Abstract
The aim of this research was to determine the concentrations of cadmium, lead, mercury, and arsenic and the essential elements iron and selenium in the tissues (muscle, kidney, liver, spleen, and fat) of fallow deer (Dama dama L.) without and with supplemental selenium addition. Another aim was to determine the effect of selenium addition on the indicators of oxidative stress, namely, the levels of superoxide dismutase, glutathione peroxidase, glutathione, and vitamin E. The research was carried out with 40 fallow deer during two research periods. Supplemental feed without selenium addition was provided during the first research period, and supplemental feed with added selenium (3 mg/kg) was provided for 60 days during the second research period. The concentration of selenium in tissues was higher in the second research period than in the first research period (in kidney tissue, 0.957 vs. 0.688 mg/kg, P < 0.05). The dietary addition of selenium decreased (P < 0.05) the concentrations of some heavy metals (lead in the spleen = 0.06 vs. 0.27 mg/kg and in the fatty tissue = 0.17 vs. 0.69 mg/kg; arsenic in the muscle tissue = 0.005 vs. 0.014 mg/kg, liver = 0.003 vs. 0.009 mg/kg, spleen = 0.004 vs. 0.013 mg/kg, and fat = 0.008 vs. 0.016 mg/kg). The activity of glutathione peroxidase was significantly higher (P < 0.05) in the second research period than in the first research period (1375.36 vs. 933.23 U/L).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lead (Pb), mercury (Hg), cadmium (Cd), and arsenic (As) are some of the most common toxic metals that enter the food chain (Cunningham and Saigo 1997) and accumulate in game meat (Długaszek and Kopczyński 2013; Maňkovská and Steinnes 1995) rather than in the meat of farm animals (Forte and Bocca 2007). Therefore, many authors refer to wild game as good indicators of environmental pollution and use wild game in biomonitoring studies (Wieczorek-Dabrowska et al. 2013).
Existing data from the literature have shown elevated levels of heavy metals in the surface agricultural soils in Croatia, mainly near airports and in areas where seasonal flooding of the Sava River occurs (Romić and Romić 2003). Elevated concentrations of Pb, Zn, and Cd, likely originating from mining and smelting, have been found (Halamić et al. 2003). Ivezić et al. (2013) did not find elevated concentrations of heavy metals in the soil in East Slavonia, the region of interest in our investigation, despite the fact that this region has more lead in forest soil than in agricultural soil. There have been few papers describing the heavy metal concentrations in fallow deer tissues. Srebočan et al. (2006) reported low Cd concentrations in the liver and kidney tissue of fallow deer in Brijuni National Park, which is under minimal influence from anthropogenic factors. Contrary to that, in roe deer in Croatia, Srebočan et al. (2011) reported higher Cd concentrations in liver and kidney tissues than allowed by the official regulations. Cadmium and Pb concentrations in red deer in eastern Croatia were below the limits prescribed by the rules, with the exception of a few kidney samples that contained Cd above the recommended value (Srebočan et al. 2012).
Selenium (Se) and iron (Fe) are essential elements required for physiological functions, and they interact with heavy metals in wild herbivores (Flueck et al. 2012). Selenium is incorporated into several key selenoproteins (Kurokawa and Berry 2013) that are important in reproduction, the regulation of thyroid hormones, the immune response, DNA synthesis, and protection against oxidative damage and infections (Shchedrina et al. 2010; Sunde 2012). The low concentrations of Se present in the soil do not provide sufficient accumulation of Se in plants. Consequently, Se deficiency has been observed in domestic animals in Croatia (Antunović et al. 2013) and in some populations of wild animals in Canada and Norway (Pollock 2005; Vikoren et al. 2011). Many studies have confirmed the interaction of Se with toxic heavy metals in vivo (Długaszek and Kopczyński 2013) and its protective role (Lazarus et al. 2010; Sørmo et al. 2011; Usuki et al. 2011). The exposure of mammals to heavy metals increases the physiological need for Se because these metals may sequester Se (Sørmo et al. 2011).
The aim of this research was to monitor the concentrations of heavy metals (Cd, Pb, As, and Hg) and essential elements (Fe and Se) in fallow deer tissues (muscle, kidney, liver, spleen, and fat) in cases when the diet is lacking in Se and when it is supplemented with Se. Another aim was to determine the effect of selenium addition on the indicators of oxidative stress, namely, the levels of superoxide dismutase, glutathione peroxidase, glutathione, and vitamin E.
Material and methods
Area of research
The research was carried out in the open hunting ground “Krndija II” XIV/23 (6.850 ha), located in the continental part of Croatia, in a hilly area with altitudes between 170 and 700 m (Fig. 1). The region is traditionally an agricultural area with mixed continental broadleaf beech and oak forests used for the natural breeding, protection, and economic exploitation of game. There were no large industries near the sampling area.
Supplemental feeding
Over 2 years of research, fallow deer were given a commercial deer feed mixture (Table 1) as a supplemental feed. In the first year of research, the deer were given a mixture without the addition of Se, and in the second year, that mixture was supplemented with organic Se (Selplex®, Alltech, USA). Supplemental feeding with the addition of Se was provided for 60 days at 14 different feeding locations. The concentrations of heavy metals were determined in the commercial feed mixture, leaves, and terrestrial flora according to the methods described in the following section (data shown in Tables 2 and 3).
