Abstract
This work aimed to develop a reliable and fast approach to estimate the plant tolerance degree to heavy metal (HM) phytotoxicity. Two independent experiments were carried out using tomato accessions, with contrasting morphological features, that were grown in a hydroponic solution containing different CdCl2 concentrations for 7 days. Plant dry weight and chlorophyll content (SPAD units) were evaluated, and tolerance degree to Cd toxicity was estimated according to the tolerance index (TI), which is a new mathematical formula based on plant biomass proposed in this study. Although with different magnitudes, tomato exhibited reductions in their dry weight concurrently with the increasing CdCl2 concentration. By contrast, chlorophyll content presented no standard response, decreasing and even increasing according to CdCl2 concentrations, indicating that only under certain conditions (particularly, at CdCl2 50 μM), this parameter can be used to estimate plant tolerance to Cd toxicity. TI was efficiently able to segregate tomato cultivars with similar performance (based on the total dry weight of plants), and such segregation was optimized when the hydroponic solution contained from 25 to 50 μM CdCl2. Within this range, data pointed at 35 μM CdCl2 as the best concentration to be employed in studies related to the tomato tolerance/sensitivity to Cd toxicity. In conclusion, TI proved to be a reliable estimator of tolerance degree to Cd exposure in genetically distinct tomato accessions. Moreover, TI can be used for this same purpose in plants under other HM-induced stresses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Contamination of agricultural lands by heavy metals (HM) has increased since the first industrial revolution, and a scenario for reductions of their release in the environment is uncertain (Carvalho 2017). Due to anthropogenic activities, HM are ranked as the main contaminators of soils (Science Communication Unit 2013), from where plants quickly absorb them (Kabata-Pendias 2011; Pompeu et al. 2017; Borges et al. 2018). Nevertheless, it is desired that certain HM, such as iron (Fe), manganese (Mn), zinc (Zn), copper (Cu), molybdenum (Mo), and nickel (Ni), exhibit high acceptable levels in plant-origin products in order to avoid mineral deficiency-related diseases in humans and animals (Brkic et al. 2004; Kovacevic et al. 2004; Teklić et al. 2013; Khan et al. 2016; Hippler et al. 2018). For this purpose, HM input in soils is usually necessary because their concentrations in plant tissues are directly proportional to their concentrations in the growing media (Kabata-Pendias 2011; Marschner 2012; Nogueirol et al. 2016). However, certain HM such as Cd, mercury (Hg), lead (Pb), silver (Ag), arsenic (As), tin (Sn), bismuth (Bi), and chromium (Cr) are extremely toxic to biological systems due to their non-essentiality and accumulation in living organisms, with some of them exhibiting high solubility in physiological conditions (Weast 1984; Kabata-Pendias 2011), so potentializing their side effects. Therefore, plants must be tolerant enough to present normal development and yield when the growing media has relatively high concentrations (nutrients) or even low concentrations (non-essential elements) of HM.
In this context, the selection of species/sub-species/populations/genotypes/lines/cultivars/varieties/ecotypes with high tolerance to HM exposure is useful for the progress of biofortification and biotechnological programs (Teklić et al. 2013; Branco-Neves et al. 2017; Norton et al. 2017), as well as for recovery of HM-contaminated soils (Rizwan et al. 2017; Bernard et al. 2018; Souza et al. 2018). Among crops, tomato (Solanum lycopersicum L.) is one of the most worldwide cultivated and consumed vegetable nowadays, as well as the best model organism for fleshy-fruited plants to be used in research programs due to its sequenced and small genome, a large set of spontaneous and artificial mutants, a short life cycle, photoperiod insensitivity, and specific morphological traits that are not shared with other model plants (The Tomato Genome Consortium 2012; Bergougnoux 2014; Piotto et al. 2014). Furthermore, tomato can be grown in different conditions (from soils to hydroponics), and also be propagated asexually by grafting, or regenerated from distinct parts of the plant (Bergougnoux 2014; Gratão et al. 2015; Nogueirol et al. 2016; Alves et al. 2017).
Interestingly, tomato exhibits a diverse performance under HM exposure, for instance, using Cd as an example, varying according to metal concentration in the growing media, time length of exposure, chemical speciation, plant features (genotype, phenological stage, and target-organ or tissue), growing media properties, association with microorganisms, among others (Gratão et al. 2008; Kabata-Pendias 2011; Dourado et al. 2013; Méndez et al. 2016; Nogueirol et al. 2016; Branco-Neves et al. 2017; Pompeu et al. 2017; Borges et al. 2018; Carvalho et al. 2018a, b).
