Abstract
Modified Hummer’s method has been used in this study to synthesize graphene oxide (GO) solution that was utilized for the fabrication of three-dimensional (3D) graphene sponges and their subsequent functionalization through a low-cost and facile vapor-based surface enhancement approach. The functionalized 3D-graphene sponge is an excellent absorbent, which can remove more than 3300 wt.% of crude oil (calculated with respect to the original sorbent mass). The functionalization of the obtained graphene sponges with trichloro (1H,1H,2H,2H-perfluorooctyl)silane enhanced their wettability properties due to the super-hydrophobic nature of the resulting materials characterized by the contact angles in water greater than 150°. Furthermore, their elastic compression modulus (estimated by conducting a series of compression tests) was about 22.3 kPa. The equilibrium modeling of the oil removal process, which was performed by plotting Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich isotherms, confirmed the properties of the fabricated 3D graphene sponges as exceptional absorbents for crude and diesel oil, which could be attributed to the oleophilic nature of graphene. Moreover, the obtained 3D graphene sponges could be regenerated via heat treatment, which was conducted to release the adsorbed species. After five adsorption-desorption cycles, the sorption capacity of the produced 3D graphene sponges towards crude oil reached 95% of the initial value.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the discovery of graphene, its potential use in various applications has been considered, owing to its excellent electrical (Chen et al. 2012; Gómez-Navarro et al. 2007; Wei et al. 2009), mechanical (Frank et al. 2007; Lee et al. 2008; Park et al. 2008; Xia et al. 2015), and optical properties (Geim 2009). After the mechanical exfoliation of graphite with cellotape performed by Andre Geim and Kostya Novoselov (Novoselov 2011), different fabrication methodologies such as crystal epitaxial growth (Vlassiouk et al. 2011; Xiao et al. 2013; Yang et al. 2013), chemical vapor deposition (Chae et al. 2009; Chen et al. 2011; Li et al. 2010), and reduction of graphene oxide (Xu et al. 2010) were proposed. Among these techniques, the reduction of graphene oxide to graphene represents the most economically feasible method, especially for large-scale production (Stankovich et al. 2007). The related procedure typically involves the oxidation of graphite powder or expandable graphite flakes via Hummer’s method (Chae et al. 2009; Marcano et al. 2010) using strong oxidants such as H2SO4 and KMnO4. The resulting graphene oxide (GO) is easily dispersible in solvents.
Various techniques for the synthesis of graphene aerogels have been proposed in the literature. For example, Chi et al. (2015) used a fishing process utilizing polystyrene as a sacrificial template during the GO self-assembly. The obtained samples exhibited low density and good thermal stability. It maintained a high adsorption capacity (93% of the initial uptake) after eight toluene-adsorbing and drying recycles. Hengchang Bi and co-workers (Bi et al. 2012) were able to synthesize spongy graphene aerogels through the hydrothermal treatment of a colloidal dispersion of GO without further surface modification. The produced samples demonstrated relatively high sorption capacities toward petroleum products and fats as well as toxic organic solvents such as toluene and chloroform. The sponge was recycled for 10 times by the heat treatment with a yield of more than 99%.Yu and co-workers (Cong et al. 2012) reported the one-step fabrication of graphene/iron oxide hydrogels for the removal of heavy oils and metal ions with excellent adsorption capacities 92%, which were achieved via the introduction of ferrous ions during the synthesis procedure. The graphene/R-FeOOH aerogel was recycled for eight times. The absorbed oil was removed by heating graphene/R-FeOOH aerogel up to 100 °C. Furthermore, Li et al. (2014) used winter melon as the only raw material to convert it into carbon aerogel characterized by high adsorption capacities toward various oils and contact angles with water of around 135°. They showed that the produced winter melon carbon aerogel exhibited relatively low density and excellent adsorption properties. The winter melon carbon aerogel found to have an absorption capacity for organic solvents and oils of 16–50 times its own weight. Various studies on the integration of graphene sheets into well interconnected macrostructures through different strategies have been conducted, and the adsorption properties of the obtained materials were investigated (Bi et al. 2012; Li et al. 2014). However, the synthesis of three-dimensional (3D) graphene sponges with modified surfaces for the efficient removal of oil and organic solvents have not been examined in sufficient detail. Using the results of the previously conducted studies on the fabrication of 3D graphene aerogel, it is possible to prepare graphene sponges from GO solutions with different concentrations and then compare their oil adsorption characteristics as well as mechanical and thermal stabilities.
Although the oil uptake process has been extensively investigated in the literature, its exact mechanism (involving either an adsorption or absorption process) requires additional clarification. For several 3D structures (such as carbon-based aerogels (Chi et al. 2015; Hashim et al. 2012; Li et al. 2014; Liang et al. 2012) and carbon fibers (Suni et al. 2004)), an absorption process was observed, whereas some carbon fiber aerogels (Yang et al. 2015; Yang et al. 2014) underwent the adsorption process. Adsorption occurs when molecules of a gas, liquid, or solute adhere to the outer or internal surface of a material. Moreover, the criteria for the adsorption process and its relation to the graphene sponge sorption capacity specified in the literature are determined by the presence of van der Waals forces, H-bonding, π − π interactions, and electrostatic interactions (Sharif and Roberts 2015; Tran et al. 2015; Yang et al. 2014; Zhao et al. 2012; Zhao et al. 2015). On the other hand, absorption occurs when a sorbent material is immersed in a liquid. In this work, the sorbent surface was functionalized to enhance its oil uptake capacity; as a result, oil molecules were expected to bond to the attached functional groups. Hence, we assumed that the oil uptake by the functionalized graphene sponge represented an adsorption process, which was subsequently verified by plotting adsorption isotherms.