Collecting and analyzing commercial feed mixtures, leaves, and terrestrial flora samples
Samples from the deer feed mixtures were taken from the middle of four bags immediately after mixing. Samples of leaves and terrestrial flora were taken from 14 different feeding locations during June in both research years. The leaf samples were composed of common beech (Fagus sylvatica), European blackberry (Rubus fructicosa), common hornbeam (Carpinus betulus), dog rose (Rosa canina), black locust (Robinia pseudoacacia), corylus (Corylus avellana), and field maple (Acer campestre). The terrestrial flora was composed of common nettle (Urtica dioica), shepherd’s purse (Capsella bursa pastoris), wild mint (Mentha longifolia), yarrow (Achilea millefolium), smooth meadow grass (Poa pratensis), wild carrot (Daucus carota), red clover (Trifolium pretense), meadow fescue (Festuca pratensis), common dandelion (Taraxacum officinale), and soft rush (Juncus effusus).
The commercial feed mixture, leaves, and terrestrial flora samples were dried and ground into a fine powder using a heavy metal-free ultracentrifugal mill (Retsch RM 200, Germany). The plants and feed mixture samples (0.5 g each) were digested with 10 mL of a 5:1 mixture of HNO3 (Suprapure, Merck, Darmstadt, Germany) and H2O2 (Suprapure, Merck, Darmstadt, Germany) at 180 °C for 60 min in a microwave oven (CEM Mars 6, USA). The total Fe, Pb, and Cd concentrations were determined using inductively coupled plasma optical emission spectrometry (ICP-OES PerkinElmer Optima 2100 DV, USA). The same device was used for the determination of As and Hg concentrations, but with the hydride technique (Bosnak and Davidowski 2004). For the pre-reduction of Se and Hg, 20 mL of the sample was placed in a clean 50-mL beaker, and 20 mL of concentrated HCl was added to reduce Se6+ to Se4+ (Antunović et al. 2012). The mixture was heated to 90 °C and allowed to cool to room temperature. The wavelengths for the determination of the concentrations of the heavy metals were as follows: Pb, 220.353 nm; Cd, 228.802 nm; As, 193.696 nm; Hg, 253.652 nm; Fe, 238.204 nm; and Se, 196.026 nm. For quality control of the analytical method, the certified reference material, cabbage (NCS ZC 73012, China National Analysis Center), was digested, and the total concentrations were determined. The recoveries of the analyzed elements were within the range of 90–110%. All samples were analyzed in triplicate.
Collecting and analyzing tissue and blood samples
Blood and tissue samples (muscle, kidney, liver, spleen, and fat) were collected from 40 randomly hunted fallow deer, with 20 fallow deer hunted before supplemental feeding with Se (first research period) and 20 fallow deer hunted after dietary addition of Se (second research period). Animals ranged in age from 3 to 9 years, with body masses ranging from 50 to 110 kg; they were not selected according to sex.
Blood was collected from the left atrium of the heart in tubes containing Li-heparin. The blood was centrifuged (10 min at 1500×g) to separate the plasma. Plasma and tissue samples were frozen at − 80 °C until further analysis.
The animal tissue samples, including muscle (M. longissimus dorsi), kidney (cortical and medullar parts), liver (left lobe), spleen, and fatty (perirenal fat) tissue, used to measure the concentrations of Pb, Cd, As, Hg, Se, Fe, As, and Hg, were prepared in the same way as the plant material. For quality control of the analytical method, the certified reference material (chicken, NCS Certified Reference Material—NCS ZC73016) was digested, and the total concentrations were determined. The recoveries of the analyzed elements were within the range of 92–108%. All samples were analyzed in triplicate.
Determination of the antioxidative status from plasma
Antioxidative status was determined by measuring the activities of antioxidative enzymes, glutathione peroxidase (GPx), and superoxide dismutase (SOD) and the concentrations of glutathione (GSH) and vitamin E in plasma. The activity of GPx was determined by a commercial RANSEL® kit (Randox, UK), and the activity of SOD was determined by a commercial RANSOD® kit (Randox, UK) with the clinical chemistry analyzer Beckman Coulter AU 400 (Beckman Coulter, USA). The concentration of GSH in the plasma was determined by a commercially available Glutathione Assay Kit (Cayman Chemical Company, Ann Arbor, MI, USA), according to the manufacturer’s instructions. The concentration of vitamin E in fallow deer serum was determined by a commercially available Bovine Vitamin E ELISA kit (BlueGene Biotech, Shanghai, China).
Statistical analysis
All statistics were performed using Dell Statistica (Dell Inc. 2016). Multifactorial ANOVA was used to test the effect of the fixed factors (tissue type, experimental year, and their interaction) on the tested parameters (P < 0.05). When the interaction between tissue and period was significant, LSD post hoc test was used. The differences between the concentrations of heavy metals in the diet and the indicators of antioxidative protection in plasma were determined by Student’s t test.
Results
We measured the concentrations of heavy metals (Pb, Cd, As, and Hg) and essential elements (Fe and Se) in five types of fallow deer tissue (muscle tissue, kidney, liver, spleen, and fat) in two research periods (Tables 4 and 5).
Lower concentrations of Pb were determined in all tissues in the second research period than in the first one, but the concentrations were only significantly lower (P < 0.05) in fat and spleen tissues. The lead concentrations were reduced two times in muscle tissue and up to four times in spleen and fat tissue in the second research period. The concentration of Cd was significantly (P < 0.01) different between tissues; the highest concentration was in the kidney tissue. We did not determine the interaction between Se addition and Cd concentration in tissues. In the first research period, a significantly (P < 0.05) higher concentration of As was determined in muscle, kidney, and fat tissues than in liver tissue. In the second research period, a significantly (P < 0.05) higher concentration of As was determined in kidney tissue than in muscle, spleen, liver, and fat tissue. In the second research period, Se supplementation lowered As concentration 2.8 times in the muscle, 3 times in the liver and spleen, and 2 times in fat tissue and almost doubled it in the kidney tissue, compared to the levels in the first period.