Although Cd concentration ranges from 0.01 to 0.8 mg kg−1 in natural areas, 1500 mg kg−1 has been detected in contaminated regions in the USA (Kabata-Pendias 2011). The major source of soil Cd is atmospheric deposition from metal smelters and phosphorous (P) fertilizers, and a substantial amount is released through mining, metal-based pesticides, industrial waste, and battery production (Kabata-Pendias 2011; Carvalho 2017). Once within the plant, Cd triggers several toxicity symptoms after short-term exposure. The visual damage consists of leaf necrosis and chlorosis, root brownish and growth reductions, symptoms that are generally preceded by Cd-induced oxidative stress, disturbances in the nutrient uptake and distribution, hormonal imbalance, and decreases in the photoassimilate production and exportation (Delpérée and Lutts 2008; Fidalgo et al. 2011; Dourado et al. 2013; Iannone et al. 2015; Cuypers et al. 2016; Bayçu et al. 2017; Souza et al. 2017; Carvalho et al. 2018a, b). In this context, researchers who aim to study the mechanisms related to plant tolerance to Cd toxicity (or to others HM) have to cope with, at least, two paramount questions: (i) What are the main factors to be used in the experimental settings in order to estimate plant tolerance degree?, and (ii) What plant features should be used to estimate it?
In the current work, plant developmental stage, time of exposure, and nutrient composition of the hydroponic solution were fixed. By contrast, different CdCl2 (Cd source) concentrations were added to the nutrient solution and tomato accessions with distinct performance to Cd toxicity were selected for the study of Cd tolerance. Data indicated that modifications in the biomass accumulation are a suitable parameter for the estimation of plant tolerance to Cd exposure; however, total plant biomass per se was not enough to segregate tomato cultivars with similar performance in more refined classes of tolerance degree. Such segregation was possible by using a tolerance index (TI), a modified biomass-based formula that is being proposed here. Our results suggested from 25 to 50 μM CdCl2 to be the best concentrations for the study of tolerance/sensitivity to Cd toxicity, because these concentrations allowed the best separation of cultivars according to TI. On the other hand, estimations based on the chlorophyll content should be used only in certain Cd concentrations, especially at 50 μM CdCl2 in which this parameter is correlated to plant dry weight and, consequently, to TI values.
Materials and methods
Plant material and growth conditions
In order to evaluate the robustness of the proposed formula for Cd tolerance estimation, two experiments were carried out. In the first assay, seeds of tomato cvs. Calabash Rouge (CR), Olirose de St Domingue (OD), Olena Ukrainien (OU), Pusa Ruby (PR), and Solymari (SO) were sown in polystyrene trays filled with thin exfoliated vermiculite. After seedling emergence, daily application of macro- and micronutrients (Peters Professional® Water Soluble Fertilizer 0.5 g L−1) was performed in order to maintain suitable seedling development. Fifteen-day-old seedlings were removed from the trays and their roots were washed and then transferred to dark plastic trays (550 × 350 × 120 mm) containing Hoagland and Arnon (1950) nutritive solution at 10% of ionic strength at pH 6.0. Seedlings were fixed in styrofoam plates using foam pieces, being spaced from each other by 8 cm (each tray contained 24 seedlings).
As an adaptation period, plants were grown in hydroponics for 8 days before the beginning of Cd exposure in order to mitigate the stress generated by seedling transplantation. During the adaptation period, nutrient concentration was gradually increased from 10 to 50% ionic strength (i.e., increments of 10% each 2 days) to decrease the stress associated to high salt concentrations in solution. After 8 days of plant adaptation, solutions were replaced by new solution with the same composition (50% of ionic strength at pH 6.0), and Cd was added to the hydroponic system at 0, 5, 10, 15, 20, 25, 50, and 100 μM CdCl2. Seedlings were grown under control (Cd-free) and Cd-containing hydroponic solutions for 7 days. During the experiment, distilled-deionized water was added to the trays daily, in order to replace water lost through evapotranspiration. The homogeneous distribution of nutrient solution and suitable oxygenation level was maintained by an air pump system. During the experiment, tomato plants grew under global solar energy 24.9 ± 6.4 MJ m−2 day−1, temperature 22.5 ± 2.3 °C, and relative humidity of 77.4 ± 10.2%.
A second trial with 35 distinct tomato accessions (S. lycopersicum and S. pimpinellifolium) was carried out using as a reference the information obtained in the first experiment described above. In this second experiment, plants were grown in hydroponic solution containing 0 or 35 μM CdCl2 for 7 days. All the procedures concerning seed sowing, seedling cultivation, and transplantation to the hydroponics as well as plant adaptation period were the same as described above. During the second experiment, tomato plants grew under global solar energy 20.6 ± 8.1 MJ m−2 day−1, temperature 22.0 ± 2.9 °C, and relative humidity of 79.6 ± 14.1%. Both experiments were conducted in a greenhouse.
Chlorophyll content
On the 7th day of plant exposure to Cd, chlorophyll content was indirectly evaluated in tomato leaves by using a soil plant analysis development (SPAD) chlorophyll meter (Konica Minolta, SPAD-502 model) through measurements in the lateral border of central leaflet from the third completely expanded leaf (Gratão et al. 2012).