In this work, we fabricated 3D graphene sponges with enhanced oleophilic characteristics, which were obtained via a combined method of high-pressure hydrothermal reduction, freeze-drying, and chemical vapor functionalization with trichloro (1H,1H,2H,2H-perfluoro-octyl)silane. The extensive characterization of the produced 3D-graphene sponges via scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS), Fourier transform infrared spectroscopy (FTIR), and Raman spectroscopy revealed the existence of a strong network structure with excellent mechanical durability and enhanced regeneration capacity, which resulted from the surface modification procedure (Xu et al. 2010; Zhang et al. 2011). In addition, the GO material used during fabrication produced a strong effect on the sponge stiffness. The macroscopic parameters of the 3D graphene sponges (including their shapes and sizes) were easily tailored by varying the shape and volume of the Teflon-lined autoclave used for the hydrothermal treatment. Finally, the adsorptions of diesel oil, crude oil, and hexane on the surfaces of the produced graphene sponges were examined by plotting Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich isotherms.
Experimental
Materials
All the chemicals used in this work were purchased from Sigma-Aldrich. They included graphite powder, 99% potassium permanganate (KMnO4), 98% sulfuric acid (H2SO4), 97% trichloro(1H,1H,2H,2H-perfluorooctyl)silane (CF3(CF2)5CH2CH2SiCl3), and 30% (w/w) hydrogen peroxide (H2O2). Arabian crude oil and diesel oil were provided by the Abu Dhabi National Oil Company (ADNOC). Hexane was purchased from Sigma-Aldrich, and olive oil was obtained from LULU hypermarket—a local distributor. All materials and chemicals were used as received without further purification.
Preparation of GO
The corresponding procedure was performed using a modified Hummers’ method. Its detailed description is provided elsewhere (Bi et al. 2012; Bi et al. 2014b).
Synthesis of reduced GO aerogel
Aqueous GO solution of 0.5 mg mL−1 was poured into a 25-mL Teflon-lined autoclave and subjected to hydrothermal reduction at a temperature of 180 °C for 12 h. After the gel formation, the autoclave was removed from the oven and cooled down to room temperature of 25 °C. Afterwards, the wet gel was removed from the autoclave using a pair of forceps, washed in deionized water, frozen for 12 h, and finally freeze-dried for 48 h to produce the reduced GO (rGO) aerogel. The purpose of the freeze-drying procedure was to preserve the 3D gel structure that was formed by the interlinking of the graphene sheets.
Surface modification of rGO aerogel
A solution of 97% trichloro(1H,1H,2H,2H-perfluorooctyl)silane purchased from Sigma-Aldrich was used for the surface modification of the rGO aerogel. The latter was placed on a glass slide inside a desiccator together with a few drops of the silane solution placed on a different glass slide. The resulting system was subjected to vacuum treatment in order to promote the deposition of silane species onto the gel surface. Subsequently, the functionalized rGO aerogel was washed with deionized water and vacuum-dried inside an oven at a temperature of 40 °C.
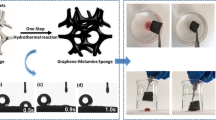
The mechanism of the simple and facile fabrication of the elastic graphene aerogel is shown in Fig. 1. During the hydrothermal treatment at 180 °C, wet hydrogel was formed, which contained a significant amount of water (about 97 wt.%) inside its pores. In order to remove this water while preserving the 3D gel structure, a freeze-drying procedure was performed. After this step, the vapor deposition of trichloro (1H,1H,2H,2H-perfluoro-octyl)silane was conducted to make the sponge surface more hydrophobic. The sponge shape was varied by selecting the desired shape and size of the Teflon-lined autoclave.
a Schematic illustration describing the preparation of the surface-enhanced spongy graphene aerogel. Panels b, c, and d contain the photographic images of the aqueous GO solution obtained b before and c after the hydrothermal reduction at 180 °C for 12 h and d graphene aerogels with similar shapes and sizes
The mechanism of the simple and facile fabrication of the elastic graphene aerogel is shown in Fig. 1. During the hydrothermal treatment at 180 °C, wet hydrogel was formed, which contained a significant amount of water (about 97 wt.%) inside its pores. In order to remove this water while preserving the 3D gel structure, a freeze-drying procedure was performed. After this step, the vapor deposition of trichloro (1H,1H,2H,2H-perfluoro-octyl)silane was conducted to make the sponge surface more hydrophobic. The sponge shape was varied by selecting the desired shape and size of the Teflon-lined autoclave.
Characterization
Morphology studies and energy dispersive X-ray spectroscopy analysis (EDS) of the prepared 3D three-dimensional graphene samples were performed using a Quanta 250 scanning electron microscope (SEM). Raman spectra were obtained using a Witec Alpha 300 RAS instrument equipped with a 532-nm green laser. Contact angle measurements were performed using KYOWA DM-501 and environmental SEM (ESEM) instruments using a Quanta 250 SEM. Mechanical compression tests were conducted using an INSTRON 5982 testing machine and a 5-kN load cell. XPS analysis was performed using a KRATOS system equipped with an Al K 1486.6 eV source gun, which produced a 400-μm spot size in the standard lens mode. FTIR spectra were recorded under vacuum using a Bruker Vertex 80v system.
