Abstract
Copper contamination of soils is a global environmental problem. Soil components (organic matter, clay minerals, and microorganisms) and retention time can govern the adsorption, fixation, and distribution of copper. This study evaluated the interaction effects of soil components and aging on the distribution of exogenous copper. Three typical Chinese soils (Ultisol, Alfisol, and Histosol) were collected from Hunan, Henan, and Heilongjiang Provinces. Soils were incubated with rice straw (RS) and engineered bacteria (Pseudomonas putida X4/pIME) in the presence of exogenous copper for 12 months. Sequential extraction was employed to obtain the distribution of Cu species in soils, and the mobility factors of Cu were calculated. The relationships between soil properties and Cu fractions were analyzed with stepwise multiple linear regression. The results show that organic carbon plays a more important role in shaping the distribution of relatively mobile Cu, and iron oxides can be more critical in stabilizing Cu species in soils. Our results suggest that organic matter is the most important factor influencing copper partitioning in Ultisols, while iron oxides are more significant in Alfisols. The mobility of exogenous Cu in soils depends largely on organic carbon, amorphous Fe, and aging. The introduction of both rice straw and rice straw + engineered bacteria enhanced the stabilization of Cu in all the three soils during aging process. The introduction of bacteria could reduce copper mobility, which was indicated by the lowest mobility factors of Cu for the treatment with bacteria in Black, Red, and Cinnamon soils at the first 4, 8, and 8 months, respectively. Different measures should be taken into account regarding the content of organic matter and iron oxides depending on soil types for the risk assessment and remediation of Cu-contaminated soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal contamination of soils, as a result of fertilizer, pesticide, manure, and sewage sludge application or stemming from industrial emissions, has become a severe issue over the past decades (Sparks 2002; Bolan et al. 2014). Copper is one of the most common heavy metals in soils and can be toxic at elevated concentrations (Maderova et al. 2011; Zeng et al. 2011).
Soil components, such as organic matter, minerals, and bacteria, can govern the adsorption, fixation, and distribution of heavy metals (Du et al. 2016; Qu et al. 2017). Solid organic matter may form complexes with heavy metals due to its high cationic exchange capacity and large number of functional groups (Diagboya et al. 2015; Qi et al. 2018). Achiba et al. (2009) found that the application of compost significantly increased the organic-bound Cu, namely from 3.8 to 11, 19, and 33%, respectively, after the addition of 40, 80, and 120 t/ha of compost to the soil. Dissolved organic matter serves as chelates and increases metal availability; thus, soil particle-adsorbed metals decrease (Fan et al. 2016; Mesquita and Carranca 2005). Cui et al. (2008) revealed that regardless of soil types, soluble Cu in their study increased from 0.26–2.4 μmol L−1 to 0.7–3.60 μmol L−1 in soils with application of rice straw. Iron oxides are efficient sorbents for the immobilization of heavy metals because of their large, active surface areas. Li et al. (2016) found that Fe-Mn oxide-bound Se was 62.0, 33.3, and 7.6% in fluvo-aquic soil, black soil, and krasnozem, respectively, with the concentration of Fe oxides being two to four times higher in krasnozem soil and black soils compared to that in the fluvo-aquic soil. Moreover, Fe-Mn oxide-bound Se was not affected by aging effects in all the three soils. Yu et al. (2016) indicated that the content of amorphous iron oxides was significantly and negatively correlated with exchangeable Cd (r = − 0.40, P < 0.01, n = 73) and carbonate-bound Cd (r = −0.50, P < 0.01, n = 73) in paddy soil contaminated with acid mine drainage.
Aging processes can decrease the extractability, bioavailability, and toxicity of copper in soils. From day 3 to day 56, the exchangeable copper was decreased by 143 mg kg−1 and transformed to reducible and oxidizable fractions in slightly acidic soil with a high concentration of organic matter (Lu et al. 2009). Jalali and Khanlari (2008) noted that the exchangeable copper decreased rapidly by 49.7 to 68.8% after an initial phase of 3 h with freshly spiked copper in five calcareous soils, and the amounts of Fe-Mn oxides and organic matter (OM) fractions increased consistently during the incubation period of 25 days. The effects of aging differed between soils with varying properties. In a similar experiment, 500 mg kg−1 of Cu were added in three types of soils. Lu et al. (2005) indicated that exchangeable copper decreased by 26 to 142 mg kg−1 from 3 h to 8 weeks in soils from Beijing, Jiangxi, and Heilongjiang, China. In their experiment, the effect of aging was largely dependent on the amount of soil organic matter.