Plant biomass
Roots, stems, and leaves were collected just before the CdCl2 addition to the hydroponics (six plants), and also after 7 days of plant exposure to this metal. Subsequently, samples were dried at 55 °C for 72 h for determination of plant biomass (dry weight—DW).
Tolerance index
A formula for the determination of plant tolerance to Cd toxicity is proposed here:
Where:
- TI i :
-
Tolerance index of the plant i under Cd exposure;
- \( {Dw}_{s_i} \) :
-
Total dry weight of the plant i under Cd exposure;
- \( {\overline{Dw}}_{ns_i}=\frac{\sum_{i=1}^n{Dw}_{ns_i}}{n} \) :
-
Average dry weight of control plants i;
- \( C=\frac{\sum_{i=1}^n{Dw}_i}{n} \) :
-
Correction factor, expressed as the average dry weight of plants before the onset of Cd exposure.
The tolerance index (TI) is based on the net biomass accumulated in Cd-stressed plants (\( {Dw}_{s_i} \)) when compared to the control plants (\( {\overline{Dw}}_{ns_i} \)), which are used as reference for the potentially maximum biomass accumulation. It also contains a correction factor (C) that reduces differences inherent to each genotype, such as the initial biomass of plants (i.e., before the beginning of stress).
Cadmium speciation in hydroponics
In the first experiment, the influence of CdCl2 concentrations on Cd speciation in hydroponic solution was determined by the Visual MINTEQ® software (Visual MINTEQ ver 3.0 n.d.). Such estimation was performed because Cd accumulation in plant tissues depends on Cd availability, which is correlated to the Cd speciation and growth media properties (Kabata-Pendias 2011).
Experimental design and statistical analysis
Seedlings of different tomato cultivars were arranged according to the complete randomized blocks into the trays. In the first assay, six replicates with one plant per plot were used for determination of SPAD index and plant biomass before Cd exposure. For the plant dry weight (biomass), three replicates with two plants per plot were used. The one-way analysis of variance—ANOVA (P < 0.05)—was performed, and least significant difference between two means (LSD) was obtained at the P < 0.05.
Before ANOVA, data were subjected to tests in order to check the assumptions for the ANOVA performance (i.e., normal distribution, variance homogeneity, and error independence). Regression analyses were also carried out (P < 0.05), and the best models were selected using Akaike’s information criterion (Akaike 1974). Moreover, Pearson’s correlation analysis (P < 0.05) was employed to evaluate the association among variables, and confidence intervals (IC95%) were estimated using \( IC=\overline{x}\pm t\frac{s}{\sqrt{n}} \). All data analyses were performed by employing R 3.1.0 software (R Development Core Team 2014).
Results
Biomass accumulation in plants before Cd exposure
Tomato cultivars exhibited different plant biomasses before the beginning of Cd exposure (Table 1). Tomato cv. Calabash Rouge (CR) presented the highest shoot and root dry weights when compared to the others cultivars, and contained 32, 17, 15, and 16% more biomass than Olirose de St Domingue (OD), Olena Ukrainien (OU), Pusa Ruby (PR), and Solymari (SO), respectively (Table 1).
Cadmium side effects on tomato development
Reductions in root, stem, and leaf growth were detected in tomato seedlings after Cd exposure for 7 days, when compared to the control plants (Figs. 1a and 2a). The higher CdCl2 concentration in the hydroponic solutions was, the higher were the Cd-induced impacts on tomato development (Figs. 1a and 2a). Chlorosis was observed only in young leaves, while brown spots along leaf veins were detected in matures leaves of all cultivars under exposure to 100 μM CdCl2 (Fig. 1b).
Cd-induced effects on 30-day-old seedlings of tomato (Solanum lycopersicum cv. Calabash Rouge) after exposure to 0, 5, 10, 15, 20, 25, 50, and 100 μM CdCl2 for 7 days (a). Leaf chlorosis in 30-day-old seedlings of tomato (S. lycopersicum cv. Olena Ukrainien) after exposure to 100 μM CdCl2 for 7 days (b)
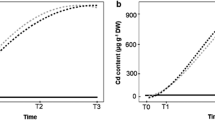
Plant biomass (a—total dry weight) and chlorophyll content (b—SPAD index) of 30-day-old tomato cultivars (Solanum lycopersicum) that were grown in hydroponics with different CdCl2 concentrations for 7 days. Single asterisk indicates confidence interval (IC95%) for CdCl2 concentrations that decreased by 50% of the plant biomass of these tomato cultivars. Double asterisks indicate confidence interval (IC95%) for CdCl2 concentrations that inhibit the plant growth. Triple asterisks indicate confidence interval (IC95%) for CdCl2 concentrations that cause the highest reductions in the chlorophyll content in tomato leaves. The equations of the curves for each cultivar were presented in Tables 2 and 3
Based on the biomass accumulation in different CdCl2 concentrations, variations in the plant sensitivity to Cd toxicity were detected (Fig. 1a), and such variations were amplified at intermediary Cd concentrations—from 25 to 50 μM CdCl2 (Fig. 2a). The equations of cultivar-dependent curves can be observed in Table 2. When all tomato cultivars were considered (Table 2), the average Cd concentration that caused reductions by 50% on plant biomass (growth reduction—GR50) was estimated as 37 μM CdCl2 with IC95% that ranged from 21 to 53 μM CdCl2 (Fig. 2a, single asterisk). Additionally, the average lethal Cd concentration (GR100) was calculated as 119.30 μM CdCl2 with IC95% ranging from 113.16 to 125.44 μM CdCl2 (Fig. 2a, double asterisks).