Oil uptake studies
Oil uptakes by the prepared aerogel samples were measured from the corresponding weight gains. During measurements, the samples were completely soaked with various oils stained with Sudan red 7B dye and then removed from the oil container, cleaned to eliminate any surface species, and weighed. Sorption capacities were calculated using the following formula:
where Q is the sorption capacity after saturation, while Wi and Wf are the initial and final weights of the sample, respectively (Bi et al. 2014b; Chi et al. 2015).
The recyclability process is carried out by physically squeezing the elastic sponge to get rid of the oil. In addition, the sponge has been heated as part of another recyclability process to make sure that we get rid of the oil from the sponge.
Results and discussion
Raman spectroscopy was used as it is a useful tool to study carbon nanomaterials. Figure 1 shows the Raman spectra of graphite and GO. The spectrum of graphite shows an intense G peak at about 1574.7 cm−1, which is likely due to the first-order scattering of the E2g mode of vibration (Ferrari 2007). In contrast, the Raman G peak obtained for GO is broader, and its position is slightly shifted to about 1583 cm−1. In addition, a noticeable increase in the intensity of the D band at 1350.0 cm−1 was observed for these two samples, which can be attributed to the reduction in size of the in-plane sp2 domains that occurred during the oxidation stage of the modified Hummer’s method (Ferrari 2007).
During the synthesis procedure, it was necessary to confirm whether the vapor deposition of trichloro(1H,1H,2H,2H-perfluoro-octyl)silane-CF3(CF2)5CH2CH2SiCl3 had occurred. Therefore, the corresponding EDS spectra were recorded before and after vapor deposition. In Fig. 2, spectrum (a) exhibits a relatively high intensity of the carbon peak since it is the predominant element in the synthesis process of graphene sponges. However, the same spectrum also shows some contamination resulting from the contact with the surrounding oxygen species, which occurred before and during the EDS measurements. On the contrary, after the vapor deposition of the silane solution, a strong silicon peak, whose intensity was much greater than that of the carbon peak, was observed, owing to the presence of CF3(CF2)5CH2CH2SiCl3 species on the sponge surface (the Cl peak was detected for the same reason). The absence of the fluorine peak can be explained by the fact that EDS does not detect light elements, while the observed K peak is due to the presence of contaminants before the EDS experiment.
The FTIR characterization of the produced graphene sponge with a concentration of 0.5 mg/mL was performed before and after its functionalization (FTIR is highly sensitive to the electronic structure and represents a valuable tool for characterizing carbon-based materials). A strong C–F stretching vibration was observed at 1200 cm−1 for the silanized graphene sponge (Fig. 3d), owing to the presence of fluorine atoms in the CF3(CF2)5CH2CH2SiCl3 functional groups. Moreover, due to the effective vapor deposition of silane molecules onto the graphene sponge surface, the observed C=C stretching vibration was less intense in the silanized sponge as compared to that in the non-functionalized sponge since the C=C group of graphene was delocalized across its hexagonal lattice structure. The effectiveness of the graphene sponge functionalization was further verified via XPS characterization (the obtained results are shown in Fig. 4 below).
a C 1s XPS spectra of the silanized and unsilanized graphene sponges produced at GO concentrations of 2 mg/mL. A comparison between the Si 2p XPS spectra recorded for the silanized sponges synthesized at GO concentrations of b 2.0 mg/mL and c 0.5 mg/mL. XPS general survey spectra obtained for the d silanized and unsilanized sponges synthesized at GO concentrations of 2.0 mg/mL
Figure 4a shows the C 1s XPS spectra recorded for the silanized and unsilanized graphene sponges, which were synthesized at GO concentrations of 2 mg/mL. The C 1s peak obtained before silanization exhibited a relatively high intensity of 70,000 counts/s as compared to that of the peak obtained after silanization (40,000 counts/s) because silane molecules effectively covered the sponge surface during vapor deposition.
In addition, silane vapor deposition procedure was performed for the samples fabricated at GO concentrations of 2.0 and 0.5 mg/mL. After analyzing the corresponding Si 2p XPS peaks (see Fig. 4c), it was found that its intensity was greater for the silanized sample produced at a GO concentration of 0.5 mg/mL (7500 counts/s) than for the silanized sample fabricated at a GO concentration of 2.0 mg/mL (1250 counts/s, see Fig. 4b). The observed difference can be explained by the fact that the sponge synthesized at a GO concentration of 2.0 mg/mL was more compact; as a result, silane molecules covered its entire porous surface less effectively. On the other hand, the sponge depicted in Fig. 4c was less compact, which resulted in a more uniform distribution of silane molecules across its surface. Hence, the sponge synthesized at a GO concentration of 0.5 mg/mL was more porous than the sponge synthesized at a GO concentration of 2.0 mg/mL. The XPS general surveys conducted for the 2.0-mg/mL silanized and unsilanized samples are presented in Fig. 4d, e, respectively. The silanized sample (Fig. 4d) shows the presence of the Si 2p and Si 2s peaks, which are absent from the spectrum recorded for the unsilanized sample (Fig. 4e), thus confirming the efficiency of the performed vapor deposition procedure. In addition to the C 1s and O 1s peaks, the F 1s peak was also detected for the silanized sample (the presence of fluorine in the unsilanized sample likely resulted from the contamination during sample transfer).
