Abstract
This work found that the removal of chromium by a straw-derived biochar was significantly promoted or inhibited by various surfactants. For example, the anionic surfactant sodium dodecyl benzene sulfonate (SDBS) inhibited the removal of Cr(VI) by the biochar but significantly promoted the removal of Cr(III) by the biochar. The nonionic surfactant Triton X-100 (TX-100) promoted the removal of Cr(VI) at low concentrations (< 100 mg L−1) but inhibited the removal at high concentrations. Different mechanisms were found for the two surfactants. As an anionic surfactant, surface-sorbed SDBS changed the surface functional groups of the biochar, making the biochar negative charged and changing the sorption ability of the biochar. For the nonionic TX-100, monomers and micelles in the aqueous phase had a major influence on the sorption of chromium due to the impact on the interfacial tension between the biochar and the solution phase as well as the solution pH. The results suggest that when biochar is used to treat heavy metal wastewater containing coexisting surfactants, the type and concentration of surfactants must be considered as important factors. Under certain surfactant conditions, biochar will enable the simultaneous and efficient removal of heavy metals and surfactants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium (Cr) is introduced to the environment from the effluents of corrosion control and the electroplating, leather tanning, metal cleaning, and dyeing industries (Warchoł et al. 2006). It appears in aqueous systems mainly in its trivalent and hexavalent forms (Aydın and Aksoy 2009). Chromium in aqueous environments leads to adverse effects on the cell membranes of living organisms and affects human health by eventually passing into the food chain (Wang et al. 2016). Various methods such as reduction-precipitation (Cheung and Gu 2003), ion exchange (Tiravanti et al. 1997), and sorption (Gupta and Rastogi 2009) have been employed to remove chromium from aqueous environments. Sorption is considered a particularly competitive and effective method (Babel and Kurniawan 2003; Beesley et al. 2011; Ming et al. 2012). Biochar (Zhou et al. 2016), activated carbon (Monser and Adhoum 2002), and clays (Ma et al. 2016) have been proposed to be effective sorbents for chromium removal.
Biochar is a low-cost, carbon-rich material that is pyrolyzed from various biomass feedstocks under limited-oxygen or anaerobic conditions (Marousek et al. 2017). It is generally accepted that the large surface area and ion exchange capacity of biochar, which are largely determined by the raw materials and pyrolysis temperatures, enable effective sorption of both organic and inorganic contaminants (Ahmad et al. 2013). Previous studies on the application of biochar to control heavy metal pollutants have mostly considered the properties of the biochar, such as the pyrolysis temperature, specific surface area, and adsorption process parameters, e.g., pH and temperature (Mohan et al. 2014). Little attention has been paid to the influence of coexisting compounds on the sorption of heavy metals by biochar.
In fact, wastewater produced by the electroplating, leather tanning, metal cleaning, and dyeing industries always contains surfactants with heavy metals due to the excellent wetting, foaming, emulsification, dispersion, solubilization, and dye leveling features of surfactants (Esumi and Ueno 1997). Therefore, the effects of surfactants must be considered in adsorption treatments of heavy metals, including chromium. However, the performance and mechanism of biochar for chromium removal from surfactant-containing wastewater is not yet clear. It is necessary to understand the influence of coexisting surfactants on the sorption of chromium by biochar.
Although the effects of surfactants on the sorption of chromium by biochar are not clear, some studies have examined the interaction between metals and coexisting surfactants. For example, in the studies on micelle-enhanced ultrafiltration, binding interactions were found between Cr3+ and an anionic surfactant micelles (Aoudia et al. 2003), as well as between chromate and a cationic surfactants micelles (Baek and Yang 2004).
Some researchers have indicated that surfactants may introduce new functional groups on the surface of adsorbents, such as clays (Zeng et al. 2010; Song et al. 2015) and activated carbon (Choi et al. 2009), and provide additional adsorption site for chromate. Although the composition and structure of biochar are different with clays or activated carbon, it can be speculated that surfactants may also affect the surface structure of biochar and thus affect its sorption ability for heavy metals.
