Abstract
Antimicrobials have been widely used in food animals for growth promotion since the 1950s. Antimicrobial resistance emerges in animal production settings and frequently spreads to humans through the food chain and direct contact. There have been international efforts to restrict or ban antimicrobials used for both humans and animals. Denmark has taken positive strides in the development of a comprehensive database DANMAP to track antimicrobial usage and resistance. Although food animals are sources of antimicrobial resistance, there is little evidence that antimicrobial resistance originates from food animals. This review comprehensively introduces the history and trends of antimicrobial use, the emergence and spread of antimicrobial resistance in food animals provides suggestions to tackle the problems of the spread of antimicrobial resistance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The World Health Organization (WHO) has recognized antimicrobial resistance as one of the top three major threats to public health. The use of antimicrobials in livestock feed has been a major factor in the emergence and spread of antimicrobial resistance. Increased surveillance of antimicrobial use and bacterial resistance is necessary to lessen the impact of excessive and unregulated use of antimicrobials in food animals. Nonetheless, antimicrobials are effective tools to prevent and treat disease and have had profound effects on morbidity and mortality due to bacterial infections.
History of antimicrobial use in food animals
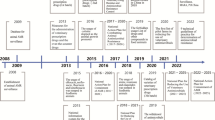
The original use of antimicrobials since the World War II was in large-scale efforts to treat human diseases. However, the use of antimicrobials as growth promoters in livestock has resulted in an ever-increasing threat to both human and animal health. Penicillin was extensively used during the World War II and veterinarians used a penicillin preparation to treat bovine mastitis (Gustafson and Bowen 1997). As early as 1946, growth improvements in chicks were documented after feeding streptomycin (Moore et al. 1946). The commercial implications were soon recognized as improvements in the growth performance of chickens and swine that were fed chlortetracycline (Aureomycin) (Stokstad et al. 1949; Jukes et al. 1950) (Fig. 1). In the 1950’s, similar positive effects of other antimicrobials on food animals including chickens, swine, and cattle were observed. The Food and Drug Administration (FDA) in the USA approved the use of antimicrobial growth promoters (AGP) in animals in 1951.
The widespread use of antimicrobials in the animal feed surged as manufacturing and production costs decreased. Subtherapeutic doses enhanced animal growth rates and feed efficiency while reducing mortality (Teillant and Laxminarayan 2015). Antimicrobial consumption by animals is currently twice than that used by humans (WHO 2012). The global average consumption equals 172, 148, and 45 mg for every kilogram of pig, chicken, and cattle mass, respectively. The total global consumption of antimicrobials in food animals was 63,151 tons in 2010 with a 67% projected increase by 2030 (Van Boeckel et al. 2015). In the USA, approximately 80% of all antimicrobials are used in food animals primarily at subtherapeutic doses and 14,600 tons were sold for animal use in 2012 (FDA 2010 and 2014). China is the world’s largest producer and consumer of antimicrobials and uses about 97, 000 tons in animal agriculture annually (Hu and Cheng 2014).
A European Union joint commission of antimicrobial use in agriculture reported that human and animal usage in the EU is currently 3821 and 8927 tons, respectively (European Medicines Agency 2017). The penicillins, macrolides, and fluoroquinolones were the most frequently used classes for human consumption and the tetracyclines, penicillins, and sulfonamides were the most frequent for animals. The average usage of the 3rd- and 4th-generation cephalosporins in people and livestock was 3.8 and 0.2 mg/kg, respectively. They are effective against both gram-positive and gram-negative bacteria, including Escherichia (E.) coli, Salmonella (S.) Enteritidis, S. Typhimurium, monophasic S. Typhimurium, and S. Infantis. The WHO classifies these antimicrobials as highest priority critically important antimicrobials (CIA). Cephalosporin resistance in invasive E. coli from humans was first reported in 2013 and was linked to the overuse of these antimicrobials (European Medicines Agency 2017).
Emergence of antimicrobial resistance
Extensive use of antimicrobials in animal production comes as a cost: it promotes antimicrobial resistance that threatens both animal health and human health. One of the first observations of resistance in animals was the report on streptomycin resistance of coliform bacteria from turkeys fed streptomycin in 1951 (Starr and Reynolds 1951). The association of resistance to tetracycline in chickens fed this antimicrobial to promote growth was reported in the late 1950s (Barnes 1958; Elliott and BARNES 1959) (Fig. 1). Antimicrobial resistance in food animals has a great impact of animal health and may be associated with resistant infections in humans. Multiple drug-resistant pathogens emerged worldwide in the 1980s. In the USA, more than two million infections and 23,000 deaths are caused by antimicrobial-resistant pathogens annually (Hampton 2013). In Europe, 25,000 people die annually as a consequence of multidrug-resistant bacterial infections at a cost of €1.5 billion (Davies 2013). Resistance to antimicrobial agents is a significant global threat to public health (WHO 2014).
