heavy metal. The biochar can reduce the mobility of heavy metals ions and its biological effectiveness (Chen et al. 2013; Jin et al. 2011). The biochar from wood materials can fasten Cd from soil mainly through ion exchange function (Gomez-Eyles et al. 2011). In this experiment, distribution of heavy metals fraction has provided important information about their mobility in soil. The treatment of pine biochar has significantly increased soil pH value. The soil pH has the strong effects on solubility and mobility of metals, so pH is viewed as the most important factor for metal in environment (Wang et al. 2015a; Lin et al. 2018). The biochar alkalinity was associated with surface organic functional groups, soluble organic compounds, carbonates, and other inorganic alkali in the biochar, among which the functional groups such as phenolic, hydroxyl, and carboxyl groups might contribute to the alkalinity of biochar (Fidel et al. 2017; Liang et al. 2017; Li et al. 2018a, b). Biochar can change soil properties such as increasing soil pH and cation exchange capacity, and thus could indirectly reduce metal mobility (Yin et al. 2016). The treatment of pine biochar has decreased Zn, Cu, and Cd OX fraction proportion. Pine biochar has significantly decreased OX fraction of Cu and increased OM fraction. Other study observed that Cu was easy to be combined with organic matter and its mobility could be improved by dissolved organic carbon (Zhao et al. 2013). Biochar reduced metal mobility in contaminated soils, which was attributed to the substantial decreases in the acid-soluble fractions of Cr, Mn, Cu, and Zn (72.20, 70.38, 50.43, and 29.78%, respectively) (Zhou et al. 2017). The acid-soluble state of Cu in soils was significantly decreased with application of different kind of crop straw biochars (Jiang and Xu 2013). The content of exchange fraction was highly reduced with maximum application of biochar. The fraction of NH4OAc extraction and weak acid extraction Cd content decreased significantly by 17.9 and 10.4% respectively with application of 25 g kg−1 content of rice husk biochar compared with control (CK) (Wang et al. 2015b).
The clay mineral particles with large specific surface and mineral rich negative surface have strong ability of adsorption and ion exchange (Mcgowen et al. 2001). The kaolin treatment has reduced total of EXC and CAR fraction in Zn, Ni, Cu, and Cd. Application of kaolin has decreased EXC fraction of Cu by 2.7% and enhanced OX fraction of Cu by 4.71%. The treatment of kaolin has reduced EXC fraction of Cd by 7.79%, CAR fraction was boost up by 2.19%, and OX fraction enhanced from 29.61 to 35.32%. The release of elements due to application of both zeolite and bentonite followed the order Cd2+ > Cu2+ > Ni2+ (Wahba et al. 2016). Soil fraction of EXC Cu content was decreased by 8.6% and fraction of CAR Cu was reduced by 36.6% compared with treatment of non-bentonite. Fraction of OX, OM, and RES was enhanced by 8.9%, 31.5 and 14.5%, respectively (Jia et al. 2013).