Based on the previous studies, it can be speculated that surfactants may have a significant impact on the biochar sorption of heavy metals. And we hypothesis that the mechanism by which surfactants affect the biochar sorption of heavy metals may occur by two routes. One is the possible change on the surface structure of the biochar due to the surfactant, while the second is the possible influence of surfactants in the solution phase on the morphology and dispersibility of heavy metals. In this study, chromium was used as a representative of heavy metals, sodium dodecyl benzene sulfonate (SDBS) and Triton X-100 (TX-100) were used as a representative of anionic and nonionic surfactants, respectively, to study the effects and mechanisms of surfactants on the sorption of heavy metal by biochar. The purpose of this work was to gain a further understanding of chromium sorption by biochar and to provide a reference for chromium removal from complex surfactant-containing wastewater.
Materials and methods
Chemicals
Sodium dodecyl benzene sulfonate (SDBS) and Triton X-100 (TX-100) were selected as representative anionic and nonionic surfactants, and the physical and chemical properties of the surfactants are listed in Table S1. All chemicals, including HCl, NaOH, H2SO4, H3PO4, CH3COCH3, C13H14N4O, KBr, SDBS, TX-100, CrCl3, and K2Cr2O7, were purchased from Aldrich Chemical Co. with analytical grade and used as received without further purification.
Materials
The biochar was produced from a rice straw, which is the most common agricultural waste in southern China. The straw was supplied by a farm in Hangzhou City, Zhejiang Province, China, and the feedstock cost was about 45 USD t−1. The straw was air-dried at room temperature and crushed to pass through a 100-mesh sieve, and then pyrolyzed at 300 °C using a muffle furnace with a tubular reactor. The muffle furnace was programmed to heat at a rate of 5 °C min−1 until it reached 300 °C, and this temperature was maintained for 2 h. The sample was treated with 1 mol L−1 HCl and followed with several rinses of distilled water to remove any residual acid. The sample was dried at 70 °C for 24 h and passed through a 200-mesh sieve. In this paper, by using the small-scale preparation method, the yield of the biochar was about 61% and the production cost was about 350 USD t−1. In the future work, the biochar produced by some specifically designed and optimized industrial-scale productions (Marousek 2014; Ahmed et al. 2016) will be employed in the actual wastewater treatment, and its costs will be significantly reduced (Maroušek et al. 2015a, b).
The dried biochar was stored in an airtight desiccator prior to use and was abbreviated as SB300, according to the pyrolysis temperature. The elemental composition and surface area of SB300 were characterized using an elemental analyzer and Brunauer–Emmett–Teller (BET) analysis. The zero point of charge (ZPC) of SB300 was evaluated using a mass titration procedure (Valdés et al. 2002). The selective parameters of SB300 are listed in Table 1. The surface functional groups of the biochar were characterized by Fourier transform infrared spectroscopy (FTIR).
Sorption experiments
Batch experiments were conducted in triplicate to determine the sorption of Cr(VI) onto the biochar. Briefly, 20.0 mg of biochar was weighed into 20-mL glass tubes with Teflon-lined screw caps. A 20-mL solution containing a certain concentration of K2Cr2O7 and surfactant was added to each tube. The initial concentration of K2Cr2O7 was 25–1000 mg L−1. The initial concentrations of SDBS and TX-100 were 50~200 mg L−1 and 25~200 mg L−1, respectively. The solution was pre-adjusted to the desired pH (2~11) using 1.0 mol L−1 HCl and 1.0 mol L−1 NaOH. All tubes were equilibrated on a reciprocating shaker for a certain time at 25 ± 1 °C and 200 rpm to ensure sorption equilibrium. The experimental procedure for Cr(III) sorption was basically the same as for Cr(VI) but with CrCl3 instead of K2Cr2O7.
Analytical methods
The total Cr was determined using an atomic adsorption spectrophotometer (TAS-990, Beijing Persee, China). The Cr(VI) concentrations were measured by a UV–vis spectrophotometer (TU-1901Beijing Persee, China) at 540 nm using the 1,5-diphenylcarbazide method. The concentrations of Cr(III) were calculated as the difference between the total Cr and Cr(VI). The concentrations of TX-100 were measured by a UV–vis spectrophotometer at 275 nm. The concentration of SDBS was analyzed by an Agilent1260 HPLC (USA) with a UV detector (224 nm) and a 4.6 × 250 mm reverse-phase C18 column. The surface tension and pH of the chromium solutions were determined using a digital tensiometer (BZY-201, Shanghai Fangrui, China) and pH meter.