SPAD values exhibited no standard response, decreasing and even increasing with the increasing in CdCl2 concentration in the growing media (Fig. 2b). Based on the equations from Table 3, the average concentration that could trigger the highest reduction in the chlorophyll content (CLmax) was 26.35 μM CdCl2 with IC95% that ranged from 21.26 to 31.44 μM CdCl2 (Fig. 2b, triple asterisks). Changes in the plant biomass were generally not correlated to modifications in the SPAD index (Table 4). However, this correlation was near to significant (P = 0.0513) at 50 μM CdCl2 (r = 0.88, Table 4).
Cadmium speciation
Chemical speciation analysis showed that more than 80% of CdCl2 added to the hydroponics was presented as free ions, Cd+2, regardless CdCl2 concentration (Table 5). In addition, the second and third most abundant species were, on average, CdSO4 (9.3%) and CdHPO4 (5.3%) (Table 5).
Tolerance index as an estimator of tomato tolerance degree to Cd toxicity
In general, TI was able to rank tomato cultivars according to their ability for biomass accumulation in Cd-containing solution when compared to the control condition, allowing the identification of trends associated to plant tolerance/sensitivity to Cd toxicity (Fig. 3). However, certain tomato cultivars such as Pusa Ruby and Olena Ukrainien presented considerable variations in their TI values, especially when CdCl2 concentrations were lower than 25 μM (Fig. 2).
Tolerance index (TI) estimation under Cd-stress after exposure to 0, 5, 10, 15, 20, 25, 50, and 100 μM CdCl2 for 7 days. Single asterisk indicates confidence interval (IC95%) for CdCl2 concentrations that inhibit the plant growth. Double asterisks indicate intersection between CdCl2 concentrations containing higher TI stability (provides great TI rank stability among cultivars)
The intersection between CdCl2 concentrations containing higher TI stability (Fig. 3, single asterisk) with the confidence interval that was associated to reductions by 50% in plant growth (GR50, Fig. 3, double asterisks) revealed 25 to 50 μM CdCl2 as the best concentrations for segregation of tomato cultivars according to their tolerance degree after Cd exposure for 7 days. Accordingly, when plants under exposure to 50 μM CdCl2 were evaluated, TI was able to segregate tomato cultivars with similar performance, when based on their total dry weight (Fig. 3).
Among the range of CdCl2 concentrations that were considered the most suitable for determination of plant tolerance, 35 μM CdCl2 was estimated as the concentration that can amplify, at maximum, the separation of tomato cultivars according their TI. Since this concentration was not included in the primary experimental settings, and the initial set of tomato cultivars was relatively small (5 cultivars), an extra trial with 35 distinct tomato accessions (S. lycopersicum and S. pimpinellifolium) was run in order to check TI efficiency as an estimator of tomato tolerance degree to Cd toxicity. After 7 days of plant exposure to 35 μM CdCl2, accession-dependent variations in the total plant biomass were observed, and TI ranged from negative to positive values (Table 6).
Discussion
Tomato tolerance to Cd toxicity depends on genotype-specific mechanisms, which are not always associated with the lowest Cd concentration in plant tissues
Cadmium is one of the most toxic metals to biological systems because it is a non-essential element with high water solubility, maintaining this feature under physiological conditions (Weast 1984; Singh et al. 2016). The direct consequence is its great potential to react with biological molecules and to impair their functions, a phenomenon that can be enhanced due to the chemical similarities shared by Cd and some nutrients (Dong et al. 2006; Cherif et al. 2012; Marschner 2012; Sebastian and Prasad 2016). In soils, most of the Cd (55 to 90%) is presented as a free metal ion that is readily available to plants (Kabata-Pendias 2011; Melo et al. 2011; Nogueirol et al. 2016). Similarly, Cd2+ ions were the most common Cd-species (> 80%) in hydroponics, regardless of the CdCl2 concentration in the nutritive solution (Table 5). From the growth media, Cd is passively and actively absorbed by plants, in which this metal frequently damages leaf tissues (Gratão et al. 2008), impairs photosynthetic activity (Gratão et al. 2015), and/or inhibits photoassimilate exportation (Delpérée and Lutts 2008), so preventing growth of Cd-stressed plants (Alves et al. 2017; Borges et al. 2018). Consistently, all tomato cultivars exhibited chlorosis and necrotic black spots in leaves (Fig. 1a, b), and reductions in their biomass after 7 days of Cd exposure, especially at highest CdCl2 concentrations in the hydroponic solution (Fig. 2a).