Fig. 5 shows the Raman spectra of graphite, GO, and the fabricated graphene aerogels. After the hydrothermal reduction of the aqueous GO solution to the graphene aerogel, its 2D peak became more pronounced, and the intensities of the defect and G peaks increased.
The spongy graphene aerogel fabricated from the GO aqueous solution with a concentration of 0.5 mg/mL is highly hydrophobic in nature. The intrinsic contact angle of the graphene aerogel fabricated at a GO concentration of 0.5 mg/mL was 145°, whereas the intrinsic contact angle of the graphene aerogel fabricated from the GO solution with a concentration of 2 mg/mL was 120° (see Fig. 6).
The functionalization of the fabricated graphene sponge with trichloro(1H,1H,2H,2H-perfluoro-octyl)silane made its surface more hydrophobic (the corresponding contact angle was as high as 150°; see Figs. 6a and 7c). Moreover, the ESEM condensation experiments conducted at foggy conditions resulted in even higher contact angles exceeding 170° (Fig. 7d). This figure shows the SEM image of the cross-section of the graphene sponge, which confirms its spongy structure.
SEM micrographs showing the interconnected layers inside the sponges fabricated at GO concentrations of a 2.0 mg/mL and b 0.5 mg/mL. c, e Results of the contact angle measurements conducted for the 3D graphene sponge (c, e) in excess of 150°. d An ESEM micrograph of the graphene sheets showing the contact angles exceeding 170°
The SEM micrographs of the graphene sponges fabricated at GO concentrations of 2.0 and 0.5 mg/mL are displayed in Fig. 7a, b, respectively. They show that the microstructure of the graphene sponge prepared from the GO solution with a concentration of 2.0 mg/mL contained larger pores as compared to that of the sponge fabricated from the GO solution with a concentration of 0.5 mg/mL (the sponge synthesized at a larger concentration of GO also contained a greater number of graphene layers inside its walls).
Mechanical compression tests were performed on the two graphene sponges manufactured at different GO concentrations (0.5 and 2.0 mg/mL). The obtained results (Fig. 8) showed that the graphene sponge fabricated at 2.0 mg/mL was stiffer and possessed better mechanical properties.
According to Fig. 8, the compressive Young modulus of the cylindrically shaped graphene sponge with a diameter of 1 cm synthesized from the aqueous GO solution with a concentration of 2.0 mg/mL was 2.7 MPa as compared to the value of 22.3 kPa obtained for the sponge synthesized from the GO solution with a concentration of 0.5 mg/mL. However, it should be noted that although the mechanical properties of the sponge manufactured at a higher GO concentration were better, its sorption capacity for oils and organic solvents was reduced. In this work, oil adsorption experiments were conducted for both the 0.5 and 2.0 mg/mL sponges. Figure 9a illustrates the adsorption of crude oil by the graphene aerogel monitored after 10 and 30 s.
a Adsorption of crude oil on the graphene aerogel surface from the oil-water mixture after 10 and 30 s. Weight gain (%) measured for the adsorption of various oils by the graphene sponges fabricated at GO concentrations of b adsorption capacity g/g for the 2.0 mg/mL and 0.5 mg/sponges. c Weight gain % for the 2.0 and 0.5 mg/mL sponges
The adsorption procedure described above was also performed for various oils and both sponges (the obtained results are depicted in Fig. 9b, c). They show that the adsorption capacity of the 0.5 mg/mL sponge was systematically higher for all the tested oils. Furthermore, its value obtained for crude oil and 0.5 mg/mL sponge was 385% greater than the magnitude obtained for crude oil and the sponge manufactured at a GO concentration of 2.0 mg/mL. In addition, the regeneration capacities of the manufactured graphene sponges were investigated using crude oil and hexane sorbates. It was found that the produced graphene aerogels exhibited excellent regeneration capacities after the removal of the adsorbed oil species via heating. Figure 10 shows that the spongy graphene aerogels retain a relatively high adsorption capacity of above 90% after five crude oil and hexane adsorption-desorption cycles.
The regeneration efficiency for both crude oil and hexane adsorbed on the surface of the spongy graphene aerogel manufactured at a GO concentration of 0.5 mg/mL (95% of the initial intake) was higher than that of the aerogel manufactured from the GO solution with a concentration of 2.0 mg/mL (90% of the initial intake). For example, for crude oil, the weight gain of the sponge manufactured at a GO concentration 0.5 mg/mL measured after the first cycle was 3380.7% as compared to 700% obtained for the sponge fabricated at a GO concentration of 2.0 mg/mL due to the larger surface area of the first sample. Since oil droplets were distributed uniformly across the surface of the 0.5 mg/mL sample, they were relatively easy to release via heating. Therefore, fabricating spongy graphene aerogels from the GO solutions with smaller concentrations improves their regeneration efficiency towards the adsorbed oils and organic solvents. Thus, the graphene aerogels fabricated at a GO concentration of 0.5 mg/mL exhibited a 5% increase in the regeneration efficiency as compared to those of the aerogels manufactured at a GO concentration of 2.0 mg/mL. As shown in Table 1, the oil uptakes measured for the silane-functionalized graphene sponge are 25–86 times greater than its own weight. The listed adsorption capacities are much higher than those of conventional absorbers such as microporous gels (Hayase et al. 2013), nonwoven polypropylene (Wei et al. 2003). Although the adsorption capacities reported for crude oil are higher in the cases of exfoliated graphite (Toyoda and Inagaki 2000) and the carbon fibers derived from bamboo (Yang et al. 2015), the fabrication process of these materials is more complex as compared to the procedure utilized for the preparation of the functionalized graphene sponges in this study. The experimental adsorption capacities measured for different oils in this work are higher than those obtained for carbon fibers (Liang et al. 2012; Suni et al. 2004).