The concentrations of Hg were numerically lower in the second period, but the difference was not statistically significant. The largest concentration of Hg was measured in the kidney tissue.
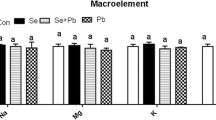
Of the investigated essential elements, the Fe concentration did not differ between the research periods. There was a significant difference (P < 0.05) in Fe concentration between tissues, with the highest concentration in the spleen and the lowest in the fat tissue (Table 4). The addition of Se to the feed increased the Se concentration in tissues in the second research period. A significantly higher (P < 0.05) Se concentration was found in kidney tissue in the second research period than in the first period. The Se concentration was higher in the muscle, kidney, liver, and spleen tissue than in the fat tissue in the second research period.
Because Se has an important role in antioxidative activity, our aim was to determine whether Se addition in feed mixture influences antioxidative capacity in animals. Therefore, the activities of SOD and GPx and the concentrations of GSH and vitamin E in the plasma of fallow deer were measured before and after supplemental feeding with the addition of Se (Table 6).
GPx activity was significantly higher (P < 0.05) in the second research period, while the activity of SOD was not different between the two research periods. The concentration of GSH was numerically lower in the second research period than in the first research period (7.53 vs. 9.54 μM, respectively). The plasma concentration of vitamin E did not differ between research periods (13.20 vs. 12.88 mg/L, respectively).
Discussion
Heavy metals in fallow deer tissue
Wild animals living in their natural ecosystems are exposed to various environmental influences. The environment is a major factor that influences the health, condition, and population of game. Heavy metals, which are easily absorbed from feed, rapidly accumulate in animal tissues (muscles, liver, kidney, and fat).
Lead enters an organism in various ways, for example, through an organism inhaling contaminated dust or ingesting contaminated food and water (Beyer et al. 2007; Ma 2011). After resorption, Pb is distributed by the blood, and it is usually very slowly excreted in feces and urine (Skerfving and Bergdahl 2007). Of the soft tissues, the liver and kidneys contain the highest concentrations of Pb, although it is present in most soft tissues, as well as in muscles (Bąkowska et al. 2016; Rudy 2009; Thompson 2012). According to the regulations regarding the maximum levels for certain contaminants in food by EC No 629/2008, the allowed concentrations of Pb are 0.5 mg/kg in the liver and kidneys and 0.1 mg/kg in muscle tissue. The concentration of Pb in muscle tissue in our research (0.149 mg/kg) was close to the allowed level and higher than that in a study by Bilandžić et al. (2009), who reported 0.04, 0.01, 0.07, and 0.03 mg/kg in red deer muscle tissue in four Croatian areas. The concentration of Pb in kidney tissue during the first research period was within the regulatory limits and lower (0.27 mg/kg) than the concentration reported by Bilandžić et al. (2009) in red deer (2.28, 2.54, 1.33, and 1.04 mg/kg). While Mulero et al. (2016) reported the highest concentration of Pb in bones and kidney tissue, in our research, the highest concentrations of Pb were detected in fatty tissue (0.69 mg/kg) and spleen tissue (0.26 mg/kg). The concentration of Pb in fatty tissue in our research was higher than that reported by Caggiano et al. (2005) in sheep fatty tissue (0.31 μg/g). Previous studies reported that high concentrations of Se in plasma reduced the toxicity of Pb (Nehru and Dua 1997; Xie et al. 1998). Although the basic mechanism for such an effect of Se is not completely clear, some studies have indicated that Se forms complexes with toxic metals, thus reducing or eliminating their negative effects. Selenium also competes with Cd and Pb for absorption in the gastrointestinal tract (Peraza et al. 1998; Xie et al. 1998). Compared to that in the first research period, the concentration of Pb in the tissues of fallow deer was lower in the second research period. A significantly lower (P < 0.05) concentration of Pb was found in fat and spleen tissue. Research by Wang et al. (2013), performed on rats, proved that treatment with Se could significantly reduce the concentration of Pb in blood. It should be emphasized that Se, when binding with heavy metals, exhibits reduced biological activity.
Low Cd excretion efficiency explains its long retention times and its tendency to accumulate, especially in kidney tissue (Srebočan et al. 2006). Srebočan et al. (2006) reported similar Cd concentrations in muscle tissue but lower concentrations in kidney tissue to those in our study in both experimental periods. Our findings are in agreement with those of Toman and Massanyi (1996), who found similar Cd concentrations in kidney and muscle tissues. In the first research period, the level of Cd was above the allowed limit because the maximum allowed concentration is 1.0 mg/kg for kidney tissue. Concentrations of Cd in kidney tissue (2.43 mg/kg) and in liver tissue (0.10 mg/kg) were higher in our research than the concentrations reported by Kramárová et al. (2005), which were 0.35 and 0.06 mg/kg, respectively, and lower than those reported by Gizejewska et al. (2017), which were 4.974 and 0.256 mg/kg, respectively. Bodies accumulate significant amounts of Cd over time. Many of the toxic effects of Cd occur because of its interaction with essential elements. It is assumed that Cd enters endothelial cells by using the transportation system of essential elements (Himeno et al. 2009), thus reducing their absorption (Peraza et al. 1998). Interaction between Cd and Se occurs on two levels. First, Se forms a complex with Cd, forming inert cadmium-selenide (Lazarus et al. 2006). Second, Se protects liver tissue against the toxicity of Cd, since it acts as an antioxidant and reduces the malondialdehyde (MDA) level (Newairy et al. 2007; Santos et al. 2005). After supplemental feeding with the addition of Se in our research, the concentration of Cd was reduced in kidney tissue (2.433:1.861 mg/kg), although the decrease was not significant. A similar case was reported by Lazarus et al. (2009), who found that perioral supplementation of Se and exposure to Cd reduced the concentrations of Cd in the kidney tissue of suckling rats. We found that, although the terrestrial flora contained higher (P < 0.05) concentrations of Cd in the second period, the concentrations of Cd in fallow deer tissues did not increase. In studies that reported no effect of Se on the Cd level in liver tissue, the animals were exposed for several weeks to Se (0.1 to 3 mg/kg) and Cd (0.006 to 200 mg/kg) in feed and/or water (Jihen et al. 2008). That proves that the duration of exposure has a significant role in chronic, sub-chronic, and acute exposure to Se and Cd.