Although quite a number of studies have investigated the mobility of exogenous heavy metals into soils, most results were obtained in a limited time regime. At a relative longer aging period, the effects of soil components interactions on the distribution of exogenous copper are still poorly understood. Crop straws have been used as conventional amendments in agricultural soils and show great potential for the immobilization of heavy metals (Rizwan et al. 2016; Mousa et al. 2013). The numbers of bacteria in soils are enormous; therefore, bacteria may have substantial influences on the behavior of heavy metals via various processes (Huang et al. 2005; Qu et al. 2018). The number of bacteria in soils may vary depending on the environmental variation. In the current study, we examined the distribution of exogenous copper as influenced by the introduction of rice straw and bacteria in three soils with different physiochemical properties. The exogenous copper was aged in soils for up to 12 months. Soil copper was fractionated, and pertinent soil characteristics were measured. The aim of the work was to establish multiple regression models between copper fractions and soil properties, thus determining the factors influencing copper distribution in different soils during the aging process.
Materials and methods
Soil sampling and characterization
Soils were collected from the surface layer of farmlands located in Hunan (subtropical), Henan (north subtropical), and Heilongjiang (temperate) provinces, China. The three types of soils are Red soil (Ultisol, USDA classification), Cinnamon soil (Alfisol), and Black soil (Histosol), respectively. Soil samples were air-dried and ground to pass through a 2-mm sieve. Soil texture was analyzed using the pipette method. Soil organic matter was determined using the potassium dichromate method. Cation exchange capacity (CEC) was measured by saturation with 1 M NH4OAc at pH 7. Soil pH was determined with a pH meter in a suspension of 1:2.5 soil to water ratio (w/v) (Denver, USA). Detailed descriptions of the analysis procedures can be found in Bao (2000). In our study, soil pH values were from 5.79 to 7.43, while soil organic matter (SOM) concentrations ranged from 6.70 to 55.16 g kg−1 (Table 1).
Incubation experiment
After sieving with a 2-mm sieve, 1 kg of air-dried soil was placed in ceramic pots. The soils were incubated with rice straw (RS) and rice straw + engineered bacteria (RS + EB). For the rice straw treatment, RS (46.2 ± 3.4% organic carbon) was grounded to less than 2 mm and mixed into the soils at the rate of 50 g kg−1. For the RS + EB treatments, the amount of rice straw applied was the same as the RS treatment, and the engineered bacteria (EB) were added at the rate of 1 × 107 CFU/g. The engineered bacteria are monkey metallothionein surface displayed Pseudomonas putida X4/pIME (He et al. 2012). Copper was introduced as Cu (NO3)2 solution to all the treatments including control (without addition of RS and RS + EB) at the rate of 200 mg kg−1. Each soil treatment was mixed thoroughly, and the pots were placed in the green house and incubated at 25 °C. The soil water content was maintained at about 60% of water holding capacity by periodically weighing the pots and adjusting the weight by addition of distilled water. The soils were homogenized in the ceramic pots with a shovel, and 80 g soil was then collected from each pot. The soil samples were air-dried and sieved through 20-mesh or 100-mesh for the subsequent chemical analysis. Soils were sampled after 4, 8, and 12 months.
Extraction of DOM and humic substances
Approximately 2 g soil was collected from each treatment and was put in the centrifuge tube; 20 ml ultrapure water was added. The tubes were shaken at 200 rpm, 25 °C for 24 h, and centrifuged at 4000 rpm for 10 min. The liquid supernatants were filtered through a 0.45-μm membrane. All the processes were kept in the dark. The content of dissolved organic carbon (DOC) was determined by TC analyzer (multi N/C 2100, analyticjena, Germany).