Mechanisms of antimicrobial resistance
Antimicrobials used in livestock are major drivers of spread of antimicrobial resistance gene (ARG) and particularly for those antimicrobials administered at subtherapeutic levels (Xiong et al. 2018; Kanwar et al. 2014). Antimicrobials in feed select for the corresponding ARG and increase the appearance of ARGs unrelated to resistance for the administered antimicrobials (Looft et al. 2012). This “cross-selection” of ARGs is an important parameter contributing to antimicrobial resistance as well as the corresponding evolution of genetic and gene transfer mechanisms (Oz et al. 2014). Antimicrobials when present even at infinitesimally low concentrations can maintain resistant phenotypes. Feed additives containing copper, zinc, and arsenic also provide co-selective pressures on ARGs in animal production settings. Copper and zinc are feed additives and possess antimicrobial properties. The combination of antimicrobials and metals in a polluted environment near animals might be sufficient to select for bacteria harboring multi-resistance plasmids (Gullberg et al. 2014).
The animal gut is a reservoir but not the origin of antimicrobial resistance
Intestinal tracts of food animals serve as important ARG reservoirs. However, these ARGs are present in healthy animals regardless of prior antimicrobial exposure (Looft et al. 2012). Genes for resistance to tetracycline, sulfonamides, quinolones, β-lactams, aminoglycosides, and vancomycin have all been identified in animals (Zhu et al. 2013; Wichmann et al. 2014). Indeed, even with a global ban on chloramphenicol in animal feed, chloramphenicol resistance genes have been detected on farms and in feedlots (He et al. 2014; Li et al. 2013).
Antimicrobial-resistant bacteria and corresponding ARGs are continually circulating in plants, soil, and animals. Resistant pathogens and ARGs can pass to humans via foodborne routes as well as through contaminated soil, water, crops, and animal protein (Xiong et al., 2015a, 2015b). There are numerous examples of the presence of antimicrobial-resistant bacteria in animal production settings. These include vancomycin-resistant Enterococcus (VRE), methicillin-resistant Staphylococcus aureus (MRSA), extended-spectrum β-lactamase (ESBL) producing bacteria, multidrug-resistant E. coli and Salmonella (Barton 2014). Importantly, ARG types such as KPC-2 (Klebsiella pneumoniae carbapenemase-2), NDM-1 (New Delhi metallo-β-lactamase-1) and MCR-1 (plasmid-mediated colistin resistance) in food animals and on farms pose grave threats to human and animal health (Liu et al. 2016; Munoz-Price et al. 2013; Wang et al. 2017).
Although animals are important ARG reservoirs, there is not enough evidence to conclude that clinical ARGs originated from animals (Allen 2014; Mather et al. 2013). The identical gene sequences found in animals and humans illustrate that the same genetic conduit can be open to more than one microorganism. For example, this process was documented between Bacteroides spp. from humans and Prevotella ruminicola found in the rumen and intestines of livestock (Durso and Cook 2014). Livestock-associated bacteria can also contribute to clinical resistance without directly causing human infection and zoonotic transmission of resistant bacteria that occurs frequently (Smillie et al. 2011). Human extraintestinal-expanded spectrum cephalosporin-resistant E. coli infections have originated from food animals particularly from poultry (Lazarus et al. 2015). High-density livestock production has been associated with human MRSA infections and zoonotic transmission was confirmed (Casey et al. 2013; Casey et al. 2014; Harrison et al. 2013). However, there is much less evidence for food animals as the original sources of bacterial resistance in humans (Bonten and Mevius 2015). The CTX-M-5 ESBL gene from the human and animal commensal Kluyvera ascorbata was originated in humans (Humeniuk et al. 2002). Additionally, although MRSA isolated from animals were sporadically reported before 2005, several MRSA strains associated with animals originated from humans (Smith and Pearson 2011; Lowder et al. 2009).
Technical advances in analysis methods have also increased awareness of animal ARG reservoirs. Whole genome sequencing is currently an economical alternative to traditional culture-based methods and has become a powerful epidemiological tool. For example, the recent clonal transmission of cephalosporin-resistant E. coli from poultry to humans was disproven using whole genome sequencing (de Been et al. 2014). This data challenged current views on the contribution of local animal reservoirs as sources of S. Typhimurium (Mather et al. 2013). In addition, the same resistant bacterial strains circulating between animals and humans may be genetically distinct. Therefore, food animals are not necessarily the origins of antimicrobial resistance in humans (Hunter et al. 2010; Allen 2014). The natural environments may contain the original ARG sources since antimicrobial resistance is a ubiquitous and ancient phenotype (D’Costa et al. 2011; Finley et al. 2013). The soil “resistome” is a natural ecological phenomenon that predates the era of antimicrobial use in humans and animals (Blair et al. 2015; D’Costa et al. 2011). The soil environment has the largest apparent diversity of ARG determinants especially among complex samples such as animal manure, activated sludge, and human feces (Nesme et al. 2014).