The adsorption amounts of biochar for Cr(VI), Cr(III), and surfactants were calculated as follows:
where Qe is the equilibrium sorption amount of sorbate onto the sorbent (mg·g−1), C0 and Ce are the initial and equilibrium concentrations of the solute in aqueous solution (mg·L−1), respectively, V is the volume of the solution (mL), and m is the amount of sorbent (g).
Two kinetic models and two sorption isotherm models were chosen to fit the experimental data (Albadarin et al. 2012).
The pseudo-first-order and pseudo-second-order models were expressed as follows:
where t (min) is the contact time; Qt (mg·g−1) is the amount of Cr(VI) or Cr(III) sorbed by biochar at a particular moment; Qe.exp(mg·g−1) and Qe(mg·g−1) are, respectively, the experimental and calculated equilibrium sorption amount of Cr(VI) or Cr(III) by biochar; and k1 and k2 are the rate constants of the pseudo-first-order and pseudo-second-order models, respectively.
The Freundlich and Langmuir equations can be expressed as follows:
where Qe (mg·g−1) is the equilibrium Cr(VI) or Cr(III) sorption amount, Ce (mg·L−1) is the equilibrium concentration of Cr(VI) or Cr(III), 1/n is the Freundlich exponent, qm is the maximum adsorbed amount for monolayer sorption, and KF ((mg·g−1) ·(mg·L−1)-1/n) and KL (mg·L−1) represent the Freundlich affinity coefficient and the Langmuir bonding term related to interaction energies, respectively.
Results and discussion
Removal of chromium by biochar without a surfactant
The removal of Cr(VI) and Cr(III) by SB300 from aqueous solution is illustrated in Fig. 1. As shown in Fig. 1a, b, the sorption amounts of Cr(VI) and Cr(III) onto SB300 increased rapidly in the first 2 h and then gradually leveled off at equilibrium. It took approximately 20 h for Cr(VI) and 16 h for Cr(III) to reach equilibrium. The data for both Cr(VI) and Cr(III) were fitted better with a pseudo-second-order model than a pseudo-first-order model (Table S2), which indicated that the rate-controlling process was at least partially a chemically mediated process (Ho 2006). Similar results were obtained in many other previous publications (Dong et al. 2011; Aliabadi et al. 2012; Han et al. 2016; Wang et al. 2016).
Removal of Cr by SB300 biochar (a sorption kinetic curve of Cr(VI) with an initial concentration of 100 mg/L; b sorption kinetic curve of Cr(III) with an initial concentration of 100 mg/L; c equilibrium sorption isotherms of Cr(VI) and Cr(III) under neutral conditions. d Equilibrium sorption isotherms of Cr(VI) under different pH conditions)
The sorption isotherms (pH = 7) are shown in Fig. 1c. The sorption amounts of Cr(VI) and Cr(III) by SB300 increased with the increase in the equilibrium concentrations and did not reach a saturation value in the experiment. It is worth to mention that the sorption removal of chromate by this biochar was much more effective than most activated carbons, especially under neutral or near-neutral conditions (Babel and Kurniawan 2004; Kobya 2004; Dobrowolski and Otto 2010; Legrouri et al. 2017). Both the Langmuir and Freundlich equations were used to fit the experimental data, and the results are shown in Table 2.
From the calculated parameters listed in Table 2, the equilibrium data for Cr(III) were fitted well by a Langmuir model, and the maximum sorption capacity obtained from the Langmuir model was 36.4 ± 7.63 mg g−1. The results suggested that the sorption of Cr(III) was predominantly monolayer adsorption. As shown in Table 1, the high H/C, O/C, and (O + N)/C atomic ratios of SB300 implied that the biochar was not fully carbonized and had some surface polar functional groups (Chen and Chen 2009). The absorption peaks at 3450 cm−1 (-OH), 1400 cm−1 (carboxylic acid -OH), and 1110 cm−1 (ester C=O and C-O-C) in the FTIR spectrum (Fig. 2a) also confirmed the presence of surface polar functional groups. The removal of Cr(III) by SB300 was attributed to the electrostatic attraction between Cr(III) and the acidic groups of the biochar.