For extraction of humus, soil samples (1.00 g) were shaking during 2 h with 20 ml 0.1 M NaOH+Na4P2O7. The mixture was centrifuged, and the soluble fraction was labeled as total humic substances-like. All the supernatant filtered using a 0.45-μm membrane to remove undissolved particulate materials. The organic carbon was determined by external-heated potassium dichromate method.
Iron oxides
Soil crystalline, amorphous, and chelated iron oxides were extracted by dithionite-citrate-bicarbonate (Fed), ammonium oxalate (Feo), and Na-pyrophosphate (Fep), respectively. The extraction method of iron oxides was described by Lu (2000). The concentrations of iron were measured with atomic absorption spectrometer (Z-2000, Hitachi, Japan).
Sequential extraction of Cu
A modified Tessier procedure was performed to assess copper distribution in soils (Tessier et al. 1979; Tang et al. 2006). The detailed procedures are shown in Table 2. Metal concentration in the supernatant was measured using a flame atomic absorption spectrometer (Z-2000, Hitachi, Japan).
Mobility factor calculation
To evaluate the percentages of metals extracted in liable forms, we introduced the Mobility factor (MF), which is calculated by the following equation (Sablu et al. 1998; Narwal and Salbu 1999; Kabala and Singh 2011):
Statistical analysis
All incubation experiments were carried out in triplicate, and the data were presented as means with standard errors. Two-way analysis of variance (ANOVA) was performed on the experimental data. The means were separated by Duncan’s test, considering a significant level of P < 0.05 throughout the study.
Stepwise multiple linear regression analysis was used to test for significant relationships between the copper fractions and soil properties (e.g., soil pH, OM, iron fractions). Regression coefficient (R2) represents the percentage of the variance accounted for by the regression models. All statistical analyses were performed using the package SPSS 17.0.
Results
Soil pH
The original pH values for the Red soil, Cinnamon soil, and Black soil were 5.97, 7.43, and 5.79, respectively. Over the incubation period of 12 months, pH values of the Cinnamon soil were 1.3 to 1.5 units higher than those of the Red and Black soils (Table 3). The pH values increased by 0.2 to 0.6 units with the addition of RS and RS + EB in the Red soil, but decreased by 0.1 to 0.4 units in the Cinnamon soil at the end of the experiment compared to 4-month incubation.
Soil organic matter
In the Black soil, SOM was about four and five times higher than in the Cinnamon and Red soil, respectively (Table 3). The addition of RS and RS + EB increased SOM by 151 to 265%, 56 to 93%, and 24 to 38% for Red, Cinnamon, and Black soils, respectively. In the control treatment, no significant changes in SOM were observed for the three soils during the incubation period of 12 months. In the RS and RS + EB treatments, SOM dramatically decreased with time in the Red soil. In the Cinnamon and Black soils, SOM increased from the fourth to the eighth month, but decreased from then until the end of the experiment.
In terms of organic carbon in humic substances (HS-OC), values were highest in the Black soil; in particular, 9.3 to 19.1 times higher than in the Red soil and 8.9 to 10.5 times higher than in the Cinnamon soil (Table 3). With the incorporation of RS and RS + EB, HS-OC increased by 1.63 to 3.77 times for the Red soil and by 1.04 to 2.25 times for the Cinnamon soil. In the Red soil, the amount of HS-OC doubled throughout the incubation period, while this effect was not observed for the two other soil types. Application of RS and RS + EB resulted in a significant increase of HS-OC from the eighth to the twelfth month in the Red soil.
In the control treatment without additives, DOC decreased significantly in Cinnamon and Red soils over the duration of the incubation (Table 3). The addition of RS and RS + EB resulted in significant DOC increases in all three soil types; however, DOC concentrations declined with time (Table 3).