However, there is evidence that correlates antimicrobial use in food animals to increase antimicrobial-resistant bacteria in humans and is well-recognized (Schechner et al. 2013). Antimicrobials used in food animals promote the emergence and persistence of specific resistant bacteria. The level of the use of a specific antimicrobial was strongly correlated with the level of corresponding resistance in commensal E. coli isolates in swine, poultry, and cattle (Chantziaras et al. 2014). Metagenomic data at the population level also demonstrated that antimicrobial use in animals contributes to resistance development in human commensal bacteria (Forslund et al. 2013). Third-generation cephalosporins used in poultry may contribute to human deaths caused by resistant E. coli (Collignon et al. 2013). However, this evidence does not prove that clinical ARGs originated from animals. It is difficult to conclude that antimicrobial use in animals has caused the emergence, spread, and persistence of ARGs in humans. This is due to the complexity of the transmission pathways involved in livestock-human, human-human, and human-livestock spread.
Drivers of antimicrobial resistance
Horizontal gene transfer is an important driver of antimicrobial resistance and occurs between different bacterial species via mobile genetic elements such as plasmids, integrases, and transposases (Gaze et al. 2011). Such transfer can occur between animal bacteria and between animal bacteria and human pathogens. Class 1 integrons involved in the transfer of sul resistance genes has been demonstrated in swine feedlots and natural rivers (Chen et al. 2015; Hsu et al. 2014). Horizontal transfer of ARGs can be enhanced by increased levels of antimicrobials and metals in animal feedlots (Zhu et al. 2013).
Vertical transmission of antimicrobial resistance through imported chickens is also a concern. For example, an extended spectrum cephalosporinase-producing E. coli was prevalent in animals originating from a country with only limited antimicrobial and no cephalosporin use. The bacterial resistance was transferred with imported animals (Nilsson et al. 2014; Agersø et al. 2014). This phenomenon provides direct evidence for the international spread of antimicrobial resistance and emphasizes the importance of international standards restricting the use of antimicrobials in food animals.
Spread of antimicrobial resistance
Several vehicles contribute to the spread of antimicrobial resistance from animal feedlots to the environment. Soil, water, crops, and animal protein (e.g., meat, milk, and fish) are the traditional physical vehicles. Recently, livestock insects have spread ARGs from animal farms to urban environments (Zurek and Ghosh 2014). Bacteriophages are most likely the prime molecular vehicles for ARG transfer in the environment (Balcazar 2014). In addition, airborne particulate matter derived from beef cattle feedlots has facilitated ARG spread (McEachran et al.2015). Compared with traditional vehicles, these emerging vehicles may pose higher risks to human health since control is problematic (Fig. 2).
Humans can be exposed to antimicrobial resistance via the food chain through consuming contaminated water, crops, seafood, and animal protein. Exposure can also occur through direct contact in recreational and agricultural activities such as swimming, plowing, and sowing (Wellington et al. 2013; Xiong et al. 2015c) (Fig. 2). Farmers and animal feedlot workers are at higher risk to acquire antimicrobial resistance than the general population. It is possible that horizontal ARG transfer from environmental microbes to human commensals and pathogens occurs and leads to resistant infections in the clinic (Singer and Williams-Nguyen 2014). The rate of routine acquisition of antimicrobial resistance from foodstuffs and the environment must be reduced to control ARG transfer.
National and international preventive strategies to combat antimicrobial resistance
Several countries have restricted or banned the use of antimicrobials in food animals (Table 1). In 1969, recommendations for the restriction of subtherapeutic use of antimicrobials in animal feeds were discussed due to oxytetracycline-resistant S. enterica found in food animals (Swann 1969). Following this, Great Britain restricted the use of some antimicrobials in feed. The use of AGPs was first banned by Sweden in 1986, followed by Denmark, the UK, and EU countries (Fig. 1). In 1997, the EU banned all agricultural use of avoparcin due to the prevalence of vancomycin-resistant Enterococcus (VRE) in patients in Europe. In 1999, EU officials imposed a ban on the use of bacitracin, spiramycin, tylosin, and virginiamycin as AGPs because they are used in human medicine. In 2006, the EU withdrew the use of all AGPs based on a cautionary principle.
The FDA in the USA has historically approved antimicrobials for use in food animals (Allen and Stanton 2014). This body initiated guidelines for the industry to voluntarily withdraw medically important antimicrobials for use as AGPs in 2012. China banned the nontherapeutic use of 150 medically important antimicrobials in food animals (Hu and Cheng 2014). In addition, China banned colistin use as an AGP in 2016 due to the emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals first reported by Chinese scientists (Liu et al. 2016).