Since Cd toxicity is frequently associated with its concentration in plant tissues (Zheng et al. 2018), it is tempting to suppose that variations in Cd absorption, translocation, and accumulation provide a differential tomato sensitivity to Cd toxicity. However, low Cd accumulation was not enough to avoid major reductions in TI of tomato cv. Olena Ukrainien in comparison to the other cultivars tested (Fig. 3), indicating that extra events may influence tomato sensitivity to Cd exposure in addition to the own Cd accumulation, such as magnitude of oxidative stress, mineral profile misbalances, and hormonal disequilibrium (Souza et al. 2018). For instance, Borges et al. (2018) showed that differential management of the antioxidant machinery was associated to improvements in tomato performance under short Cd exposure. Tolerance degree can also be related to differential regulation of nutrient status to counteract Cd toxicity, which frequently triggers iron (Fe) and zinc (Zn) deficiencies most likely due to the Cd ability to use the same transporters of these micronutrients (Bækgaard et al. 2010; Chou et al. 2011; Hermans et al. 2011; Kudo et al. 2015; Gao et al. 2016). A recent study showed, however, that reductions in Fe status in leaves can take part of a protective mechanism against Cd toxicity by enhancing proline production in Cd-treated plants (Sharmila et al. 2017), so modifications in nutrient status can be a protective mechanism actively modulated by stressed plants rather than a merely Cd-induced side effect. Anyways, the main focus of this work was the development of a reliable estimator of tolerance degree to Cd exposure, rather than to investigate tolerance mechanisms per se.
Tolerance index: a biomass-based formula modified to estimate the sensitivity degree to metal toxicity in distinct plant genotypes
The results obtained in this study indicated that the magnitude of Cd-induced effects on plant biomass was strongly dependent on tomato accessions (Table 6). If tolerance was only based on the final biomass of Cd-stressed plants, USP0037, USP0002, and USP0026A (624.83, 601.67, 586.67 mg plant−1 DW, respectively) might be classified as the most tolerant accessions, while USP0078, USP0075, and USP0079 (23.83, 29.33, and 43.67 mg plant−1 DW, respectively) as the most sensitive plants (Table 6). In a way, this classification is not so wrong because these accessions were placed into the group of plants with “superior” and “inferior” tolerance degrees, respectively, when based on TI values. The segregation of plants with divergent performance is not a problem; however, the classification of accessions with similar plant biomass after Cd exposure is a very hard task. In this context, TI can be a valuable tool for ranking accessions in more refined tolerance classes. For instance, USP0084, USP0082, and USP0036 exhibited similar biomass in Cd-stressed plants (366.00, 360.00, and 377.00 mg plant−1 DW, respectively), but different TI values (1.10, 0.56, and 0.23, respectively—Table 6). This means that, although these accessions were able to accumulate biomass under Cd exposure (TI with positive values—compare “initial biomass” vs “Cd biomass” at Table 6), such plant’s ability was differently affected by Cd-induced stress (compared “Control biomass” vs “Cd biomass” at Table 6).
Accordingly, USP0084 was able to maintain a similar rate of biomass accumulation between Cd-stressed and control plants (TI~1), but USP0082 and USP0036 presented a decreased biomass accumulation in plants under Cd exposure, when compared to the plants that were grown in optimal, Cd-free hydroponic solution (Table 6). Although TI is based on the net biomass production in Cd-stressed in comparison to control plants, its mathematical formula takes into account differences in the initial plant biomass (i.e., before the stress onset) by using a correction factor (C). From the practical point of view, this means that different genotypes (i.e., with intrinsically distinct capacity for biomass accumulation) can be comparable to each other. Furthermore, the use of C in the formula provides robustness to the classification of tolerance degree based on TI because it standardizes the Cd-induced effects according to genotype. In this way, tomato accessions with different biomass losses can present similar tolerance degree, as observed in USP0001 and USP0042 that exhibited TI = 0.31, despite Cd-induced reductions in their biomass by 12.44% and 23.17%, respectively. In addition, TI can measure when a stressor is able to enhance the plant performance (TI > 1), a phenomenon frequently observed in “mild stress”. This index can also exhibit negative values (TI < 0), which indicate that some genotypes are so sensitive to Cd toxicity that a short-term exposure can trigger organ/tissue losses (e.g., abscission of cotyledons and leaves), hence decreasing plant dry weight to levels lower than their biomass before the onset of Cd-induced stress (Table 6).