Isotherm modeling
The adsorption of diesel and crude oils on the surfaces of the 3D graphene sponges were modeled by plotting Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich isotherms. The main purpose of constructing adsorption isotherms is to correlate the obtained experimental results with the empirical theoretical parameters. In addition, these isotherms can be used to quantify the heat and energy of adsorption as well as the adsorption mechanism of the oil molecules on the spongy surface. If the obtained experimental results are close to the empirical theoretical parameters of a specific adsorption isotherm, it can be concluded that the studied adsorption process can be described by the corresponding theoretical model. Thus, the Langmuir adsorption model describes the process of adsorption by assuming that the adsorbate behaves as an ideal gas at the ideal conditions. During the adsorption of a single adsorbate molecule on the surface of a solid, it is assumed that (1) all its sites are equivalent, (2) the sorbent surface is flat and homogeneous, (3) each adsorption site can accommodate only one molecule, and (4) little or no interactions occur between the adsorbate molecules attached to the adjacent sites. The Langmuir isotherm is expressed as follows:
After linearization, this equation becomes
where qe is the amount of adsorbate adsorbed at equilibrium, Ce is the concentration of adsorbate in the bulk fluid at equilibrium, Q is the constant that represents the maximum amount of adsorbate that can be adsorbed on the surface, and b is the isotherm constant. The Langmuir model is applicable if the term Qb is larger than 1. Furthermore, the results obtained for the adsorption of diesel and crude oils in this study were also modeled using the Freundlich isotherm model, which is often utilized for the adsorption on heterogeneous surfaces. It can be described by the following equation:
After linearization, the following form can be obtained:
where k and n are the isotherm parameters that depend on the adsorbate and adsorbent. The constant k is related to the adsorption capacity of the sponge. If n>1, the adsorption on a heterogeneous surface is favorable. If n = 1, the partition between the adsorbate oil and surface of the graphene sponge is independent of the oil concentration. On the other hand, if 1/n is greater than 1, cooperative adsorption is observed. The Temkin isotherm model is also used to describe the adsorption process and quantify the heat of sorption (Chen 2015). It assumes that the heat of sorption of all adsorbate molecules decreases linearly with coverage (Chen 2015; Eriksson et al. 1997; Johnson and Arnold 1995). This model can be characterized by the following equation:
where AT is the equilibrium binding constant (L/g), bT is the constant, R is the universal gas constant (8.314 J/mol/K), T is the temperature, and B is the heat of sorption (J/mol). The Temkin equation emphasizes uniform distribution of the binding energies measured for the oil molecules adsorbed on the graphene sponge surface. Finally, the Dubinin-Radushkevich (DRK) isotherm model is applied to investigate the adsorption process on a heterogeneous surface corresponding to the Gaussian energy relationship (Chen and Yang 1994; Pinto et al. 2010; Rudziński et al. 1974). Furthermore, this model is also used to characterize the physical and chemical adsorption processes using the mean free energy E of sorption parameter. The DRK isotherm can be expressed by the following equation:
where qe is the amount of adsorbed molecules at equilibrium (mg/g). qs is the theoretical saturation capacity (mg/g), Kad is the isotherm constant (mol2/kJ2), and ε is another isotherm constant.
The mean free energy of sorption E and constant ε can be calculated as follows:
where BDR is the isotherm constant and Ce represents the adsorbate equilibrium concentration (mg/L). By performing the linear regression analysis (such as Inqe = In(qs) − (Kadε2)), four different isotherms are plotted in Fig. 11.
The slope and y-intercept of the Langmuir plot depicted in Fig. 11a were used to determine Q (which quantifies the adsorption capacity) and b (which describes the affinity of the binding sites on the spongy graphene aerogel surface). According to Fig. 11, Q = 107.99 g diesel/g sponge, b = 0.554 L/g diesel, and Qb = 59.82. Therefore, since Qb ≫ 1, the Langmuir isotherm model is applicable to the studied process. For the Freundlich isotherm plot (Fig. 11b), linear regression analysis was used to estimate the values n and k, which were equal to 3.04 g diesel/g sponge and 41.00 g diesel/g sponge, respectively. From the Temkin isotherm plot, the following values were obtained: AT = 11.41 L/mg, B = 17.871 J/mol, and bT = 138.644. Therefore, it can be concluded that the adsorption of diesel oil onto the graphene sponge surface represents a physisorption process with a heat of sorption of 17.871 J/mol. Finally, from the DRK isotherm plot, the following parameters were obtained: qs = 83.0 mg/g, Kad = 2.918 × 10−7 mol2/kJ2, and adsorption energy per mole of adsorbate equal to 1.29 kJ/mol, which corresponded to the physisorption of diesel oil on the graphene sponge surface. In addition to the described relations between the obtained isotherms and the adsorption of diesel oil on the synthesized graphene sponges, the same models were applied to crude oil in Figs. 12 and 13. The Langmuir isotherm parameter Qb equal to 8.48 for crude oil was greater than 1, indicating monolayer adsorption of crude oil on the outer sponge surface and, therefore, a finite number of identical surface sites.
The isotherm parameters obtained for crude oil, diesel oil, and hexane are listed in Table 2 .