Denmark, a representative country banning AGP use has gained some success in lessening the rate of ARG transfer. A > 50% reduction in the usage of antimicrobials per kilogram of pigs was achieved from 1992 to 2008 while the over-all productivity increased (Levy 2014). The levels of VRE markedly declined after avoparcin was banned (Agersø et al. 2011). Levels of extended spectrum cephalosporinase-producing bacteria were also effectively reduced after a voluntary ban on cephalosporin in Danish pig production (Agersø and Aarestrup 2013). Furthermore, DANMAP (www.danmap.org) was established as a comprehensive surveillance system to track antimicrobial usage and resistance in animals, food products, and humans (Aarestrup 2012). Although resistance of key zoonotic bacteria has not decreased, the total consumption of antimicrobials in food animals has declined (Jensen and Hayes 2014). This is an example of solid progress in reducing the prevalence of antimicrobial resistance (Levy 2014).
However, the Denmark model may be difficult for other countries to duplicate. In most countries, animal production still depends heavily on widespread use of antimicrobials. An acute and complete ban on antimicrobial use in food animals would have serious consequences on animal health, productivity, and welfare as well as food prices (Woolhouse et al. 2015). For example, a US ban of AGPs in swine and chickens would result in higher costs for the consumer and producer, including an estimated 4.5% increase of overall pig production costs (Hayes and Jensen 2003). Animal nutrition and hygiene management should be considered when implementing an AGP ban. Improvements of hygiene and feed management can reduce the harmful effects of such a ban on animal health and productivity (Larson 2015; Teillant and Laxminarayan 2015). Economic impacts would be potentially higher in poorer countries with less developed hygiene and production practices, while it could be limited in industrialized countries with optimized production systems (Laxminarayan et al. 2015).
Better surveillance is an essential feature of antimicrobial resistance control. Global monitoring systems such as the U.S. National Antimicrobial Resistance Monitoring System for Enteric Bacteria and the European Antimicrobial Resistance Surveillance Network are making efforts to collect antimicrobial consumption and antimicrobial resistance information (Grundmann et al. 2011). The Food and Agriculture Organization (FAO), the World organization for Animal Health (OIE), and the WHO are cooperating to control antimicrobial use in animals (Maron et al. 2013). However, local national efforts are disconnected and patchy (Woolhouse and Farrar 2014). DANMAP is a comprehensive surveillance system that may be as a good model for other countries. It is urgent that counties establish such programs in collaboration with international agencies.
The global trend of increasing antimicrobial use can be limited by prudent application in both humans and animals. The development of new drugs should be enhanced worldwide, but the discovery of novel antimicrobials is not a sustainable strategy due to the increasing technical difficulties and a lack of novel compounds. Several antimicrobial alternatives such as vaccines, phage therapy, and pre- and probiotics are in the developmental stages but they may not be able to cover the antimicrobial spectrum of antimicrobials in food animals (Woolhouse and Farrar 2014; Allen et al. 2013). For example, although the use of vaccines has a long history, vaccines are not widely used as alternatives to antimicrobials due to the increased cost and technical difficulties in vaccination of food animals.
Although antimicrobial use in animals varies by country, those listed as “critically important” by the OIE include representatives of all major classes of antimicrobials used in human medicine and should be restricted or banned globally. In addition, antimicrobials that co-select or cross select for clinical antimicrobial resistance should be not used in food animals. For example, ceftiofur and cefquinome should be banned in animals due to the emergence of animal sources of ESBL.
Issues and perspectives
The establishment of standard diagnostic approaches for ARG identification will allow a more comprehensive view of global threats to the dissemination of antimicrobial resistance. Metagenomic analyses based on modern sequencing technologies are a promising approach if sample collection, nucleic acid extraction, and sequence assembly steps are standardized. We should also strive to identify indicators of environmental ARG pollution. Bacterial indicators, such as E. coli and fecal Enterococci are already used to assess environmental quality. However, there needs to be a consensus on accepted indicators for resistance determinants. Finally, the magnitude of the threat risk of antimicrobial resistance to public health must be assessed. Although animals are of important reservoirs of antimicrobial resistance that threaten human health, the magnitude of the risk to public health via different exposure pathways such as the food chain and direct contact is still unknown, and needs to be quantified. Management options such as the judicious or prudent use of antimicrobials, good management of animal health and effective treatment of manure containing antimicrobials could aid in mitigating risks of antimicrobial resistance released from farms. However, bearing in mind that antimicrobial resistance is global and cross-sectorial, effective action on tackling antimicrobial resistance requires a coordinated response from agriculture, industry, and governments as well as international agencies. It also requires collaboration between pharmacists, farmers, veterinarians, physicians, and patients.