Arsenic is absorbed by the digestive tract, but more than 90% disappears quickly from the blood because it is excreted within 48 h. This means that it does not significantly accumulate in the organism. That might explain why the level of As in our research was low (muscle, 0.01 mg/kg; kidney, 0.02 mg/kg; liver, 0.01 mg/kg; fatty tissue, 0.01 mg/kg; spleen, 0.01 mg/kg). Findo et al. (1993) in Slovakia found similar values in the kidney tissue of fallow deer (0.01 mg/kg). In our research, significantly lower concentrations of As were determined in the kidney, fat, and spleen tissue of fallow deer after supplemental feeding with the addition of Se. In an experiment in rabbits, it was found that Se lowers the concentrations of As in blood by creating non-toxic conjugates in the liver and gall bladder (Gailer et al. 2000). In our research, the concentrations were below the allowed maximum levels (0.1 mg/kg in muscle, 0.5 mg/kg in kidney, and 0.5 mg/kg in liver tissue) and adequately safe for human consumption.
All animal tissues contain low concentrations of Hg, which is normal for terrestrial animals, unlike aquatic animals, in which Hg accumulates substantially (Ropero et al. 2016). In general, the concentrations of Hg in the examined tissues of fallow deer were small. Findo et al. (1993), Falandysz (1994), and Lazarus et al. (2008) recorded similar values in the muscles, kidneys, and liver of red deer. The small concentrations of Hg in the examined fallow deer are an indicator of low Hg contamination in the environment. In our research, very low concentrations of Hg were determined in all tissues, and there was no decrease in the second research period after Se supplementation. Some studies have confirmed the formation by selenoprotein P of mercury-selenium, silver-selenium, and cadmium-selenium complexes in plasma (Sasakura and Suzuki 1998), and those complexes play roles in reducing Hg toxicity.
Essential elements in fallow deer tissue
Balanced mineral nutrition is essential for appropriate growth, reproduction, and health. This complex process has been studied mostly in domestic animals and rarely in wild animals. Kwong et al. (2004) have found that Fe deficiency may increase susceptibility to Pb poisoning, which could be prevented by high Fe intake or sufficient Fe stores. The highest concentration of Fe is found in the liver, spleen, and bone marrow. In our investigation during the first research period, the highest concentration of Fe was found in the spleen (424.80 mg/kg). The average concentration of 91.24 mg/kg in the liver was less than that reported by Vengušt and Vengušt (2004), but higher than the concentration determined by Morse et al. (2009), while Puls (1994) stated that the concentrations of Fe in the liver were up to 1500 mg/kg. The concentrations of Fe did not decrease in the second research period, which shows that Se addition had no detrimental effect on Fe availability. While supplementary iron adversely affected the selenium concentration in young women, Se supplementation had no effect on the iron concentration (Viita et al. 1989), which confirms our results. In the Se-deficient rats, the Fe concentration in liver tissue was higher than in normal rats in the same age group, and the Fe concentration in spleen tissue was 1–2-fold higher than in other organs (Matsumoto et al. 2009), which agrees with our research. Bjorklund et al. (2017) recommended assessing the status of trace elements such as Cu, Mn, Zn, and Se in parallel with the indices of Fe homeostasis.
The bioavailability of Se depends on its chemical form, other dietary components, the concentration of Se in an organism, and its physiological condition (Thomson 1998). Selenomethionine and selenocysteine are more bioavailable than the inorganic forms of Se. In our research, the concentration of Se in liver tissue was very low (0.14 mg/kg). Puls (1994) determined the reference range of Se in the liver to be between 0.25 and 1.5 mg/kg. In research by Humann-Ziehank et al. (2008), the concentrations of Se in deer liver tissues were higher than in our study (0.21 and 0.27 mg/kg), but these concentrations were also considered low. The low concentration of Se in tissues was probably a consequence of low concentration of Se in plants that were the main source of feed for deer. Intestinal resorption of Se in ruminants is lower than that in monogastric animals. It is assumed that bacteria in the digestive tract of ruminants reduce Se into insoluble forms inappropriate for absorption (Underwood 1977). For different metabolic functions, most ruminants require 0.10 mg/kg Se (Anonymous 1985). This could explain why our animals did not obtain sufficient amounts of Se to completely fill all reserves in all tissues. Therefore, Se supplementation by commercial feed mixture of fallow deer should be above the limit prescribed by EU legislation.