According to Table 2, the 3D graphene sponges adsorb a higher amount of diesel oil per mass of sponge as compared to the magnitudes obtained for crude oil and hexane. For instance, the experimental sorption capacity measured for diesel oil was obtained as 86 g diesel oil/g sponge, in which this value was close to the theoretical DRK isotherm saturation capacity of 83.0 g diesel oil/g sponge. Therefore, an adsorption, not absorption phenomena, was observed in this work. Furthermore, the experimental sorption capacities estimated for crude oil and hexane were obtained as 34 g crude oil/g sponge and 25 g hexane/g sponge, respectively. The experimental sorption capacity obtained for crude oil was close to the theoretical Langmuir isotherm maximum monolayer coverage capacity of 33.39 g crude/g sponge, whereas the experimental sorption capacity of hexane was close to the theoretical DRK isotherm saturation capacity of 29.78 g hexane/ g sponge. Therefore, regions of the surface of the graphene sponge had a fixed contained a limited number of adsorption sites, which could retain with each site holding only one molecule of crude oil molecule during the formation of the monolayer on the thin outer surface. From these obtained correlations, it can be noted that the adsorption of crude oil adsorption closely mainly follows the Langmuir adsorption isotherm model, while the hexane adsorption closely follows the DRK isotherm model.
Second, according to the obtained parameters of the Langmuir and Freundlich isotherms, the adsorption capacity of diesel oil was about three times higher than that of crude oil because the Q value determined for crude oil from the Langmuir isotherm was roughly three times greater than that of diesel oil, suggesting a higher efficiency of the sponge surface towards diesel adsorption. Furthermore, the k value obtained for crude oil from the Freundlich isotherm was more than three times higher than that of diesel oil. Diesel oil exhibits lower affinity for water because it is less viscous and consists of 75% saturated hydrocarbons and 25% aromatic hydrocarbons (Lanský) with the common formula C12H23. On the other hand, crude oil (which is mainly composed of alkanes (Powell and McKirdy 1973)) has a higher affinity for water than diesel because it represents a mixture of a various molecules with different viscosities boiling points.
Moreover, the heat of sorption (B) determined from the Temkin isotherm for diesel oil was more than two times greater than the heat of sorption obtained for crude oil (7.051 J/mol), which could be attributed to the higher affinity of the sponges for diesel oil. The adsorption energy of diesel oil determined from the DRK isotherm was higher than the value obtained for crude oil due to the lower viscosity of diesel oil (4.5 × 10−3 versus 2.3 × 10−2 Pa ⋅s obtained for Arabian crude oil) (O’Donnell 1977).
Finally, the Langmuir isotherm parameters listed in Table 2 show that the produced graphene sponge exhibits a higher hexane adsorption capacity (50.25 g hexane/g sponge) as compared to that of crude oil (33.39 g oil/g sponge). Furthermore, because the Qb value determined for the hexane adsorption was greater than 1 (5.59), it could be described using the Langmuir adsorption model. The plotted Temkin isotherm revealed that the heat of sorption of hexane was higher than that of crude oil, which was consistent with the results obtained from with the Langmuir isotherm. Further, the n and k values obtained from the Freundlich isotherm plotted for hexane were lower than the corresponding parameters determined for crude oil, owing to the volatile nature of hexane (which is also the reason for its relatively low heat of adsorption obtained from the DRK isotherm).
Conclusion
Functionalized 3D graphene sponges have been successfully fabricated from the GO solutions with different concentrations and trichloro(1H,1H,2H,2H-perfluorooctyl) silane. Lower concentrations of GO were observed to have higher adsorption performances. 3D graphene sponges with higher GO concentration resulted in stiffer graphene sponges as the compression modulus of 2.0 mg/mL GO concentration was 2.7 MPa (as compared to the magnitude of 22.4 kPa obtained for the 3D graphene sponges of 0.5-mg/mL GO concentration). The functionalized 3D graphene sponges adsorbed diesel oil much better than crude oil. Their affinity for diesel oil was more than three times higher than the affinity for crude oil. Diesel oil exhibits lower affinity for water because it is less viscous and composed of 75% saturated hydrocarbons and 25% aromatic hydrocarbons. On the other hand, crude oil shows higher affinity for water in its unrefined state due to the presence of various constituents such as alkanes, paraffin, bitumen, light fuels, and heavy fuels. The heat of sorption obtained for diesel oil was 17.87 J/mol, which was three times higher than that of diesel oil. Furthermore, the Temkin isotherm revealed that the heat of sorption of hexane was higher than that of crude oil due to the volatile nature of hexane (which was also the reason for the lower heat of adsorption obtained for hexane from the DRK isotherm). Hence, the fabricated 3D graphene sponges exhibited better adsorption efficiency of hexane solution as compared to that of crude oil obtained using the Langmuir isotherm model. This work describes a low-cost method for the fabrication of mechanically elastic and highly absorbent graphene sponges for oil-water separation.