Discussion and conclusions
The relationship between antimicrobial usage and antimicrobial resistance for many types of pathogens is complex. The use of antimicrobials on farms is linked to ARG emergence but whether animals are the sources of ARG transfer to human pathogens is an open question. ARG exposure is not only a food safety problem but also a problem involving public health and environmental exposure through air, soil, and water. Furthermore, it is crucial to establish a diagnostic standard such as whole genome sequencing for ARG detection.
There are two measures to control antimicrobial resistance: (1) Reduction of the antimicrobial volumes currently used by humans and livestock and limiting the use of broad spectrum and critically important antimicrobials, (2) Prohibiting the dumping of antimicrobial waste into the environment and eliminating antimicrobial residues that exceed the standard limit in foods and water. We need the assistance from all sectors that use antimicrobials to control antimicrobial use and to limit the spread of antimicrobial-resistant bacteria locally and internationally.
References
Aarestrup F (2012) Sustainable farming: get pigs off antibiotics. Nature 486(7404):465–466
Agersø Y, Aarestrup FM (2013) Voluntary ban on cephalosporin use in Danish pig production has effectively reduced extended-spectrum cephalosporinase-producing Escherichia coli in slaughter pigs. J Antimicrob Chemother 68(3):569–572
Agersø Y, Hald T, Helwigh B, Høg BB, Jensen LB, Jensen VF, Korsgaard H, Larsen LS, Seyfarth AM, Struve T (2011) DANMAP 2011: use of antimicrobial agents and occurrence of antimicrobial resistance in Bacteria from food animals, foods and humans in Denmark. The Danish Integrated Antimicrobial Resistance Monitoring and Research Program, Copenhagen Available: http://goo.gl/XoxPPm [accessed 20 May 2014]
Agersø Y, Jensen JD, Hasman H, Pedersen K (2014) Spread of extended spectrum cephalosporinase-producing Escherichia coli clones and plasmids from parent animals to broilers and to broiler meat in a production without use of cephalosporins. Foodborne Pathog Dis 11(9):740–746
Allen HK (2014) Antibiotic resistance gene discovery in food-producing animals. Curr Opin Microbiol 19:25–29
Allen HK, Stanton TB (2014) Altered egos: antibiotic effects on food animal microbiomes. Annu Rev Microbiol 68:297–315
Allen HK, Levine UY, Looft T, Bandrick M, Casey TA (2013) Treatment, promotion, commotion: antibiotic alternatives in food-producing animals. Trends Microbiol 21(3):114–119
Balcazar JL (2014) Bacteriophages as vehicles for antibiotic resistance genes in the environment. PLoS Pathog 10(7):e1004219
Barnes EM (1958) The effect of antibiotic supplements on the faecal streptococci (Lancefield group D) of poultry. Br Vet J 114:333–344
Barton MD (2014) Impact of antibiotic use in the swine industry. Curr Opin Microbiol 19:9–15
de Been M, Lanza VF, de Toro M, Scharringa J, Dohmen W, Du Y, Hu J, Lei Y, Li N, Tooming-Klunderud A (2014) Dissemination of cephalosporin resistance genes between Escherichia coli strains from farm animals and humans by specific plasmid lineages. PLoS Genet 10(12):e1004776
Blair JM, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJ (2015) Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol 13(1):42–51
Bonten M, Mevius D (2015) Less evidence for an important role of food-producing animals as source of antibiotic resistance in humans. Clini Infect Dis: Off Publ Infect Dis Soc Am 60:1867
Casey JA, Curriero FC, Cosgrove SE, Nachman KE, Schwartz BS (2013) High-density livestock operations, crop field application of manure, and risk of community-associated methicillin-resistant Staphylococcus aureus infection in Pennsylvania. JAMA Intern Med 173(21):1980–1990
Casey JA, Shopsin B, Cosgrove SE, Nachman KE, Curriero FC, Rose HR, Schwartz BS (2014) High-density livestock production and molecularly characterized MRSA infections in Pennsylvania. Environ Health Perspect 122(5):464–470
Chantziaras I, Boyen F, Callens B, Dewulf J (2014) Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: a report on seven countries. J Antimicrob Chemother 69(3):827–834