Antioxidant status in fallow deer
Oxidative stress is caused by different pathophysiological mechanisms, i.e., by increased production of ROS in inflammatory processes. Protective enzymatic and non-enzymatic antioxidative defense mechanisms reduce oxidative stress by degrading reactive oxygen species (ROS). The main intracellular antioxidative enzymes are SOD, catalase, and GPx. Their effects occur in two steps. In the first step, SOD converts the highly active superoxide radical into hydrogen peroxide and oxygen. After that, CAT and GPx independently convert hydrogen peroxide into water and oxygen (Sies 1993). Glutathione is not only a co-factor of GPx but also acts as a direct deactivator of ROS. At the non-enzymatic level, vitamins and other antioxidants can remove free radicals and delay the oxidation of molecules. In our research, the concentrations of SOD, GPx, GSH, and vitamin E were determined in the plasma and serum of fallow deer before and after supplemental feeding with the addition of Se. In mammalian metabolism, Se is a part of the selenoprotein of the GPx enzyme, which acts as a peroxide scavenger, so its activity is a measure of its ability to neutralize radicals and to prevent cell damage due to oxidation processes. Significantly higher activity of GPx was determined during the second research period, unlike in Calamari et al. (2011), who did not find an increase of GPx activity after treatment with different dietary Se concentrations. The increase of GPx activity in our research may be a result of the increased bioavailability of Se due to supplementation of Se in feed for fallow deer, which directly affects the activity of enzymes that are dependent on Se.
Most GSH is present in the cytosol, and the extracellular concentrations are relatively low (from 2 to 20 μM/L in plasma). In our research, the GSH concentration in plasma was within the mentioned limits. After supplemental feeding with the addition of Se, the concentration of GSH was lower, although not significantly. Glutathione also has the ability to form poorly soluble mercaptides with the ions of heavy metals through its –SH groups. Jovanović et al. (2013) studied the effects of GSH on the accumulation of Cd and Pb and the effects of lipid peroxidation in liver and kidney tissue in intoxicated rats, and proved that GSH has a protective role during and after intoxication with Cd and Pb. It contains a free –SH group, which forms a stable bond with ions of Cd and Pb, blocking them and reducing their toxic effects.
Vitamin E is important for normal growth and development and for preventing damage to muscles caused by physical stress. Many studies have confirmed the antioxidative, protective effect of vitamin E against several metals that cause hepatotoxicity, such as Cu, Pb, Cd, sodium fluoride, Hg, and iron sulfate (Chinoy et al. 2004; Osfor et al. 2010; Gaurav et al. 2010; Al-Attar 2011). The vitamin E concentration in fallow deer in our research was higher than the reference range (from 1 to 5 mg/L) in the plasma of domestic ruminants (Puls 1994). Vitamin E is not stable in feed, and its content depends on the season and feed supplies. It is known that in the fall, the concentration of vitamin E decreases in grass, and in winter, when feed consists of dried hay, the supply of vitamin E is deficient. The high concentration of vitamin E found in fallow deer in this study can be explained by the fact that deer are selective ruminants. Their feed consists of buds, leaves, and fresh grass that contain higher concentrations of vitamin E than the feed available to domestic ruminants. In wild animals, there are great individual differences in vitamin E status because of seasonal variations and different habitats. There is a synergy between Se and vitamin E in protecting against cellular damage by ROS (Saito et al. 2003). The metabolic function of Se is associated with the function of GPx in the cell cytosol, while vitamin E is a component of the lipid membrane (Surai 2002). Some studies have shown that supplementation with combined vitamin E and Se significantly increases the activity of GPx (Surai 2002; Ebeid 2012). In our research, the concentration of vitamin E after supplemental feeding with the addition of Se was not significantly different in the second experimental period.
Conclusion
The beneficial effect of dietary selenium addition in fallow deer was confirmed by lower heavy metal concentrations in tissues, especially Pb in the spleen and fat tissue and As in the muscle, liver, spleen, and fat tissue. Concurrently, better antioxidative protection was provided. Consequently, the risk of heavy metal toxicity could be reduced by Se supplementation in fallow deer.
Dietary Se supplementation of fallow deer in the amount of 3 mg/kg DM over the course of 2 months was not enough to sufficiently increase Se in all organs. Therefore, either a higher concentration or a longer supplementation time is required.
References
Al-Attar AM (2011) Vitamin E attenuates liver injury induced by exposure to lead, mercury, cadmium and copper in albino mice. Saudi J Biol Sci 18(4):395–401. https://doi.org/10.1016/j.sjbs.2011.07.004