Robust result as advantageous feature of TI and its related approaches
TI robustness was supported by subsequent studies carried out with the contrasting tomato cultivars, Yoshimatsu (tolerant—TI = 0.91) and Tropic Two Orders (sensitive—TI = 0.07), that were grown from the seedling stage to fruit production in soil containing 3.77 mg kg−1 of available Cd (~ 33.5 μM CdCl2) (Carvalho et al. 2018a, b). Although both genotypes were able to acclimate to Cd exposure, a very distinct plant performance was detected: the tolerant cultivar was able to produce bigger and heavier fruits, and also seeds with increased germination in comparison to control plants, while the sensitive cultivar presented no modifications in fruit parameters and seed vigor after Cd exposure (Carvalho et al. 2018a). In another work, in which tomato accessions were grown in hydroponic solution containing 35 μM CdCl2 for 7 days, the maintenance of the classification of Calabash Rouge as a sensitive genotype when compared to Pusa Ruby (Borges et al. 2018) indicates that TI-related approaches generate reproducible results. Summarizing, these methods yielded data that (i) can be comparable to each other, even from independent studies, and (ii) can select plants with distinct strategies to cope with Cd toxicity. Due to its features, TI can be also be used for estimation of tolerance degree of plants under exposure to other stressors, but trials should be carry out to select the best parameter for such estimation, since Cd uptake depends on both environmental and hereditary factors (Kovacevic and Vragolovic 2011). These parameters must be an ordinarily and rapidly detected side effect from the stressor, and such trials should consider a representative set of accessions from a given species, as well as plant age, time length of exposure, and stress levels.
In the present study, for instance, chlorophyll content (SPAD index) was initially selected as one of the parameters for estimation of tomato tolerance/sensitivity to Cd toxicity. Although the chlorophyll content could be used to indirectly estimate the tolerance degree of 30-day-old tomato seedlings under exposure to 50 μM CdCl2 for 7 days (Table 4), the use of SPAD index for this purpose should be meticulously weighted because it exhibited large variations depending on CdCl2 concentrations (Fig. 1b). Differently, changes in plant biomass exhibited a trend: as higher the Cd concentration in the growing media, the lower the plant biomass (Fig. 1a, b). In conclusion, the estimation of tomato tolerance to Cd exposure can be efficiently performed by using TI, an improved mathematical formula based on plant biomass that proved to be reliable to calculate plant sensitivity degree to metal toxicity by using a short-term exposure method. In the current work, plant developmental stage, time of exposure, and nutrient composition of the hydroponic solution were fixed. By contrast, tomato accessions with distinct performance to Cd toxicity and different CdCl2 (Cd source) concentrations in the nutrient solution were used for evaluation of Cd tolerance. The results indicated 25 to 50 μM CdCl2 as the best concentrations to be used in studies of tolerance/sensitivity to Cd toxicity in tomato seedlings; within this range, 35 μM CdCl2 provided the most enhanced segregation of cultivars according to TI values.
References
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19:716–723
Alves LA, Monteiro CC, Carvalho RF, Ribeiro PC, Tezotto T, Azevedo RA, Gratão PL (2017) Cadmium stress related to root-to-shoot communication depends on ethylene and auxin in tomato plants. Environ Exp Bot 134:102–115
Bækgaard L, Mikkelsen MD, Sørensen DM, Hegelund JN, Persson DP, Mills RF, Yang Z, Husted S, Andersen JP, Buch-Pedersen MJ, Schjoerring JK, Williams LE, Palmgren MG (2010) A combined zinc/cadmium sensor and zinc/cadmium export regulator in a heavy metal pump. J Biol Chem 285:31243–31252
Bayçu G, Rognes SE, Özden H, Gören-Saglam N, Csatári I, Szabó S (2017) Abiotic stress effects on the antioxidative response profile of Albizia julibrissin Durazz. (Fabaceae). Braz J Bot 40:21–32
Bergougnoux V (2014) The history of tomato: from domestication to biopharming. Biotechnol Adv 32:170–189
Bernard F, Dumez S, Lemière S, Platel A, Nesslany F, Deram A, Vandenbulcke F, Cuny D (2018) Impact of cadmium on forage kale (Brassica oleracea var. viridis cv “Prover”) after 3-, 10- and 56-day exposure to a Cd-spiked field soil. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-018-1636-8