Copper fraction
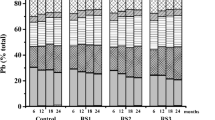
Figure 1 shows the effects of the tested organic soil amendments on the different copper fractions over time. In the RS and RS + EB treatments, water-soluble copper (WS-Cu) was 4.6 to 13.6 times, 1.9 to 4.2 times, and 0.5 to 3.0 times higher in Red, Cinnamon, and Black soils, respectively, compared to the control treatment. In the Red soil, exchangeable copper (E-Cu) increased by 30.3 and 32.0% in RS and RS + EB treatments, respectively, after 4 months of incubation, then decreased by 18.3 to 41.1% until the end of the incubation. A similar trend could be observed for the Black soil. Both RS and RS + EB increased exchangeable copper by 84.4 to 160% in Cinnamon soils. However, specifically adsorbed/carbonate-bound Cu (SP/CA-Cu) decreased by 11.3 to 34.9 and by 36.0 to 58.5%, respectively, with the addition of RS and RS + EB to Red and Cinnamon soils. In the Black soil, CA-Cu was 27.9 to 41.6% lower than in the control between 8 and 12 months. The application of RS and RS + EB resulted in increased humic substance-bound Cu (HS-Cu) in Red and Cinnamon soils.
Copper fractions as a function of soil types, organic amendments, and time. a Water-soluble Cu. b Exchangeable Cu. c Specifically adsorbed/carbonate-bound Cu. d Humic substance-bound Cu. e Iron-manganese oxide-bound Cu. f Residual Cu. The content of SO-Cu was below the detection limit. Different letters in each group (CK, RS, RS + EB) at the same sampling time indicate significant difference at P < 0.05 by Duncan’s test
In the control treatments, WS-Cu copper was constant in all the three soils. In contrast, SP/CA-Cu declined with time in Red and Black soils. In all three soils, HS-Cu decreased from the fourth to the eighth month and then increased; a similar trend was found for CA-Cu in the Cinnamon soil. RES-Cu increased with time in all three soils. In the treatments RS and RS + EB, WS-Cu decreased in Red and Cinnamon soils. In all three soils, HS-Cu sharply decreased from the fourth to the eighth month and subsequently increased.
Cu mobility
The Cu mobility factor was highest in the Cinnamon soil, reaching an absolute value of 44.3% of total extractable Cu (Table 4). After 8 months, the mobility values of Red and Cinnamon soils were similar.
The addition of rice straw decreased Cu mobility in all soils; the extent of the mobility reduction was a function of both soil type and incubation time. After 4 months of incubation, Cu mobility was reduced by 13.7 to 20.8% compared to the control treatment. These values nearly doubled after 8 months of incubation. At the end of the experiment, Cu mobility was constant in the Cinnamon soil, but decreased in the Red and Black soils.
Compared to the treatment RS, RS + EB did not significantly affect Cu mobility after an incubation period of 4 months. However, after 8 months of incubation, RS + EB resulted in a greater decrease of Cu mobility in than RS, albeit this could only be observed for the Red soil. The efficiencies of RS versus RS + EB treatments diverged significantly after 12 months of incubation. In all three soils, Cu mobility in the RS + EB treatments was significantly higher than in the RS treatments (Table 4).
In the control treatment, Cu mobility decreased steadily over time in all three soils, indicating that aging contributes to Cu immobilization. At the end of the experiment, after 12 months of incubation, Cu mobility was reduced similarly in all three soils in the control treatment. In the RS treatment, Cu mobility was decreased by 36.8% (Red soil) and 43.6% (Black soil) at the end of the experiment compared to 4 months of incubation. In contrast, the treatment RS + EB in Red and Cinnamon soils resulted in the lowest Cu mobility after 8 months (Table 4), which indicates that the bacteria play important roles in reducing copper mobility could sustain for 8 months. The lowest mobility in Black soil was found after 4-month incubation in Black soil. These results confirmed that bacteria are synergistic with other soil components in depressing Cu mobility at the first 4 to 8 months, while this effect was reduced afterward in the three soils.