Anonymous (1985) Nutrient requirements of domestic animals. Nutrient Requirements of Sheep. National Academy of Science-National Research Council, Washington
Antunović Z, Klapec T, Čavar S, Mioč B, Novoselec J, Klir Ž (2012) Changes of heavy metal concentrations in goats milk during lactation stage in organic breeding. Bulgarian J Agr Sci 18:166–170
Antunović Z, Klapec T, Čavar S, Šperanda M, Pavić V, Novoselec J, Klir Z (2013) Status of selenium and correlation with blood GSH-px in goats and their kids in organic breeding fed with different levels of organic seleniumium. Arch Tierz 56:167–177
Bąkowska M, Pilarczyk B, Tomza-Marciniak A, Udała J, Pilarczyk R (2016) The bioaccumulation of lead in the organs of roe deer (Capreolus capreolus L.), red deer (Cervus elaphus L.), and wild boar (Sus scrofa L.) from Poland. Environ Sci Pollut Res 23:14373–14382. https://doi.org/10.1007/s11356-016-6605-5
Beyer WN, Gaston G, Brazzle R, O’Connell AF, Audet DJ (2007) Deer exposed to exceptionally high concentrations of lead near the Continental Mine in Idaho, USA. Environ Toxicol Chem 26(5):1040–1046. https://doi.org/10.1897/06-304R.1
Bilandžić N, Sedak M, Vratarić D, Perić T, Šimić B (2009) Lead and cadmium in red deer and wild boar from different hunting grounds in Croatia. Sci Total Environ 407:4243–4247
Bjorklund G, Aaseth J., Skalny VA, Suliburska J, Skalnaya G., Nikonorov AA, Tinkoy AA (2017) Interaction of iron and manganese, zinc, chromium and selenium as related to prophylaxis and treatment of iron deficiency. J Trace Elem Med Biol 41:41–53
Bosnak CP, Davidowski I (2004) Continuous flow hydride generation using the optima ICP. Field application report. PerkinElmer Life and Analytical Science. http://www.perkinelmer.co.kr/files/AP00059.pdf. Accessed 15 December 2017
Caggiano R, Sabia S, Emilio M, Macchiato M, Anastasio A, Ragosta M, Paino S (2005) Metal levels in fodder, milk, dairy productions, and tissues sampled in ovine farms of Southern Italy. Environ Res 99:48–57
Calamari L, Petrera F, Abeni F, Bertin F (2011) Metabolic and hematological profiles in heat stressed lactating dairy cows fed diets supplemented with different seleniumum sources and doses. Livest Sci 142(1-3):128–137. https://doi.org/10.1016/j.livsci.2011.07.005
Chinoy N, Sharma A, Patel T, Memon R, Jhala D (2004) Recovery from fluoride and aluminium induced free radical liver toxicity in mice. Fluoride 12:14–16
Cunningham WP, Saigo BW (1997) Environmental science a global concern, 4th edn. WMC Brown Publisher, New York
Dell Inc. (2016). Dell Statistica (data analysis software system), version 13. software.dell.com
Długaszek M, Kopczyński K (2013) Elemental composition of muscle tissue of wild animals from central region of Poland. Int J Environ Res 7:973–978
Ebeid TA (2012) Vitamin E and organic seleniumium enhances the antioxidative status and quality of chicken cockerel semen under high ambient temperature. Br Poult Sci 53(5):708–714. https://doi.org/10.1080/00071668.2012.722192
Falandysz J (1994) Some toxic and trace metals in big game hunted in the northern part of Poland in 1987–1991. Sci Total Environ 141(1-3):59–73. https://doi.org/10.1016/0048-9697(94)90018-3
Findo S, Hell P, Farkas J, Mankovska BZ, Ilinec M, Stanovsky M (1993) Akkumulation von ausgewahlten Schwermetallen beim Rot-und Rehwild im zentralen Teil der Westkarpaten (Mittelslowakei). Z Jagdwiss 39:181–189
Flueck WT, Smith-Flueck JM, Mionczynski J, Mincher BJ (2012) The implications of seleniumium deficiency for wild herbivore conservation: a review. Eur J Wildl Res 58(5):761–780. https://doi.org/10.1007/s10344-012-0645-z
Forte G, Bocca B (2007) Quantification of cadmium and lead in offal by SF-ICP-MS: method development and uncertainty estimate. Food Chem 105(4):1591–1598. https://doi.org/10.1016/j.foodchem.2007.03.043
Gailer J, George GN, Pickering IJ, Prince RC, Ringwald SC, Pemberton JE, Aposhian HV (2000) A metabolic link between arsenite and selenite: the seleno-bis(S-glutathionyl) arsinium ion. J Am Chem Soc 122(19):4637–4639. https://doi.org/10.1021/ja993064m
Gaurav D, Preet S, Dua K (2010) Chronic cadmium toxicity in rats: treatment with combined administration of vitamins, amino acids, antioxidants and essential metals. J Food Drug Anal 18:464–470
Gizejewska A, Szkoda J, Nawrocka A, Zmudzki J, Gizejewski Z (2017) Can red deer antlers be used as an indicator of environmental and edible tissues’ trace element contamination? Environ Sci Pollut Res Int 23:11630–11638. https://doi.org/10.1007/s11356-017-8798-7
Halamić J, Galović L, Šparica M (2003) Heavy metal (As, Cd, Cu, Hg, Pb and Zn) distribution in topsoil developed on alluvial sediments of the Drava and Sava rivers in NW Croatia. Geologia Croat 56:215–232
Himeno S, Yanagiya T, Fujishiro H (2009) The role of zinc transporters in cadmium and manganese transport in mammalian cells. Biochimie 91:1218–1222
Humann-Ziehank E, Ganter M, Hennig-Pauka I, Binder A (2008) Trace mineral status and liver and blood parameters in sheep without mineral supply compared to local roe deer (Capreolus capreolus L.) populations. Small Rumin Res 75(2-3):185–191. https://doi.org/10.1016/j.smallrumres.2007.10.006