References
Bi H, Xie X, Yin K, Zhou Y, Wan S, He L, Xu F, Banhart F, Sun L, Ruoff RS (2012) Spongy graphene as a highly efficient and recyclable sorbent for oils and organic solvents. Adv Funct Mater 22:4421–4425
Bi H, Huang X, Wu X, Cao X, Tan C, Yin Z, Lu X, Sun L, Zhang H (2014a) Carbon microbelt aerogel prepared by waste paper: an efficient and recyclable sorbent for oils and organic solvents. Small 10:3544–3550
Bi H, Xie X, Yin K, Zhou Y, Wan S, Ruoff RS, Sun L (2014b) Highly enhanced performance of spongy graphene as an oil sorbent. J Mater Chem A 2:1652–1656
Chae SJ, Güneş F, Kim KK, Kim ES, Han GH, Kim SM, Shin HJ, Yoon SM, Choi JY, Park MH (2009) Synthesis of large-area graphene layers on poly-nickel substrate by chemical vapor deposition: wrinkle formation. Adv Mater 21:2328–2333
Chen X (2015) Modeling of experimental adsorption isotherm data. Information 6:14–22
Chen S, Yang R (1994) Theoretical basis for the potential-theory adsorption-isotherms-the Dubinin-Radushkevich and Dubinin-Astakhov equations. Langmuir 10:4244–4249
Chen Z, Ren W, Gao L, Liu B, Pei S, Cheng H-M (2011) Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat Mater 10:424–428
Chen J, Sheng K, Luo P, Li C, Shi G (2012) Graphene hydrogels deposited in nickel foams for high-rate electrochemical capacitors. Adv Mater 24:4569–4573
Chi C, Xu H, Zhang K, Wang Y, Zhang S, Liu X, Liu X, Zhao J, Li Y (2015) 3D hierarchical porous graphene aerogels for highly improved adsorption and recycled capacity. Mater Sci Eng B 194:62–67
Cong H-P, Ren X-C, Wang P, Yu S-H (2012) Macroscopic multifunctional graphene-based hydrogels and aerogels by a metal ion induced self-assembly process. ACS Nano 6:2693–2703
Eriksson M, Lundström I, Ekedahl L-G (1997) A model of the Temkin isotherm behavior for hydrogen adsorption at Pd–SiO 2 interfaces. J Appl Phys 82:3143–3146
Ferrari AC (2007) Raman spectroscopy of graphene and graphite: disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid state communications 143:47–57
Frank I, Tanenbaum DM, van der Zande A, McEuen PL (2007): Mechanical properties of suspended graphene sheets. Journal of Vacuum Science & Technology B: Microelectronics and Nanometer Structures Processing, Measurement, and Phenomena 25, 2558–2561
Ge B, Men X, Zhu X, Zhang Z (2015) A superhydrophobic monolithic material with tunable wettability for oil and water separation. J Mater Sci 50:2365–2369
Geim AK (2009) Graphene: status and prospects. Science 324:1530–1534
Gómez-Navarro C, Weitz RT, Bittner AM, Scolari M, Mews A, Burghard M, Kern K (2007) Electronic transport properties of individual chemically reduced graphene oxide sheets. Nano Lett 7:3499–3503
Gui X, Zeng Z, Lin Z, Gan Q, Xiang R, Zhu Y, Cao A, Tang Z (2013) Magnetic and highly recyclable macroporous carbon nanotubes for spilled oil sorption and separation. ACS Appl Mater Interfaces 5:5845–5850
Hashim DP, Narayanan NT, Romo-Herrera JM, Cullen DA, Hahm MG, Lezzi P, Suttle JR, Kelkhoff D, Munoz-Sandoval E, Ganguli S (2012) Covalently bonded three-dimensional carbon nanotube solids via boron induced nanojunctions. Sci Rep 2:363
Hayase G, Kanamori K, Fukuchi M, Kaji H, Nakanishi K (2013) Facile synthesis of marshmallow-like macroporous gels usable under harsh conditions for the separation of oil and water. Angew Chem Int Ed 52:1986–1989
Johnson RD, Arnold FH (1995) The Temkin isotherm describes heterogeneous protein adsorption. Biochim Biophys Acta (BBA)-Protein Struct Mol Enzymol 1247:293–297
Lee C, Wei X, Kysar JW, Hone J (2008) Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 321:385–388
Li X, Magnuson CW, Venugopal A, An J, Suk JW, Han B, Borysiak M, Cai W, Velamakanni A, Zhu Y (2010) Graphene films with large domain size by a two-step chemical vapor deposition process. Nano Lett 10:4328–4334
Li Y-Q, Samad YA, Polychronopoulou K, Alhassan SM, Liao K (2014) Carbon aerogel from winter melon for highly efficient and recyclable oils and organic solvents absorption. ACS Sustain Chem Eng 2:1492–1497
Liang HW, Guan QF, Chen LF, Zhu Z, Zhang WJ, Yu SH (2012) Macroscopic-scale template synthesis of robust carbonaceous nanofiber hydrogels and aerogels and their applications. Angew Chem Int Ed 51:5101–5105
Liu C, Yang J, Tang Y, Yin L, Tang H, Li C (2015) Versatile fabrication of the magnetic polymer-based graphene foam and applications for oil–water separation. Colloids Surf A Physicochem Eng Asp 468:10–16
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A, Alemany LB, Lu W, Tour JM (2010) Improved synthesis of graphene oxide. ACS Nano 4:4806–4814
Niu Z, Chen J, Hng HH, Ma J, Chen X (2012) A leavening strategy to prepare reduced graphene oxide foams. Adv Mater 24:4144–4150
Novoselov KS (2011) Graphene: the magic of flat carbon. ECS transactiones 20:45–46
O'Donnell K 1977: Chemical and physical properties of refined petroleum products
Park S, Lee K-S, Bozoklu G, Cai W, Nguyen ST, Ruoff RS (2008) Graphene oxide papers modified by divalent ions—enhancing mechanical properties via chemical cross-linking. ACS Nano 2:572–578