Chen B, Liang X, Nie X, Huang X, Zou S, Li X (2015) The role of class I integrons in the dissemination of sulfonamide resistance genes in the Pearl River and Pearl River Estuary, South China. J Hazard Mater 282:61–67
Cogliani C, Goossens H, Greko C (2011) Restricting antimicrobial use in food animals: lessons from Europe. Microbe 6(6):274
Collignon P, Aarestrup FM, Irwin R, McEwen S (2013) Human deaths and third-generation cephalosporin use in poultry, Europe. Emerg Infect Dis 19(8):1339–1340
D’Costa VM, King CE, Kalan L, Morar M, Sung WW, Schwarz C, Froese D, Zazula G, Calmels F, Debruyne R (2011) Antibiotic resistance is ancient. Nature 477(7365):457–461
Davies S (2013) Annual report of the chief medical officer, volume two, 2011, infections and the rise of antimicrobial resistance. Department of Health, London
Durso LM, Cook KL (2014) Impacts of antibiotic use in agriculture: what are the benefits and risks? Curr Opin Microbiol 19:37–44
Elliott S, BARNES EM (1959) Changes in serological type and antibiotic resistance of Lancefield group D streptococci in chickens receiving dietary chlortetracycline. J Gen Microbiol 20(2):426–433
European Medicines Agency (EMA) (2017) ECDC/EFSA/EMA second joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2017/07/WC500232336.pdf
Finley, R. L., P. Collignon, D. J. Larsson, S. A. McEwen, X.-Z. Li, W. H. Gaze, R. Reid-Smith, M. Timinouni, D. W. Graham, and E. Topp. 2013. The scourge of antibiotic resistance: the important role of the environment. Clinical infectious diseases: cit355
Food and Drug Administration. 2010. CVM Updates-CVM Reports on Antimicrobials Sold or Distributed for Food-Producing Animals (Food Drug Admin, Silver Spring. MD). Available at www.fda.gov/AnimalVeterinary/NewsEvents/CVMUpdates/ucm236143.htm.Accessed March 10, 2015
Food and Drug Administration. 2014. FDA annual summary report on antimicrobials sold or distributed in 2012 for use in food-producing animals. Available online: http://www.fda.gov/AnimalVeterinary/NewsEvents/CVMUpdates/ucm416974.htm
Food and Drug Administration. 2017. FDA Announces Implementation of GFI #213, Outlines Continuing Efforts to Address Antimicrobial Resistance. Available online: https://www.fda.gov/AnimalVeterinary/NewsEvents/CVMUpdates/ucm535154.htm
Forslund K, Sunagawa S, Kultima JR, Mende DR, Arumugam M, Typas A, Bork P (2013) Country-specific antibiotic use practices impact the human gut resistome. Genome Res 23(7):1163–1169
Gaze WH, Zhang L, Abdouslam NA, Hawkey PM, Calvo-Bado L, Royle J, Brown H, Davis S, Kay P, Boxall AB (2011) Impacts of anthropogenic activity on the ecology of class 1 integrons and integron-associated genes in the environment. ISME J 5(8):1253–1261
Grundmann H, Klugman KP, Walsh T, Ramon-Pardo P, Sigauque B, Khan W, Laxminarayan R, Heddini A, Stelling J (2011) A framework for global surveillance of antibiotic resistance. Drug Resist Updat 14(2):79–87
Gullberg E, Albrecht LM, Karlsson C, Sandegren L, Andersson DI (2014) Selection of a multidrug resistance plasmid by sublethal levels of antibiotics and heavy metals. MBio 5(5):e01918-01914
Gustafson R, Bowen R (1997) Antibiotic use in animal agriculture. J Appl Microbiol 83(5):531–541
Hampton T (2013) Report reveals scope of US antibiotic resistance threat. JAMA 310(16):1661–1663
Harrison EM, Paterson GK, Holden MT, Larsen J, Stegger M, Larsen AR, Petersen A, Skov RL, Christensen JM, Zeuthen AB (2013) Whole genome sequencing identifies zoonotic transmission of MRSA isolates with the novel mecA homologue mecC. EMBO Mol Med 5(4):509–515
Hayes, D. J., and H. H. Jensen. 2003. Lessons from the Danish ban on feed-grade antibiotics
He L-Y, Liu Y-S, Su H-C, Zhao J-L, Liu S-S, Chen J, Liu W-R, Ying G-G (2014) Dissemination of antibiotic resistance genes in representative broiler feedlots environments: identification of indicator ARGs and correlations with environmental variables. Environ Sci Technol 48(22):13120–13129
Hsu J-T, Chen C-Y, Young C-W, Chao W-L, Li M-H, Liu Y-H, Lin C-M, Ying C (2014) Prevalence of sulfonamide-resistant bacteria, resistance genes and integron-associated horizontal gene transfer in natural water bodies and soils adjacent to a swine feedlot in northern Taiwan. J Hazard Mater 277:34–43
Hu Y, Cheng H (2014) Research opportunities for antimicrobial resistance control in China’s factory farming. Environ Sci Technol 48(10):5364–5365