Ivezić V, Singh BR, Rossebø AA, Lončarić Z (2013) Water extractable concentrations of Fe, Mn, Ni, Co, Mo, Pb and Cd under different land uses of Danube basin in Croatia. Acta Agric Scand Sect B Soil Plant Sci 61:747–759
Jihen EH, Imed M, Fatima H, Abdelhamid K (2008) Protective effects of selenium (Se) and zinc (Zn) on cadmium (Cd) toxicity in the liver and kidney of the rat: Histology and Cd accumulation. Food Chem Toxicol 46(11):3522–3527. https://doi.org/10.1016/j.fct.2008.08.037
Jovanović JM, Nikolić RS, Kocić GM, Krstić NS, Krsmanović MM (2013) Glutathione protects liver and kidney tissue from cadmiumand lead-provoked lipid peroxidation. J Serb Chem Soc 78(2):197–207. https://doi.org/10.2298/JSC120214053J
Kramárová M, Massányi P, Jančová A, Toman R, Slamečka J, Tataruch F, Kováčik J, Gašparik J, Nad P, Skalická M, Koréneková B, Juričik R, Čubon J, Haščik P (2005) Concentration of cadmium in the liver and kidneys of some wild and farm animals. Bull Vet Inst Pulawy 49:465–469
Kurokawa S, Berry MJ (2013) Selenium. Role of the essential metalloid in health. Met Ions Life Sci 13:499–534. https://doi.org/10.1007/978-94-007-7500-8_16
Kwong WT, Richard PF, Semba D (2004) Interactions between iron deficiency and lead poisoning: epidemiology and pathogenesis. Sci Total Environ 330(1-3):21–37. https://doi.org/10.1016/j.scitotenv.2004.03.017
Lazarus M, Orct T, Aladrović J, Beer-Ljubić B, Jurasović J (2010) Effect of seleniumium pre-treatment on antioxidative enzymes and lipid peroxidation in Cd-exposed suckling rats. Biol Trace Elem Res 142:611–622
Lazarus M, Orct T, Blanuša M, Kostial K, Piršljin J, Beer-Ljubić B (2006) Effect of selenium pre-tretment on cadmium content and enzymatic antioxidants in tissues of suckling rat. Toxicol Lett 164:S191. https://doi.org/10.1016/j.toxlet.2006.07.055
Lazarus M, Orct T, Blanuša M, Vicković I, Šoštarić B (2008) Toxic and essential metal concentrations in four tissues of red deer (Cervus elaphus L.) from Baranja, Croatia. Food Addit Contam 25:270–283
Lazarus M, Orct T, Jurasović J, Blanuša M (2009) The effect of dietary seleniumium supplementation on cadmium absorption and retention in suckling rats. Biometals 22(6):973–983. https://doi.org/10.1007/s10534-009-9249-9
Lazarus M, Prevendar-Crnić A, Bilandžić N, Kusak J, Reljić S (2014) Cadmium, lead and mercury exposure assessment among Croatia consumers of free-living game. Arc Hig Rada Toksikol 65:281–292
Ma WC (2011) Lead in mammals. In: Beyer WN, Meador JP (eds) Environmental Contaminants in Biota: Interpreting Tissue Concentrations, 2nd edn. CRC Press, Boca Raton, pp 595–608. https://doi.org/10.1201/b10598-19
Maňkovská B, Steinnes E (1995) Effects of pollutants from an aluminium reduction plant on forest ecosystems. Sci Total Environ 163(1-3):11–23. https://doi.org/10.1016/0048-9697(95)04489-N
Matsumoto K, Terada S, Ariyoshi M, Okajo A, Hisamatsu A, Ui I, Endo K (2009) The effect of long-running severe selenium-deficiency on the amount of iron and zinc in the organs of rats. Molecules 14:4440–4453
Morse BW, Miller DL, Miller KV, Baldwin CA (2009) Population health of fallow deer (Dama dama L.) on little St. Simons Island, Georgia, USA. J Wildl Dis 45(2):411–421. https://doi.org/10.7589/0090-3558-45.2.411
Mulero R, Cano-Mauel J, Raez-Bravo A, Perez J, Espinoza J, Soriguer R, Fandos P, Granados JE, Romero D (2016) Lead and cadmium in wild boar (Sus scrofa) in the Sierra Nevada Natural Space (southern Spain). Environ Sci Pollut Res 23(16):16598–16608. https://doi.org/10.1007/s11356-016-6845-4
Nehru B, Dua R (1997) The effect of dietary seleniumium on lead neurotoxicity. J Environ Pathol Toxicol Oncol 16:47–50
Newairy AA, El-Sharaky AS, Badreldeen MM, Eweda SM, Sheweita SA (2007) The hepatoprotective effects of seleniumium against cadmium toxicity in rats. Toxicology 242(1-3):23–30. https://doi.org/10.1016/j.tox.2007.09.001
Osfor MM, Ibrahim HS, Mohamed YA, Ahmed S, Abd El Azeem A, Hegazy AM (2010) Effect of alpha lipoic acid and vitamin E on heavy metals intoxication in male albino rats. J Am Sci 6:6–63
Peraza MA, Ayala-Fierro F, Barber DS, Casarez E, Rael LT (1998) Effects of micronutrients on metal toxicity. Environ Health Perspect 106:203–216
Pollock B (2005) Trace elements status of white-tailed red deer (Odocoileus virginianus) and moose (Alces alces) in Nova Scotia. Wildlife Damage Management, Internet Center for Canadian Cooperative Wildlife Health Centre: Newsletters & Publications, University of Nebraska–Lincoln, 45. https://digitalcommons.unl.edu/cgi/viewcontent.cgi?referer=https://www.google.hr/&httpsredir=1&article=1044&context=icwdmccwhcnews. Accessed 22 December 2017
Puls R (1994) Mineral levels in animal health: diagnostic data, 2nd edn. Sherpa International Clearbook, Abbotsford
Romić M, Romić D (2003) Heavy metals distribution in agricultural topsoils in urban area. Environ Geol 43:795–805
Ropero MJP, Farinas NR, Mateo R, Nevado JJB, Martin-Doimeadios RCR (2016) Mercury species accumulation and trophic transfer in biological systems using the Almaden mining district (Ciudad Real, Spain) as a case of study. Environ Sci Pollut Res Int 23(7):6074–6081. https://doi.org/10.1007/s11356-015-4989-2