The phosphate materials can be induced to generate precipitation of heavy metals, and its surface adsorption of heavy metals or minerals (Cao and Dai 2011). Phosphorus materials can fix Cd from soil through reaction of surface complexation (Basta et al. 2001). The treatment of TSP has significantly decreased the pH value. This is due to the phosphoric acid released by TSP which composition consists mostly of Ca(H2PO4)·2H2O (Thawornchaisit and Polprasert 2009). The treatment of TSP has increased total of EXC and CAR fraction in Zn and Ni. This may be due to TSP decreased pH. It is indicated that use TSP to remediate multiple heavy metal-contaminated soil should be considered carefully especially soil contaminated with Zn and Ni. It is reported that the application of P source as monobasic calcium phosphate was found to reduce mobility of Pb and Cd. However, Zn was slightly mobilized (Theodoratos et al. 2002), which enhanced the risk of Zn ecological environment. In Pb-Cu-Zn ternary system, competitive metal sorption occurred with sorption capacity reduction of 15.2, 48.3, and 75.6% for Pb, Cu, and Zn, respectively, compared to mono-metal system (Cao et al. 2004). The treatment of TSP has decreased proportion of Cd EXC fraction by 37.29%, proportion of OX fraction was increased by 40.25%, and proportion of RES fraction was enhanced from 0.65 to 2.56%. Application of TSP has reduced concentration of Cd by 31.3%. The treatment of calcium-magnesia phosphate fertilizer in contaminated soil has declined soil effective fraction Cd content from 10.37 to 0.19%. The substances such as calcium phosphate can be directed through the surface adsorption at the same time which has reduced soil cadmium mobility and biological effectiveness. Other study has investigated that PO43−, HPO42−, and H2PO4− can be generated with Cd2+ (Cao and Dai 2011). A dissolution–precipitation mechanism via the formation of pyromorphite-like mineral was used to explain the decrease in Pb availability in previous studies suggested that Cd immobilization could be associated with the ion exchange (Guo et al. 2017).
The Salix sp. has showed high transfer ability of heavy metals which indicated highest shoot content than root content. The highest Zn and Cd content were 606.74 mg kg−1 and 15.16 mg kg−1, respectively. Other studies revealed the same results, such as content of Zn and Cd in Salix sp. has reached 61.4 and 1940 mg kg−1, respectively. The TF of Zn and Cd were greater than 0.8 (Chen et al. 2017).
Pine biochar and kaolin have increased Zn and Cd accumulation of Salix sp. Both shoot and root were significantly improved. It is possible that pine biochar and kaolin improves water retention decrease soil density and increase soil microbe (Maroušek et al. 2017a; Maroušek et al. 2018; Varela et al. 2015). The TSP has reduced heavy metal accumulation of Salix sp. and B. microphylla. However, TSP has significantly decreased biomass of B. microphylla and Salix sp. and enhanced MDA content. It is possible that the TSP was increased bioavailability of Zn and Ni which has caused poisonous effect on plant.
Conclusion
The treatment of pine biochar has significantly enhanced soil pH. The pine biochar has positively affected at decreased mobility of Cd, Cu, and Zn in soil. The kaolin has positively affected at decreased mobility of Cd and Cu in soil. Pine biochar and kaolin can be grown for phytoremediation which remediate heavy metal-contaminated soils. The TSP transformed Cu from CAR to OX fraction and Cd from EXC to OX fraction which indicated that the TSP can improve Cu and Cd stability in soil. However, TSP has significantly decreased soil pH and increased Zn and Ni EXC fraction which enhanced Zn and Ni ecological risk. This indicated the complexity of multiple heavy metal contamination of soil due to multiple heavy metals could not remediate by single amendment. The amendments have not caused significant effect on SPAD value of plant in this study. Salix sp. as a fast-growing plant has great potential in remediation of heavy metal pollution due to its large biomass and high Cd and Zn adsorption quantity.