Borges KLR, Salvato F, Alcântara BK, Nalin RS, Piotto FA, Azevedo RA (2018) Temporal dynamic responses of roots in contrasting tomatogenotypes to cadmium tolerance. Ecotoxicology 27:245–258
Branco-Neves S, Soares C, Sousa A, Martins V, Azenha M, Gerós H, Fidalgo F (2017) An efficient antioxidant system and heavy metal exclusion from leaves make Solanum cheesmaniae more tolerant to Cu than its cultivated counterpart. Food Energy Secur 6:123–133
Brkic I, Simic D, Zdunic Z, Jambrovic A, Ledencan T, Kovacevic V, Kadar I (2004) Genotypic variability of micronutrient element concentrations in maize kernels. Cereal Res Commun 32:107–112
Carvalho FP (2017) Mining industry and sustainable development: time for change. Food Energy Secur 6:61–77
Carvalho MEA, Piotto FA, Nogueira ML, Gomes-Junior FG, Chamma HMCP, Pizzaia D, Azevedo RA (2018a) Cadmium exposure triggers genotype-dependent changes in seed vigor and germination of tomato offspring. Protoplasma 255:989–999. https://doi.org/10.1007/s00709-018-1210-8
Carvalho MEA, Piotto FA, Gaziola SA, Jacomino AP, Jozefczak M, Cuypers A, Azevedo RA (2018b) New insights about cadmium impacts on tomato: plant acclimation, nutritional changes, fruit quality and yield. Food Energy Secur 7:e00131. https://doi.org/10.1002/fes3.131
Cherif J, Derbel N, Nakkach M, Bergmann H, Jemal F, Lakhdar ZB (2012) Spectroscopic studies of photosynthetic responses of tomato plants to the interaction of zinc and cadmium toxicity. J Photochem Photobiol B 111:9–16
Chou T-S, Chao Y-Y, Huang W-D, Hong C-Y, Kao C-H (2011) Effect of magnesium deficiency on antioxidant status and cadmium toxicity in rice seedlings. J Plant Physiol 168:1021–1030
Cuypers A, Hendrix S, Reis RA, Smet S, Deckers J, Gielen H, Jozefczak M, Loix C, Vercampt H, Vangronsveld J, Keunen E (2016) Hydrogen peroxide, signaling in disguise during metal phytotoxicity. Front Plant Sci 7:470
Delpérée C, Lutts S (2008) Growth inhibition occurs independently of cell mortality in tomato (Solanum lycopersicum) exposed to high cadmium concentrations. J Integr Plant Biol 50:300–310
Dong J, Wu F, Zhang G (2006) Influence of cadmium on antioxidant capacity and four microelement concentrations in tomato seedlings (Lycopersicon esculentum). Chemosphere 64:1659–1666
Dourado MN, Martins PF, Quecine MC, Piotto FA, Souza LA, Franco MR, Tezotto T, Azevedo RA (2013) Burkholderia sp. SCMS54 reduces cadmium toxicity and promotes growth in tomato. Ann Appl Biol 163:494–507
Fidalgo F, Freitas R, Ferreira R, Pessoa AM, Teixeira J (2011) Solanum nigrum L. antioxidant defence system isozymes are regulated transcriptionally and posttranslationally in Cd-induced stress. Environ Exp Bot 72:312–319
Gao L, Chang J, Chen R, Li H, Lu H, Tao L, Xiong J (2016) Comparison on cellular mechanisms of iron and cadmium accumulation in rice: prospects for cultivating Fe-rich but Cd-free rice. Rice 9:39
Gratão PL, Monteiro CC, Antunes AM, Peres LEP, Azevedo RA (2008) Acquired tolerance of tomato (Lycopersicon esculentum cv. Micro-Tom) plants to cadmium-induced stress. Ann Appl Biol 153:321–333
Gratão PL, Monteiro CC, Tezotto T, Carvalho RF, Alves LR, Peters LP, Azevedo RA (2015) Cadmium stress antioxidant responses and root-to-shoot communication in grafted tomato plants. Biometals 28:803–816
Gratão PL, Monteiro CC, Carvalho RF, Tezotto T, Piotto FA, Peres LEP, Azevedo RA (2012) Biochemical dissection of diageotropica and Never ripe tomato mutants to Cd-stressful conditions. Plant Physiol Biochem 56:79–96
Hermans C, Chen J, Coppens F, Inzé D, Verbruggen N (2011) Low magnesium status in plants enhances tolerance to cadmium exposure. New Phytol 192:428–436
Hippler FWR, Petená G, Boaretto RM, Quaggio JA, Azevedo RA, Mattos-Jr D (2018) Mechanisms of copper stress alleviation in Citrus trees after metal uptake by leaves or roots. Environ Sci Pollut Res 25:13134–13146
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. California Agriculture Station, Berkeley
Iannone MF, Groppa MD, Benavides MP (2015) Cadmium induces different biochemical responses in wild type and catalase-deficient-tobacco plants. Environ Exp Bot 109:20–211
Kabata-Pendias A (2011) Cadmium. In: Kabata-Pendias A (ed) Trace elements in soils and plants. CRC Press, Boca Raton, pp 287–304
Khan A, Khan S, Alam M, Khan MA, Aamir M, Qamar Z, Rehman ZU, Perveen S (2016) Toxic metal interactions affect the bioaccumulation and dietary intake of macro- and micro-nutrients. Chemosphere 146:121–128
Kovacevic V, Vragolovic A (2011) Genotype and environmental effects on cadmium concentration in maize. J Life Sci 5:926–932
Kovacevic V, Antunovic M, Bukvic G, Rastija M, Kadar I (2004) Soil and genotype influences on heavy metals status in maize. Ekol Bratislava 23:65–60