Rudy M (2009) Correlation of lead, cadmium and mercury levels in tissue and liver samples with age in cattle. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 26(6):847–853. https://doi.org/10.1080/02652030902835747
Saito Y, Yoshida Y, Akazawa T, Takahashi K, Niki E (2003) Cell death caused by selenium deficiency and protective effect of antioxidants. J Biol Chem 278:39428–39434
Santos FW, Zeni G, Rocha JBT, Weis SN, Fachinetto JM, Favero AM, Nogueira CW (2005) Diphenyl diseleniumide reverses cadmium-induced oxidative damage on mice tissues. Chem Biol Interact 151(3):159–165. https://doi.org/10.1016/j.cbi.2005.01.001
Sasakura C, Suzuki KT (1998) Biological interaction between transition metals (Ag, Cd and Hg), seleniumide/sulfi de and seleniumoprotein P. J Inorg Biochem 71(3-4):159–162. https://doi.org/10.1016/S0162-0134(98)10048-X
Shchedrina VA, Zhang Y, Labunskyy VM, Hatfield DL, Gladyshev VN (2010) Structure–function relations, physiological roles, and evolution of mammalian ER-resident selenoproteins. Antiox Redox Signal 12(7):839–849. https://doi.org/10.1089/ars.2009.2865
Sies H (1993) Strategies of antioxidant defence. Eur J Biochem 215(2):213–219. https://doi.org/10.1111/j.1432-1033.1993.tb18025.x
Sørmo EG, Ciesielski TM, Øverjordet IB, Lierhagen S, Eggen GS, Berg T, Jenssen BM (2011) Selenium moderates mercury toxicity in freeranging freshwater fish. Environ Sci Technol 45(15):6561–6566. https://doi.org/10.1021/es200478b
Srebočan E, Janicki Z, Prevendar Crnić A, Tomljanović K, Šebečić M, Konjević D (2012) Cadmium, lead and mercury concentrations in selected red deer (Cervus elaphus L.) tissues from north-eastern Croatia. J Environ Sci Health A 47(13):2101–2108. https://doi.org/10.1080/10934529.2012.695994
Srebočan E, Pompe-Gotal J, Konjević D, Prevendar-Crnić A, Popović N, Kolić E (2006) Cadmium in fallow deer tissue. Veterinarski Arhiv 76:S143–S150
Srebočan E, Prevendar Crnić A, Ekert Kabalin A, Lazarus M, Jurasović J, Tomljanović K, Andreić D, Strunjak Perović I, Čož-Rakovac R (2011) Cadmium, lead and mercury concentration in tissues of roe deer (Capreolus capreolus L.) and wild boar (Sus scrofa L.) from lowland Croatia. Czech J Food Sci 29:624–633
Sunde RA (2012) Seleniumium. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR (eds) Modern nutrition in health and disease, 11th edn. Lippincott Williams & Wilkins, Philadelphia, pp 225–237
Surai PF (2002) Selenium in poultry nutrition 2. Reproduction, egg and meat quality and practical applications. Worlds Poult Sci J 58(04):431–450. https://doi.org/10.1079/WPS20020032
Thompson L (2012) Lead. In: Gupta R (ed) Veterinary toxicology: basic and clinical principles, 2nd. Elsevier Academic Press, New York, pp 522–526. https://doi.org/10.1016/B978-0-12-385926-6.00037-5
Thomson CD (1998) Selenium speciation in human body fluids. Analyst 123(5):827–831. https://doi.org/10.1039/a707292i
Toman R, Massanyi P (1996) Cadmium in selected organs of fallow-deer (dama dama), sheep (ovis aries), brown hare (lepus europaeus) and rabbit (oryctolagus cuniculus) in Slovakia. J Env Sci Heath Part A 31(5):1043–1051
Underwood EJ (1977) Selenium. In: Underwood EJ (ed) Trace elements in human and animal nutrition, 4th edn. Academic press, New York, pp 302–346
Usuki F, Yamashita A, Fujimura M (2011) Post-transcriptional defects of antioxidant selenoenzymes cause oxidative stress under methylmercury exposure. J Biol Chem 286:6641–6649
Vengušt G, Vengušt A (2004) Some minerals as well as trace and toxic elements in livers of fallow deer (Dama dama L.) in Slovenia. Eur J Wildl Res 50(2):59–61. https://doi.org/10.1007/s10344-004-0038-z
Viita LM, Mutanen ML, Mykkanen HM (1989) Selenium-iron interaction in young women with low selenium status. J Hum Nutr Diet 2(1):39–42. https://doi.org/10.1111/j.1365-277X.1989.tb00006.x
Vikoren T, Kristoffersen AB, Lierhagen S, Handeland K (2011) A comparative study of hepatic trace element levels in wild moose, roe deer, and reindeer from Norway. J Wildl Dis 41:569–579
Wang M, Fu H, Xiao Y, Ai B, Wei Q, Wang S, Liu T, Ye L, Hu Q (2013) Effects of low-level organic seleniumium on lead-induced alternation in neural cell adhesion molecules. Brain Res 1530:76–81. https://doi.org/10.1016/j.brainres.2013.07.028
Wieczorek-Dabrowska M, Tomza-Marciniak A, Pilarczyk B, Baliska-Ramisc A (2013) Roe and red deer as bioindicators of heavy metals contamination in north-western Poland. Chem Ecol 29(2):100–110. https://doi.org/10.1080/02757540.2012.711322
Xie Y, Chiba M, Shinohara A, Watanabe H, Inaba Y (1998) Studies on lead binding protein and interaction between lead and selenium in the human erythrocytes. Ind Health 36:234–239
Funding
The authors express gratitude to the Croatian Hunting Association for providing support in funding this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Vukšić, N., Šperanda, M., Lončarić, Z. et al. The effect of dietary selenium addition on the concentrations of heavy metals in the tissues of fallow deer (Dama dama L.) in Croatia. Environ Sci Pollut Res 25, 11023–11033 (2018). https://doi.org/10.1007/s11356-018-1406-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1406-7