References
Asai H, Samson BK, Stephan HM, Songyikhangsuthor K, Homma K, Kiyono Y et al (2009) Biochar amendment techniques for upland rice production in Northern Laos: 1. Soil physical properties, leaf SPAD and grain yield. Field Crop Res 111:81–84
Basta NT, Gradwohl R, Snethen KL, Schroder JL (2001) Chemical immobilization of lead, zinc, and cadmium in smelter-contaminated soils using biosolids and rock phosphate. J Environ Qual 30:1222–1230
Boyd RS, Rajakaruna N (2013) Heavy metal tolerance. Oxford Bibliographies in Ecology 8:499–505(7)
Cao X, Dai (2011) Combined pollution of multiple heavy metals and their chemical immobilization in contaminated soils: a review. Chin J Environ Eng 5:1441–1453 (in Chinese)
Cao X, Ma LQ, Rhue DR, Appel CS (2004) Mechanisms of lead, copper, and zinc retention by phosphate rock. Environ Pollut 131:435–444
Chen WF, Zhang WM, Meng J (2013) Advances and prospects in research of biochar utilization in agriculture. Sci Agric Sin 46:3324–3333 (in Chinese)
Chen XM, Guotao HU, Yang X, Zhengqian YE, Xiaohong WU, Wang H (2017) Heavy metal accumulation and physiological response of bamboo-willow plants to soil co-contaminated with Cd and Zn. Acta Sci Circumst 37(10):3968–3976 (in Chinese)
Fidel RB, Laird DA, Thompson ML, Lawrinenko M (2017) Characterization and quantification of biochar alkalinity. Chemosphere 167:367–373
Gomez-Eyles JL, Sizmur T, Collins CD et al (2011) Effects of biochar and the earthworm Eisenia fetida on the bioavailability of polycyclic aromatic hydrocarbons and potentially toxic elements. Environ Pollut 159:616–622
Guo G, Lei M, Chen T, Yang J (2017) Evaluation of different amendments and foliar fertilizer for immobilization of heavy metals in contaminated soils. J Soils Sediments 24:1–9
Huang G, Su X, Rizwan MS, Zhu Y, Hu H (2016) Chemical immobilization of Pb, Cu, and Cd by phosphate materials and calcium carbonate in contaminated soils. Environ Sci Pollut Res Int 23:1–12
Jia LL, Chen XP, Zhang FS (2007) The comparision of spad chlorophyll meter and sap nitrate test as N diagnosis methods for winter wheat. Acta Agriculturae Boreali-Sinica 06:157–160 (in Chinese)
Jia Z, Liu X, Bu Y, Hao J, Li J (2013) Effects of bentonite on the growth and physiological resistance of rape in copper contaminated soil. J Shanxi Agric Univ 33(03):250–254 (in Chinese)
Jiang J, Xu RK (2013) Application of crop straw derived biochars to Cu(II) contaminated Ultisol: evaluating role of alkali and organic functional groups in Cu(II) immobilization. Bioresour Technol 133:537–545
Jin HP, Choppala GK, Bolan NS, Chung JW, Chuasavathi T (2011) Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 348:439
Kang H, Lin J, Zhang N, Bao L, Liu B (2015) Passivation effect of different passive materials on heavy metal polluted soil. Chin Agric Sci Bull 31(35):176–180 (in Chinese)
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—a review. Waste Manag 28:215–225
Li T, Hu Y, Du X, Tang H, Shen C, Wu J (2014) Salicylic acid alleviates the adverse effects of salt stress in Torreya grandis cv. Merrillii seedlings by activating photosynthesis and enhancing antioxidant systems. PLoS One 9(10):e109492
Li S, Islam E, Peng D, Chen J, Wang Y, Wu J et al (2015) Accumulation and localization of cadmium in moso bamboo (Phyllostachys pubescens) grown hydroponically. Acta Physiol Plant 37:56
Li YF, Hu SD, Chen JH, Müller K, Li YC, Fu WJ, Lin ZW, Wang HL (2018a) Effects of biochar application in forest ecosystems on soil properties and greenhouse gas emissions: a review. J Soils Sediments 18:546–563