Humeniuk C, Arlet G, Gautier V, Grimont P, Labia R, Philippon A (2002) β-Lactamases of Kluyvera ascorbata, probable progenitors of some plasmid-encoded CTX-M types. Antimicrob Agents Chemother 46(9):3045–3049
Hunter PA, Dawson S, French GL, Goossens H, Hawkey PM, Kuijper EJ, Nathwani D, Taylor DJ, Teale CJ, Warren RE (2010) Antimicrobial-resistant pathogens in animals and man: prescribing, practices and policies. J Antimicrob Chemother 65(suppl 1):i3–i17
Jensen HH, Hayes DJ (2014) Impact of Denmark’s ban on antimicrobials for growth promotion. Curr Opin Microbiol 19:30–36
Jukes TH, Stokstad E, Tayloe R, Cunha T, Edwards H, Meadows G (1950) Growth-promoting effect of aureomycin on pigs. Arch Biochem 26:324–325
Kanwar N, Scott HM, Norby B, Loneragan GH, Vinasco J, Cottell JL, Chalmers G, Chengappa MM, Bai J, Boerlin P (2014) Impact of treatment strategies on cephalosporin and tetracycline resistance gene quantities in the bovine fecal metagenome. Sci Rep 4
Larson C (2015) China’s lakes of pig manure spawn antibiotic resistance. Science 347(6223):704–704
Laxminarayan, R., Van Boeckel, T and A. Teillant. 2015. The economic costs of withdrawing antimicrobial growth promoters from the livestock sector
Lazarus B, Paterson DL, Mollinger JL, Rogers BA (2015) Do human extraintestinal Escherichia coli infections resistant to expanded-spectrum cephalosporins originate from food-producing animals? A systematic review. Clin Infect Dis 60(3):439–452
Levy S (2014) Reduced antibiotic use in livestock: how Denmark tackled resistance. Environ Health Perspect 122(6):A160–A165
Li J, Shao B, Shen J, Wang S, Wu Y (2013) Occurrence of chloramphenicol-resistance genes as environmental pollutants from swine feedlots. Environ Sci Technol 47(6):2892–2897
Liu Y-Y, Wang Y, Walsh TR, Yi L-X, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X (2016) Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16(2):161–168
Looft T, Johnson TA, Allen HK, Bayles DO, Alt DP, Stedtfeld RD, Sul WJ, Stedtfeld TM, Chai B, Cole JR (2012) In-feed antibiotic effects on the swine intestinal microbiome. Proc Natl Acad Sci 109(5):1691–1696
Lowder BV, Guinane CM, Zakour NLB, Weinert LA, Conway-Morris A, Cartwright RA, Simpson AJ, Rambaut A, Nübel U, Fitzgerald JR (2009) Recent human-to-poultry host jump, adaptation, and pandemic spread of Staphylococcus aureus. Proc Natl Acad Sci 106(46):19545–19550
Maron DF, Smith TJ, Nachman KE (2013) Restrictions on antimicrobial use in food animal production: an international regulatory and economic survey. Glob Health 9:48
Mather A, Reid S, Maskell D, Parkhill J, Fookes M, Harris S, Brown D, Coia J, Mulvey M, Gilmour M (2013) Distinguishable epidemics of multidrug-resistant Salmonella Typhimurium DT104 in different hosts. Science 341(6153):1514–1517
McEachran AD, Blackwell BR, Hanson JD, Wooten KJ, Mayer GD, Cox SB, Smith PN (2015) Antibiotics, bacteria, and antibiotic resistance genes: aerial transport from cattle feed yards via particulate matter. Environ Health Perspect 123:4
Moore P, Evenson A, Luckey T, McCoy E, Elvehjem C, Hart E (1946) Use of sulfasuxidine, streptothricin, and streptomycin in nutritional studies with the chick. J Biol Chem 165(2):437–441
Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK (2013) Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 13(9):785–796
Nesme J, Cécillon S, Delmont TO, Monier J-M, Vogel TM, Simonet P (2014) Large-scale metagenomic-based study of antibiotic resistance in the environment. Curr Biol 24(10):1096–1100
Nilsson O, Börjesson S, Landén A, Bengtsson B (2014) Vertical transmission of Escherichia coli carrying plasmid-mediated AmpC (pAmpC) through the broiler production pyramid. J Antimicrob Chemother 69(6):1497–1500
Oz, T., A. Guvenek, S. Yildiz, E. Karaboga, Y. T. Tamer, N. Mumcuyan, V. B. Ozan, G. H. Senturk, M. Cokol, and P. Yeh. 2014. Strength of selection pressure is an important parameter contributing to the complexity of antibiotic resistance evolution. Molecular biology and evolution:msu191
Schechner V, Temkin E, Harbarth S, Carmeli Y, Schwaber MJ (2013) Epidemiological interpretation of studies examining the effect of antibiotic usage on resistance. Clin Microbiol Rev 26(2):289–307