The sorption of Cr(VI) onto biochar was better described by the Freundlich equation than the Langmuir equation, which indicated that the sorption of Cr(VI) onto SB300 was not monolayer adsorption and was more complex than the Cr(III) adsorption (Wong et al. 2004). Previous studies have demonstrated that the reduction of Cr(VI) to Cr(III) and further complexation between Cr(III) and the surface groups of the biochar as well as the formation of surface complexes or H bonds between CrO42− and the hydroxyl groups of biochar may be responsible for Cr(VI) removal (Dong et al. 2011; Yang et al. 2017; Zhao et al. 2017).
As shown in Fig. 1d, the removal of Cr(VI) by biochar increased with the decreasing pH, because the pH can affect both the Cr speciation and the adsorption sites of biochar. At a low pH, more Cr(VI) is reduced to Cr(III) (Dong et al. 2011; Yang et al. 2017), and the converted Cr(III) was retained by the biochar. However, since the point of zero charge of SB300 was 6.81 (Table 1), the hydroxyl and carboxyl groups were protonated, and the biochar surface was positively charged under acidic conditions. Positively charged SB300 would electrostatically attract negatively charged CrO42− or HCrO4− ions (Tan et al. 2015). HCrO4− would be the main ionic form at pH 2.0–6.4, while CrO42− would be predominant at pH > 6.4 (Tytłak et al. 2015). Compared to CrO42−, the same number of adsorption sites can adsorb more HCrO4−, resulting in higher removal of Cr(VI) by SB300 at a lower pH. As a result, when considering the effects of other coexisting compounds on the sorption of Cr(VI) by biochar, the effects of the pH cannot be neglected.
Effect of SDBS on the sorption of chromium by biochar
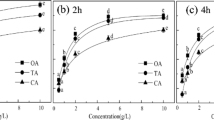
The selected anionic surfactant, sodium dodecyl benzene sulfonate (SDBS), is one of the most common detergents and a commonly used additive in the textile, dyeing, and electroplating industries. Figure 3a, b illustrate the effect of SDBS on the removal of Cr(VI) and Cr(III) by SB300. The sorption of Cr(VI) onto SB300 was inhibited by coexisting SDBS, and the degree of inhibition increased with the increasing SDBS concentration. In contrast, the sorption of Cr(III) was promoted by SDBS at all the tested concentrations (0~400 mg L−1), and 200 mg L−1 SDBS showed the strongest promotion effect. The sorption of SB300 for Cr(III) doubled with 200 mg L−1 SDBS. For example, under the same equilibrium concentration of 1000 mg L−1 Cr(III), the sorption of Cr(III) by SB300 increased from 14.2 to 31.8 mg g−1 when SDBS increased from 0 to 200 mg L−1.
From the sorption isotherms shown in Fig. 4b and the calculated parameters listed in Table 2, the sorption of SDBS by SB300 was remarkable, and the sorption data fitted the Freundlich equation better than the Langmuir equation. Previous research suggested that surfactants immobilized on the surface of a sorbent obviously affected the surface characteristics and, consequently, the sorption ability of a sorbent (Choi et al. 2009; Zeng et al. 2010). In this case, the SDBS sorbed on the surface of the biochar may also affect its sorption ability for chromium. As illustrated in Fig. 2b, the FTIR spectrum of SDBS–biochar exhibited a new sorption peak at 705 cm−1, which belonged to the deformation vibrations of the aromatic C–H of SDBS. According to the high H/C atomic ratio (Table 1) and the FTIR spectrum, SB300 contained some non-carbonized structures similar to soil organic matter, cellulose, fatty acid, or lignin. The alkyl tails of SDBS may bind to the non-carbonized part of SB300 via hydrophobic interactions (Lu and Zhu 2011), and thus, the anionic benzene sulfonate groups are exposed to the surface of SB300. As a result, surface-sorbed SDBS endows biochar with negative charges, improving the electrostatic attraction between biochar and Cr(III) and enhancing the electrostatic repulsion of biochar and Cr(VI). The result suggested that at certain concentration of SDBS, biochar will enable the simultaneous and efficient removal of SDBS and Cr(III).
The surfactant in the solution phase may also influence the sorption of chromium by SB300. Previous studies have shown that some surfactants can enhance the adsorption of metal ions by ion exchangers by reducing the interfacial tension between the sorbent and solution (Varshney et al. 2007, 2008). As Fig. 5a, b show, the addition of SDBS caused an obvious decrease in the surface tension of the solution, which may cause a reduction in the interfacial tension between SB300 and the solution phase. This may be one reason for the sorption promotion of Cr(III). However, for Cr(VI), the effect of SDBS on pH was more critical. As the concentration of SDBS increased from 0 to 400 mg·L−1, the pH of the Cr(VI) solution increased from 5 to 7. As illustrated in 3.1 (Fig. 1d), an increased pH also inhibited the removal of Cr(VI) by SB300.