Li YC, Li YF, Chang SX, Yang YF, Fu SL, Jiang PK, Luo Y, Yang M, Chen ZH, Hu SD, Zhao MX, Liang X, Xu QF, Zhou GM, Zhou JZ (2018b) Biochar reduces soil heterotrophic respiration in a subtropical plantation through increasing soil organic carbon recalcitrancy and decreasing carbon-degrading microbial activity. Soil Biol Biochem 122:173–185
Liang J, Yang Z, Tang L, Zeng G, Yu M, Li X et al (2017) Changes in heavy metal mobility and availability from contaminated wetland soil remediated with combined biochar-compost. Chemosphere 181:281–288
Lin ZW, Li YF, Tang CX, Luo Y, Fu WJ, Cai XQ, Li YC, Yue T, Jiang PK, Hu SD, Chang SX (2018) Converting natural evergreen broadleaf forests to intensively managed moso bamboo plantations affects the pool size and stability of soil organic carbon and enzyme activities. Biol Fertil Soils 54:467–480
Liu D, Li TQ, Yang XE, Islam E, Jin XF, Mahmood Q (2008) Effect of Pb on leaf antioxidant enzyme activities and ultrastructure of the two ecotypes of Sedum alfredii Hance. Russ J Plant Physiol 55:68–76
Liu D, Chen J, Mahmood Q, Li S, Wu J, Ye Z et al (2014) Effect of Zn toxicity on root morphology, ultrastructure, and the ability to accumulate Zn in Moso bamboo (Phyllostachys pubescens). Environ Sci Pollut Res Int 21:13615–13624
Liu D, Li S, Islam E, Chen JR, Wu JS, Ye ZQ et al (2015) Lead accumulation and tolerance of Moso bamboo (Phyllostachys pubescens) seedlings: applications of phytoremediation. J Zhejiang Univ Sci B 16:123–130
Liu L, Li W, Song W, Guo M (2018) Remediation techniques for heavy metal-contaminated soils: principles and applicability. Sci Total Environ 633:206–219
Maroušek J, Hašková S, Zeman R, Žák J, Vaníčková R, Maroušková A et al (2015a) Techno-economic assessment of processing the cellulose casings waste. Clean Techn Environ Policy 17:2441–2446
Maroušek J, Maroušková A, Myšková K, Váchal J, Vochozka M, Žák J (2015b) Techno-economic assessment of collagen casings waste management. Int J Environ Sci Technol 12:3385–3390
Maroušek J, Kolář L, Vochozka M, Stehel V, Maroušková A (2017a) Novel method for cultivating beetroot reduces nitrate content. J Clean Prod 168:60–62
Maroušek J, Vochozka M, Plachý J, Žák J (2017b) Glory and misery of biochar. Clean Techn Environ Policy 19:311–317
Maroušek J, Kolář L, Vochozka M, Stehel V, Maroušková A (2018) Biochar reduces nitrate level in red beet. Environ Sci Pollut Res 4:1–4
Mcgowen SL, Basta NT, Brown GO (2001) Use of diammonium phosphate to reduce heavy metal solubility and transport in smelter-contaminated soil. J Environ Qual 30:493
Niazi NK, Singh B, Minasny B (2015) Mid-infrared spectroscopy and partial least-squares regression to estimate soil arsenic at a highly variable arsenic-contaminated site. Int J Environ Sci Technol 12:1–10
Rizwan M, Ali S, Zia MUR, Rinklebe J, Tsang D, Bashir A et al (2018) Cadmium phytoremediation potential of Brassica crop species: a review. Sci Total Environ 631-632:1175–1191
Seshadri B, Bolan NS, Choppala G, Kunhikrishnan A, Sanderson P, Wang H et al (2017) Potential value of phosphate compounds in enhancing immobilization and reducing bioavailability of mixed heavy metal contaminants in shooting range soil. Chemosphere 184:197–206
Shao Y, Chen JX, Li XM, Cui JM, Wang WP (2017) The remediation of growth and yield characteristics of wheat under Pb stress by four kinds of organic materials. Ecol Environ Sci 26:315–322 (in Chinese)
Shirvani M, Sherkat Z, Khalili B, Bakhtiary S (2015) Sorption of Pb(II) on palygorskite and sepiolite in the presence of amino acids: equilibria and kinetics. Geoderma 249-250:21–27
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
Thawornchaisit U, Polprasert C (2009) Evaluation of phosphate fertilizers for the stabilization of cadmium in highly contaminated soils. J Hazard Mater 165:1109–1113