Stepwise multiple linear regression analysis
Considering all the soils, the stepwise multiple linear regression analysis (Table 5) indicates that DOC and HS-OC are the most important factors impacting the relatively mobile Cu (P < 0.01), while amorphous iron oxides (Feo) and crystalline iron oxides (Fed) are significantly correlated with the relatively stable copper forms i.e., humic substance-bound Cu and residual Cu (P < 0.05). In different soils, the results of the stepwise multiple linear regression analysis show that HS-OC, OM, and DOC are the most important factors controlling copper distribution in the Red soil (Table 6). DOC and HS-OC showed the most positive effect with WS-Cu (P < 0.01) and HS-Cu (P < 0.01), respectively. HS-OC had a significant negative correlation with E-Cu (P < 0.001) and SP-Cu (P < 0.01), showing the importance of HS-OC in decreasing the mobility of Cu. In the Cinnamon soil, Fed and Feo played a crucial role in copper distribution (Table 7), with Fed had negative association with WS-Cu (P < 0.05) and CA-Cu (P < 0.05) and positive close with RES-Cu (P < 0.001). In Black soil, DOC, OM, pH, Feo, and chelated irons (Fep) were significantly correlated with copper fractions (Table 8). The pH was found to be the most important factors controlling the content of CA-Cu (P < 0.001), HS-Cu (P < 0.05), and RES-Cu (P < 0.001). DOC and OM played the most vital role in WS-Cu (P < 0.001) and E-Cu (P < 0.001), respectively. OM was negatively correlated with E-Cu and CA-Cu (P < 0.01), indicating that OM played a significant role in reducing the mobility of Cu in this soil.
Discussion
The Pearson correlation analysis (Table S2) shows that, among the soil properties and components examined, both HS-OC and Feo correlate significantly with all Cu fractions except for iron-manganese oxide-bound copper (Fe-MnOx-Cu). Moreover, the two parameters are inversely correlated with the mobility factor (P < 0.01), indicating the important roles of solid organic matter and amorphous iron oxides in adsorbing Cu and reducing the mobility of Cu. These results imply that HS-OC and Feo are more crucial in governing Cu distribution in soils. The stepwise multiple regression analysis further indicates that DOC is the first and second factor for WS-Cu and E-Cu, while HS-OC contributes largely to E-Cu, SP/CA-Cu, and HS-Cu. Feo, and Fed are the most vital factors for RES-Cu (Table 5). These observations suggest that organic carbon plays a more important role in shaping the distribution of relatively mobile Cu, and iron oxides can be more critical in stabilizing Cu species.
Considering the relationship between soil characteristics and Cu species for each soil, some different phenomena are observed. In Red soil, all the parameters for soil organic matter including SOM, HS-OC, and DOC have higher correlations than iron oxide with all the examined Cu species, except for RES-Cu (Table 6). However, Fed and Feo are more closely linked with Cu species than organic C in Cinnamon soil (Table 7). For Black soil, no obvious pattern was found regarding the associations between soil parameters and Cu species (Table 8). These results suggest the different roles of organic C or iron oxide in governing Cu distribution and transformation in the three soils. It seems that organic matter is more important in Red soils in regulating the species and mobility of exogenous Cu. The amendment of RS and RS + EB in Red soil produced the largest increases in WS-Cu and HS-Cu, both were highly associated with DOC and HS-OC. Antoniadis and Golia (2015) addressed that total organic carbon may have a major role in heavy metal retention in acidic soil only when the content of OC is higher than a threshold. The soils they examined have an organic carbon concentrations of < 10.6 g kg−1. The total carbon content of our Red soil is 3.9 g kg−1. The simultaneous enhancement of soil organic C with WS-Cu and HS-Cu in RS and RS + EB amended soils suggest that organic matter is more important in influencing Cu distribution in acidic soils with low organic matter contents. In terms of Cinnamon soil, the amount of Fe-MnOx-Cu is similar to that of the other two soils although the contents of Feo, Fed, and Fep are the lowest among the three soils. The stepwise multiple regression analysis shows that Feo, Fed, or both work more significantly in E-Cu, HS-Cu, and RES-Cu, respectively. Moreover, the addition of Fed or Feo results in a more precise regression model for estimation of the WS-Cu and Fe-MnOx-Cu, respectively (Table 7). Therefore, it is likely that iron oxides play a more important role in Cu distribution in Cinnamon soils.
The above results may have environmental implications for the risk assessment and regulation of exogenous Cu to soils. The increase of OM contents in acid soils with low organic carbon might be an important measure to immobilize Cu although part of soluble Cu may be released. In neutral soils with low organic carbon and iron oxides, adjustment in the amounts and forms of iron oxides is a crucial strategy to control Cu distribution and mobility. It is generally accepted that pH is the most significant factor in determining the species of heavy metals (Ramos 2005; Achiba et al. 2009). This study extends our knowledge with respect to the associations of soil components and properties with the species and mobility of heavy metals. Adjusting the content of organic matter and iron oxides depending on soil types should be taken into account when considering the risk and remediation of Cu in contaminated soils.