Effect of TX-100 on the sorption of chromium by biochar
The selected nonionic surfactant TX-100 is widely used in the chemical, pulp and paper, textile, and other industries. In the experiment, the sorption capacity of SB300 for Cr(III) was remarkably enhanced by TX-100, and the extent of the enhancement increased with the increasing concentration of TX-100 (Fig. 3c). At the same Cr(III) equilibrium concentration, the sorption of Cr(III) by SB300 increased by approximately threefold when the coexisting TX-100 was 200 mg L−1. For Cr(VI), TX-100 showed a promotion at low concentrations but inhibition at high concentrations for the removal of Cr(VI) by SB300 (Fig. 3d). The promotion effect increased with the increasing concentration of TX-100 from 0 to 50 mg L−1, and then, the promotion effect decreased as TX-100 further increased from 50 to 100 mg L−1. When the concentration reached 200 mg L−1, TX-100 inhibited the removal of Cr(VI) by SB300.
The sorption of TX-100 by SB300 was much lower than that of SDBS (Fig. 4), and no obvious changes in the structure of biochar were found before and after the sorption treatment in the FTIR spectra. This suggested that the direct interaction between TX-100 and biochar was almost negligible. The monomers and micelles of TX-100 in the aqueous phase rather than TX-100 sorbed onto the biochar have a major influence on the sorption behavior of Cr(VI) and Cr(III).
For Cr(III), the reduction of interfacial tension between SB300 and the solution phase was the main reason for the sorption promotion. As Fig. 5c, d show, the surface tension of the solution was significantly reduced as the concentration of TX-100 increased from 0 to 200 mg L−1. The critical micelle concentration (CMC) of TX-100 is approximately 190 mg L−1 (Table S1). When the concentration was lower than the CMC, TX-100 was mainly in monomer form in the solution. The number of monomers increased as the concentration of TX-100 increased from zero to CMC, which was the primary reason for the decrease in the surface tension.
For Cr(VI), TX-100 had important effects on both the interfacial tension and pH (Fig. 5d). When the concentration of TX-100 was very low (0–50 mg L−1), the surface tension decreased dramatically but the pH increased gently with the increasing concentration of TX-100. The sorption promoting effect due to the decrease in the surface tension was dominant. When the concentration of TX-100 further increased from 50 to 150 mg L−1, the decreasing trend for the surface tension slowed, but the pH increased linearly with the concentration of TX-100. The negative effect of increasing the pH was gradually revealed, and the sorption promotion effect decreased. When the concentration of TX-100 was close to or exceeded the CMC, micelles of TX-100 formed; the number of monomers remained constant, and the surface tension was almost unchanged. At this point, the pH became the key limiting factor for sorption. As a result, the sorption of Cr(VI) onto SB300 was significantly inhibited by 200 mg L−1 TX-100.
Conclusions
The effects and mechanisms of the two surfactants on the removal of chromium by biochar were different. As an anionic surfactant, surface-sorbed SDBS gave biochar negative charges, which improved the electrostatic attraction between biochar and Cr(III) and enhanced the electrostatic repulsion between biochar and the Cr(VI). As a result, SDBS promoted Cr(III) sorption but inhibited Cr(VI) sorption onto SB300. As a nonionic surfactant, the sorption of TX-100 by biochar was negligible, but the monomers and micelles of TX-100 in the aqueous phase had a major influence on the chromium removal. TX-100 reduced the interfacial tension between the biochar and the solution phase, which enhanced the removal of Cr(III) by SB300. For Cr(VI), due to the opposite effects of TX-100 on the interfacial tension and pH, low concentrations (< CMC) of TX-100 promoted the sorption of Cr(VI) by SB300, but high concentrations (> CMC) of TX-100 inhibited the sorption of Cr(VI) by SB300. The results confirmed that the coexisting surfactants have a great impact on the biochar sorption of heavy metals. And suggest that when biochar is used to treat heavy metal wastewater containing coexisting surfactants, such as electroplating, textile, and dyeing wastewater, the type and concentration of surfactants must be considered as important factors. Under certain surfactant conditions, biochar, as a low-cost sorbent, will enable the simultaneous and efficient removal of heavy metals and surfactants.