Pinto ML, Mestre AS, Carvalho AP, Jo P (2010) Comparison of methods to obtain micropore size distributions of carbonaceous materials from CO2 adsorption based on the Dubinin− Radushkevich isotherm. Ind Eng Chem Res 49:4726–4730
Powell T, McKirdy D (1973) Relationship between ratio of pristane to phytane, crude oil composition and geological environment in Australia. Nature 243:37–39
Rudziński W, Jaroniec M, Sokołowski S (1974) A new isotherm equation for multilayer adsorption on heterogeneous surfaces yielding the Dubinin-Radushkevich isotherm in the submonolayer region. Phys Lett A 48:171–172
Sharif F, Roberts E (2015) Study of graphene FOAM characteristics: adsorption and electrochemical regeneration, meeting abstracts. The electrochemical. Society:1197–1197
Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia Y, Wu Y, Nguyen ST, Ruoff RS (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45:1558–1565
Sun H, La P, Zhu Z, Liang W, Yang B, Zhao X, Pei C, Li A (2014) Hydrophobic carbon nanotubes for removal of oils and organics from water. J Mater Sci 49:6855–6861
Suni S, Kosunen A-L, Hautala M, Pasila A, Romantschuk M (2004) Use of a by-product of peat excavation, cotton grass fibre, as a sorbent for oil-spills. Mar Pollut Bull 49:916–921
Toyoda M, Inagaki M (2000) Heavy oil sorption using exfoliated graphite: new application of exfoliated graphite to protect heavy oil pollution. Carbon 38:199–210
Tran DNH, Kabiri S, Sim TR, Losic D (2015) Selective adsorption of oil-water mixtures using polydimethylsiloxane (PDMS)-graphene sponges. Environ Sci: Water Res Technol 1:298–305
Tuncaboylu DC, Okay O (2009) Preparation and characterization of single-hole macroporous organogel particles of high toughness and superfast responsivity. Eur Polym J 45:2033–2042
Upadhyay RK, Dubey A, Waghmare PR, Priyadarshini R, Roy SS (2016) Multifunctional reduced graphene oxide coated cloths for oil/water separation and antibacterial application. RSC Adv 6:62760–62767
Vlassiouk I, Regmi M, Fulvio P, Dai S, Datskos P, Eres G, Smirnov S (2011) Role of hydrogen in chemical vapor deposition growth of large single-crystal graphene. ACS Nano 5:6069–6076
Wei Q, Mather R, Fotheringham A, Yang R (2003) Evaluation of nonwoven polypropylene oil sorbents in marine oil-spill recovery. Mar Pollut Bull 46:780–783
Wei D, Liu Y, Wang Y, Zhang H, Huang L, Yu G (2009) Synthesis of N-doped graphene by chemical vapor deposition and its electrical properties. Nano Lett 9:1752–1758
Xia K, Zhan H, Gu Y (2015) Two-dimensional graphene heterojunctions: the tunable mechanical properties. Carbon 95:1061–1068
Xiao K, Deng W, Keum JK, Yoon M, Vlassiouk IV, Clark KW, Li A-P, Kravchenko II, Gu G, Payzant EA (2013) Surface-induced orientation control of CuPc molecules for the epitaxial growth of highly ordered organic crystals on graphene. J Am Chem Soc 135:3680–3687
Xu Y, Sheng K, Li C, Shi G (2010) Self-assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano 4:4324–4330
Yang W, Chen G, Shi Z, Liu C-C, Zhang L, Xie G, Cheng M, Wang D, Yang R, Shi D (2013) Epitaxial growth of single-domain graphene on hexagonal boron nitride. Nat Mater 12:792–797
Yang S, Chen L, Mu L, Ma P-C (2014) Magnetic graphene foam for efficient adsorption of oil and organic solvents. J Colloid Interface Sci 430:337–344
Yang S, Chen L, Mu L, Hao B, Ma P-C (2015) Low cost carbon fiber aerogel derived from bamboo for the adsorption of oils and organic solvents with excellent performances. RSC Adv 5:38470–38478
Zhang X, Sui Z, Xu B, Yue S, Luo Y, Zhan W, Liu B (2011) Mechanically strong and highly conductive graphene aerogel and its use as electrodes for electrochemical power sources. J Mater Chem 21:6494–6497
Zhang W, Zhai X, Xiang T, Zhou M, Zang D, Gao Z, Wang C (2017) Superhydrophobic melamine sponge with excellent surface selectivity and fire retardancy for oil absorption. J Mater Sci 52:73–85
Zhao J, Ren W, Cheng H-M (2012) Graphene sponge for efficient and repeatable adsorption and desorption of water contaminations. J Mater Chem 22:20197–20202
Zhao L, Yu B, Xue F, Xie J, Zhang X, Wu R, Wang R, Hu Z, Yang S-T, Luo J (2015) Facile hydrothermal preparation of recyclable S-doped graphene sponge for cu 2+ adsorption. J Hazard Mater 286:449–456
Acknowledgments
The authors gratefully acknowledge the financial assistance provided by the Masdar Institute of Science and Technology. The authors would also like to thank the entire team of the Masdar Institute Microscopy Suite for their contribution and support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Bagoole, O., Rahman, M.M., Shah, S. et al. Functionalized three-dimensional graphene sponges for highly efficient crude and diesel oil adsorption. Environ Sci Pollut Res 25, 23091–23105 (2018). https://doi.org/10.1007/s11356-018-2248-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2248-z