Theodoratos P, Papassiopi N, Xenidis A (2002) Evaluation of monobasic calcium phosphate for the immobilization of heavy metals in contaminated soils from Lavrion. J Hazard Mater 94(2):135–146
Usman A, Kuzyakov Y, Stahr K (2005) Effect of clay minerals on immobilization of heavy metals and microbial activity in a sewage sludge-contaminated soil (8 pp). J Soils Sediments 5(4):245–252
Varela SA, Weigandt MN, Willems P, Bianchi E, Diez JP, Gyenge JE (2015) Physiological status of conifer seedlings treated with radiation, drought and frost stress mitigation techniques: a laboratory assessment. New For 47(1):1–17
Wahba MM, Labib BF, Darwish KM, Zaghloul MA (2016) Application of bentonite and zeolite to eliminate the hazards of cadmium, copper and nickel metals in contaminated soils. Clay Res 35(1):34–42
Wang ZL, Li YF, Chang SX, Zhang JJ, Jiang PK, Zhou GM, Shen ZM (2014) Contrasting effects of bamboo leaf and its biochar on soil CO2 efflux and labile organic carbon in an intensively managed Chinese chestnut plantation. Biol Fertil Soils 50:1109–1119
Wang H, Yuan X, Wu Y, Zeng G, Chen X, Leng L et al (2015a) Facile synthesis of amino-functionalized titanium metal-organic frameworks and their superior visible-light photocatalytic activity for Cr(VI) reduction. J Hazard Mater 286:187–194
Wang YH, Li MJ, Tang M, Ai SY, Yu DN (2015b) Effect of rice husk biochar on lettuce Cd uptake and soil fertility. Chin J Eco-Agric 23:207–214
Wang M, Zhu Y, Cheng L, Andserson B, Zhao X, Wang D et al (2018) Review on utilization of biochar for metal-contaminated soil and sediment remediation. J Environ Sci 63:156–173
Wu YJ, Zhou H, Zou ZJ, Zhu W, Yang WT, Peng PQ, Zeng M, Liao BH (2016) A three-year in-situ study on the persistence of a combined amendment (limestone+sepiolite) for remedying paddy soil polluted with heavy metals. Ecotoxicol Environ Saf 130:163–170
Xu X, Cao X, Zhao L, Zhou H, Luo Q (2014) Interaction of organic and inorganic fractions of biochar with Pb. RSC Adv 4:44930–44937
Xu Y, Liang X, Xu Y, Huang Q, Wang L, Sun Y (2017) Remediation of heavy metal-polluted agricultural soils using clay minerals: a review. Pedosphere 27:193–204
Yin D, Wang X, Chen C, Peng B, Tan C, Li H (2016) Varying effect of biochar on Cd, Pb and As mobility in a multi-metal contaminated paddy soil. Chemosphere 152:196–206
Zhang R, Zhang Y, Song L, Song X, Hänninen H, Wu J (2017) Biochar enhances nut quality of Torreya grandis and soil fertility under simulated nitrogen deposition. For Ecol Manag 391:321–329
Zhao S, Feng C, Wang D, Liu Y, Shen Z (2013) Salinity increases the mobility of Cd, Cu, Mn, and Pb in the sediments of Yangtze estuary: relative role of sediments’ properties and metal speciation. Chemosphere 91:977–984
Zhou D, Liu D, Gao F, Li M, Luo X (2017) Effects of biochar-derived sewage sludge on heavy metal adsorption and immobilization in soils. Int J Environ Res Public Health 14:681
Acknowledgements
The study was financially supported through a grant from the Natural Science Foundation of China (31670617), key research and development project of Science Technology Department of Zhejiang province (2015C03020-2) and key research and development project of Science Technology Department of Zhejiang province (2018C03028).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Wang, R., Shafi, M., Ma, J. et al. Effect of amendments on contaminated soil of multiple heavy metals and accumulation of heavy metals in plants. Environ Sci Pollut Res 25, 28695–28704 (2018). https://doi.org/10.1007/s11356-018-2918-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2918-x