References
Ahmad M, Rajapaksha AU, Lim JE et al (2013) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99:19–33
Ahmed M, Zhou JL, Ngo HH et al (2016) Insight into biochar properties and its cost analysis. Biomass Bioenergy 84:76–86
Albadarin AB, Mangwandi C, Al-Muhtaseb A et al (2012) Kinetic and thermodynamics of chromium ions adsorption onto low-cost dolomite adsorbent. Chem Eng J 179:193–202
Aliabadi M, Khazaei I, Fakhraee H, Mousavian MTH (2012) Hexavalent chromium removal from aqueous solutions by using low-cost biological wastes: equilibrium and kinetic studies. Int J Environ Sci Technol 9:319–326
Aoudia M, Allal N, Djennet A, Toumi L (2003) Dynamic micellar enhanced ultrafiltration: use of anionic (SDS)–nonionic(NPE) system to remove Cr3+ at low surfactant concentration. J Membr Sci 217:181–192
Aydın YA, Aksoy ND (2009) Adsorption of chromium on chitosan: optimization, kinetics and thermodynamics. Chem Eng J 151:188–194
Babel S, Kurniawan TA (2003) Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard Mater 97:219–243
Babel S, Kurniawan TA (2004) Cr(VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan. Chemosphere 54:951–967
Baek K, Yang JW (2004) Competitive bind of anionic metals with cetylpyridinium chloride micelle in micellar-enhanced ultrafiltration. Desalination 167:101–110
Beesley L, Moreno-Jimenez E, Gomez-Eyles JL et al (2011) A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ Pollut 159:3269–3282
Chen BL, Chen ZM (2009) Sorption of naphthalene and 1-naphthol by biochars of orange peels with different pyrolytic temperatures. Chemosphere 76:127–133
Cheung KH, Gu JD (2003) Reduction of chromate (CrO4(2-)) by an enrichment consortium and an isolate of marine sulfate-reducing bacteria. Chemosphere 52:1523–1529
Choi HD, Cho JM, Baek K, Yang JS, Lee JY (2009) Influence of cationic surfactant on adsorption of Cr(VI) onto activated carbon. J Hazard Mater 161:1565–1568
Dobrowolski R, Otto M (2010) Study of chromium(VI) adsorption onto modified activated carbons with respect to analytical application. Adsorption 16:279–286
Dong XL, Ma LQ, Li YC (2011) Characteristics and mechanisms of hexavalent chromium removal by biochar from sugar beet tailing. J Hazard Mater 190:909–915
Esumi K, Ueno M (1997) Structure-performance relationships in surfactants. Marcel Dekker, New York
Gupta VK, Rastogi A (2009) Biosorption of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions. J Hazard Mater 163:396–402
Han YT, Cao X, Ouyang X, Sohi SP, Chen J (2016) Adsorption kinetics of magnetic biochar derived from peanut hull on removal of Cr (VI) from aqueous solution: effects of production conditions and particle size. Chemosphere 145:336–341
Ho YS (2006) Review of second-order models for adsorption systems. J Hazard Mater 136:681–689
Kobya M (2004) Removal of Cr(VI) from aqueous solutions by adsorption onto hazelnut shell activated carbon: kinetic and equilibrium studies. Bioresour Technol 91:317–321
Legrouri K, Khouya E, Hannache H et al (2017) Activated carbon from molasses efficiency for Cr(VI), Pb(II) and Cu(II) adsorption: a mechanistic study. Chem Int 3(3):301–310
Lu L, Zhu LZ (2011) Effect of soil components on the surfactant-enhanced soil sorption of PAHs. J Soils Sediments 12:161–168
Ma L, Xi Y, He H, Ayoko GA, Zhu R, Zhu J (2016) Efficiency of Fe–montmorillonite on the removal of Rhodamine B and hexavalent chromium from aqueous solution. Appl Clay Sci 120:9–15
Marousek J (2014) Significant breakthrough in biochar cost reduction. Clean Technol Environ 16:1821–1825
Maroušek J, Hašková S, Zeman R, Žák J, Vaníčková R, Maroušková A, Váchal J, Myšková K (2015a) Techno-economic assessment of processing the cellulose casings waste. Clean Technol Environ 17:2441–2446