Singer RS, Williams-Nguyen J (2014) Human health impacts of antibiotic use in agriculture: a push for improved causal inference. Curr Opin Microbiol 19:1–8
Smillie CS, Smith MB, Friedman J, Cordero OX, David LA, Alm EJ (2011) Ecology drives a global network of gene exchange connecting the human microbiome. Nature 480(7376):241–244
Smith TC, Pearson N (2011) The emergence of Staphylococcus aureus ST398. Vector-Borne and Zoonotic Dis 11(4):327–339
Starr MP, Reynolds DM (1951) Streptomycin resistance of coliform bacteria from turkeys fed streptomycin. Am J Publ Health Nations Health 41(11_Pt_1):1375–1380
Stokstad E, Jukes TH, Pierce J, Page A, Franklin AL (1949) The multiple nature of the animal protein factor. J Biol Chem 180:647–654
Swann, M. M. 1969. Report joint committee on the use of antibiotics in animal husbandry and veterinary medicine: HM stationery office
Teillant A, Laxminarayan R (2015) Economics of antibiotic use in US swine and poultry production. Choices 30(1):1–11
Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, Robinson TP, Teillant A, Laxminarayan R (2015) Global trends in antimicrobial use in food animals. Proc Natl Acad Sci 112(18):5649–5654
Wang Y, Zhang R, Li J, Wu Z, Yin W, Schwarz S, Tyrrell JM, Zheng Y, Wang S, Shen Z (2017) Comprehensive resistome analysis reveals the prevalence of NDM and MCR-1 in Chinese poultry production. Nat Microbiol 2:16260
Wellington EM, Boxall AB, Cross P, Feil EJ, Gaze WH, Hawkey PM, Johnson-Rollings AS, Jones DL, Lee NM, Otten W (2013) The role of the natural environment in the emergence of antibiotic resistance in gram-negative bacteria. Lancet Infect Dis 13(2):155–165
WHO. Antimicrobial Resistance: Global Report on Surveillance 2014. Geneva, Switzerland:World Health Organization (2014). Available: www.who.int/drugresistance/documents/surveillancereport/en/
Wichmann F, Udikovic-Kolic N, Andrew S, Handelsman J (2014) Diverse antibiotic resistance genes in dairy cow manure. MBio 5(2):e01017-01013
Woolhouse M, Farrar J (2014) Policy: an intergovernmental panel on antimicrobial resistance. Nature 509(7502):555–557
Woolhouse M, Ward M, van Bunnik B, Farrar J (2015) Antimicrobial resistance in humans, livestock and the wider environment. Philosophical Trans Royal Soc London B: Biol Sci 370(1670):20140083
World Health Organization. The evolving threat of antimicrobial resistance: options for action (WHO, 2012)
Xiong W, Sun Y, Ding X, Zhang Y, Zhong X, Liang W, Zeng Z (2015a) Responses of plasmid-mediated quinolone resistance genes and bacterial taxa to (fluoro) quinolones-containing manure in arable soil. Chemosphere 119:473–478
Xiong W, Sun Y, Ding X, Wang M, Zeng Z (2015b) Selective pressure of antibiotics on ARGs and bacterial communities in manure-polluted freshwater-sediment microcosms. Front Microbiol 6:194
Xiong W, Sun Y, Zhang T, Ding X, Li Y, Wang M, Zeng Z (2015c) Antibiotics, antibiotic resistance genes, and bacterial community composition in fresh water aquaculture environment in China. Microb Ecol 70:425–432
Xiong W, Wang Y, Sun Y, Ma L, Zeng Q, Jiang X, Li A, Zeng Z, Zhang T (2018) Antibiotic-mediated changes in the fecal microbiome of broiler chickens define the incidence of antibiotic resistance genes. Microbiome 6:34
Zhu Y-G, Johnson TA, Su J-Q, Qiao M, Guo G-X, Stedtfeld RD, Hashsham SA, Tiedje JM (2013) Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc Natl Acad Sci 110(9):3435–3440
Zurek L, Ghosh A (2014) Insects represent a link between food animal farms and the urban environment for antibiotic resistance traits. Appl Environ Microbiol 80(12):3562–3567
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 31702290 and 31672608) and the Natural Science Foundation of Guangdong Province (No. 2016A030311029).
Author information
Authors and Affiliations
Contributions
WX wrote the manuscript. YS and ZZ reviewed and finalized the manuscript.
Corresponding authors
Additional information
Responsible editor: Diane Purchase
Rights and permissions
About this article
Cite this article
Xiong, W., Sun, Y. & Zeng, Z. Antimicrobial use and antimicrobial resistance in food animals. Environ Sci Pollut Res 25, 18377–18384 (2018). https://doi.org/10.1007/s11356-018-1852-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1852-2