Kudo H, Kudo K, Uemura M, Kawai S (2015) Magnesium inhibits cadmium translocation from roots to shoots, rather than the uptake from roots, in barley. Botany 93:345–351
Marschner P (2012) Marschner’s mineral nutrition of higher plants. Academic Press, San Diego
Melo LCA, Alleoni LRF, Carvalho G, Azevedo RA (2011) Cadmium and barium toxicity effects on growth and antioxidant capacity of soybean (Glycine max L.) plants, grown in two soil types with different physicochemical properties. J Plant Nutr Soil Sci 174:847–859
Méndez AAE, Pena LB, Benavides MP, Gallego SM (2016) Priming with NO controls redox state and prevents cadmium-induced general up-regulation of methionine sulfoxide reductase gene family in Arabidopsis. Biochimie 131:128–136
Nogueirol RC, Monteiro FA, Gratão PL, Silva BKA, Azevedo RA (2016) Cadmium application in tomato: nutritional imbalance and oxidative stress. Water Air Soil Pollut 227:210
Norton GJ, Travis AJ, Danku JMC, Salt DE, Hossain M, Islam MR, Price AH (2017) Biomass and elemental concentrations of 22 rice cultivars grown under alternate wetting and drying conditions at three field sites in Bangladesh. Food Energy Secur 6:98–112
Piotto FA, Tulmann Neto A, Franco MR, Boaretto LF, Azevedo RA (2014) Rapid screening for selection heavy metals tolerant plants. Crop Breed Appl Biotechnol 14:1–7
Pompeu GB, Vilhena MB, Gratão PL, Carvalho RF, Rossi ML, Martinelli AP, Azevedo RA (2017) Abscisic acid-deficient sit tomato mutant responses to cadmium-induced stress. Protoplasma 254:771–783
R Development Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rizwan M, Ali S, Qayyum MF, Ok YS, Zia-Ur-Rehman M, Abbas Z, Hannan F (2017) Use of maize (Zea mays L.) for phytomanagement of Cd-contaminated soils: a critical review. Environ Geochem Health 39:259–277
Science Communication Unit (2013) Science for environment policy in-depth report: soil contamination: impacts on human health. Report produced for the European Commission DG Environment, 2013. Retrieved from http://ec.europa.eu/science-environment-policy
Sebastian A, Prasad MNV (2016) Modulatory role of mineral nutrients on cadmium accumulation and stress tolerance in Oryza sativa L. seedlings. Environ Sci Pollut Res 23:1224–1233
Sharmila P, Kumari PK, Singh K, Prasad NVSRK, Pardha-Saradhi P (2017) Cadmium toxicity-induced proline accumulation is coupled to iron depletion. Protoplasma 254:763–770
Singh S, Parihar P, Singh R, Singh VP, Prasad SM (2016) Heavy metal tolerance in plants: role of transcriptomics, proteomics, metabolomics, and ionomics. Front Plant Sci 6:1143
Souza LA, Monteiro CC, Carvalho RF, Gratão PL, Azevedo RA (2017) Dealing with abiotic stresses: an integrative view of how phytohormones control abiotic stress-induced oxidative stress. Theor Exp Plant Physiol 29:109–127
Souza LA, Camargos LS, Carvalho MEA (2018) Toxic metal phytoremediation using high biomass non-hyperaccumulator crops: new possibilities for bioenergy resources. In: Matichenkov V (ed) Phytoremediation: methods, management, assessment. Nova Science, New York, pp 1–25
Teklić T, Loncaric Z, Kovacevic V, Singh BR (2013) Metallic trace elements in cereal grain – a review: how much metal do we eat? Food Energy Secur 2:81–95
The Tomato Genome Consortium (2012) The tomato genome sequence provides insights into fleshy fruit evolution. Nature 485:635–641
Visual MINTEQ ver 3.0 (n.d.) Disponible at: www2.lwr.kth.se/English/OurSoftware/vminteq
Weast RC (1984) Handbook of chemistry and physics, 64th edn. CRC Press, Boca Raton
Zheng J, Gu XQ, Zhang TJ, Liu HH, Ou QJ, Peng CL (2018) Phytotoxic effects of Cu, Cd and Zn on the seagrass Thalassia hemprichii and metal accumulation in plants growing in Xincun Bay, Hainan, China. Ecotoxicology 27:517–526. https://doi.org/10.1007/s10646-018-1924-6
Acknowledgements
We are grateful to Mr. Etienne Vernet (Kokopelli Seeds Foundation) for providing the tomato seeds.
Funding
This work was funded by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP – grant no. 2009/54676-0; scholarships: 2012/03861-4, 2013/15217-5, and 2015/26640-1, 2016/05788-3) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico for the research fellowship to R.A.A. (CNPq no. 303749/2016-4).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Piotto, F.A., Carvalho, M.E.A., Souza, L.A. et al. Estimating tomato tolerance to heavy metal toxicity: cadmium as study case. Environ Sci Pollut Res 25, 27535–27544 (2018). https://doi.org/10.1007/s11356-018-2778-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2778-4