Maroušek J, Maroušková A, Myšková K, Váchal J, Vochozka M, Žák J (2015b) Techno-economic assessment of collagen casings waste management. Int J Environ Sci Technol 12:3385–3390
Marousek J, Vochozka M, Plachy J et al (2017) Glory and misery of biochar. Clean Technol Environ 19:311–317
Ming H, Zhang S, Pan B et al (2012) Heavy metal removal from water/wastewater by nanosized metal oxides: a review. J Hazard Mater 211-212:317–331
Mohan D, Sarswat A, OK YS et al (2014) Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—a critical review. Bioresour Technol 160:191–202
Monser L, Adhoum N (2002) Modified activated carbon for the removal of copper, zinc, chromium and cyanide from wastewater. Sep Purif Technol 26:137–146
Song W, Shi T, Yang D, Ye J, Zhou Y, Feng Y (2015) Pretreatment effects on the sorption of Cr(VI) onto surfactant-modified zeolite: mechanism analysis. J Environ Manag 162:96–101
Tan X, Liu Y, Zeng G, Wang X, Hu X, Gu Y, Yang Z (2015) Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 125:70–85
Tiravanti G, Petruzzelli D, Passino R (1997) Pretreatment of tannery wastewaters by an ion exchange process for Cr(III) removal and recovery. Water Sci Technol 36:197–207
Tytłak A, Oleszczuk P, Dobrowolski R (2015) Sorption and desorption of Cr(VI) ions from water by biochars in different environmental conditions. Environ Sci Pollut Res 22:5985–5994
Valdés H, Sánchez-Polo M, Rivera-Utrilla J, Zaror CA (2002) Effect of ozone treatment on surface properties of activated carbon. Langmuir 18:2111–2116
Varshney KG, Rafiquee MZA, Somya A (2007) Effect of surfactants on the adsorption behaviour of tin(IV) phosphate, cation exchanger for alkaline earths and heavy meal ions. Colloid Surface A 301:224–228
Varshney KG, Rafiquee MZA, Somya A (2008) Triton X-100 based cerium(IV) phosphate as a new hg(II) selective, surfactant based fibrous ion exchanger: synthesis, characterization and adsorption behaviour. Colloid Surface A 317:400–405
Wang C, Gu L, Liu X, Zhang X, Cao L, Hu X (2016) Sorption behavior of Cr(VI) on pineapple-peel-derived biochar and the influence of coexisting pyrene. Int Biodeter Biodegr 111:78–84
Warchoł J, Misaelides P, Petrus R (2006) Preparation and application of organo-modified zeolitic material in the removal of chromates and iodides. J Hazard Mater 137:1410–1416
Wong YC, Szeto YS, Cheung WH, McKay G (2004) Adsorption of acid dyes on chitosan—equilibrium isotherm analyses. Process Biochem 39:695–704
Yang P, Guo D, Chen Z, Cui B, Xiao B, Liu S, Hu M (2017) Removal of Cr (VI) from aqueous solution using magnetic biochar synthesized by a single step method. J Disper Sci Technol 38:1665–1674
Zeng Y, Woo H, Lee G, Park J (2010) Removal of chromate from water using surfactant modified Pohang clinoptilolite and Haruna chabazite. Desalination 257:102–109
Zhao N, Zhao C, Lv Y, Zhang W, du Y, Hao Z, Zhang J (2017) Adsorption and coadsorption mechanisms of Cr(VI) and organic contaminants on H3PO4 treated biochar. Chemosphere 186:422–429
Zhou L, Liu Y, Liu S, Yin Y, Zeng G, Tan X, Hu X, Hu X, Jiang L, Ding Y, Liu S, Huang X (2016) Investigation of the adsorption-reduction mechanisms of hexavalentchromium by ramie biochars of different pyrolytic temperatures. Bioresour Technol 218:351–359
Acknowledgements
This work was supported by the National Natural Science Foundation of China (41301327, 41501521 and 21407037).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Guilherme L. Dotto
Electronic supplementary material
ESM 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Chai, Q., Lu, L., Lin, Y. et al. Effects and mechanisms of anionic and nonionic surfactants on biochar removal of chromium. Environ Sci Pollut Res 25, 18443–18450 (2018). https://doi.org/10.1007/s11356-018-1933-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1933-2