Conclusions
Our study demonstrates that soil organic carbon, iron oxides, and aging processes have significant effects on the distribution of exogenous Cu in soils. Organic matter is the most important factor influencing copper partitioning in Ultisols, while iron oxides play a more significant role in Alfisols. The mobility of exogenous copper in the soils is dependent on soil organic carbon, amorphous Fe, and aging process. The introduction of both rice straw and bacteria could facilitate the aging and stabilization of Cu in soils. The enhanced effect of organic and microbial materials on Cu reaches maximum at the 4 to 8 months and declines afterwards. The results obtained were helpful for the risk assessment and amelioration of Cu-contaminated soils.
References
Achiba WB, Gabteni N, Lakhdar A, Laing GD, Verloo M, Jedidi N, Gallali T (2009) Effects of 5-year application of municipal solid waste compost on the distribution and mobility of heavy metals in a Tunisian calcareous soil. Agric Ecosyst Environ 130(3-4):156–163. https://doi.org/10.1016/j.agee.2009.01.001
Antoniadis V, Golia EE (2015) Sorption of Cu and Zn in low organic matter-soils as influenced by soil properties and by the degree of soil weathering. Chemosphere 138:364–369. https://doi.org/10.1016/j.chemosphere.2015.06.037
Bao S (2000) Analysis methods for soil agro-chemistry. China Agricultural Press, Beijing (in Chinese)
Bolan N, Kunhikrishnan A, Thangarajan R, Kumpiene J, Park J, Makino T, Kirkham MB, Scheckl K (2014) Remediation of heavy metal(loid)s contaminated soils—to mobilize or to immobilize? J Hazard Mater 266:141–146. https://doi.org/10.1016/j.jhazmat.2013.12.018
Cui Y, Du X, Weng L, Zhu Y (2008) Effects of rice straw on the speciation of cadmium and copper in soils. Geoderma 146(1-2):370–377. https://doi.org/10.1016/j.geoderma.2008.06.010
Diagboya PN, Olu-Owolabi BI, Adebowale KO (2015) Effects of time, soil organic matter and iron oxides on the relative retention and redistribution of lead, cadmium, and copper on soils. Environ Sci Pollut R 22(13):10331–10339. https://doi.org/10.1007/s11356-015-4241-0
Du H, Chen W, Cai P, Rong X, Dai K, Peacock CL, Huang Q (2016) Cd (II) sorption on montmorillonite-humic acid-bacteria composites. Sci Rep 6(1):19499. https://doi.org/10.1038/srep19499
Fan T, Wang Y, Li C, He J, Gao J, Zhou D, Fredman SP, Sparks DL (2016) Effect of organic matter on sorption of Zn on soil: elucidation by Wien effect measurement and EXAFS spectroscopy. Environ Sci Technol 50(6):2931–2937. https://doi.org/10.1021/acs.est.5b05281
He X, Chen W, Huang Q (2012) Surface display of monkey metallothionein α tandem repeats and EGFP fusion protein on Pseudomonas putida X4 for biosorption and detection of cadmium. Appl Microbiol Biot 95(6):1605–1613. https://doi.org/10.1007/s00253-011-3768-3
Huang Q, Chen W, Xu L (2005) Adsorption of copper and cadmium by Cu- and Cd-resistant bacteria and their composites with soil colloids and kaolinite. Geomicrobiol J 22(5):227–236. https://doi.org/10.1080/01490450590947779
Jalali M, Khanlari ZV (2008) Effect of aging process on the fraction of heavy metals in some calcareous soils from Iran. Geoderma 143(1-2):26–40. https://doi.org/10.1016/j.geoderma.2007.10.002
Kabala C, Singh BR (2011) Fractionation and mobility of copper, lead and zinc in soil profiles in the vicinity of a copper smelter. J Environ Qual 30:485–492
Li J, Peng Q, Liang D, Liang S, Chen J, Sun H, Li S, Lei P (2016) Effects of aging on the fraction distribution and bioavailability of selenium in three different soils. Chemosphere 144:2351–2359. https://doi.org/10.1016/j.chemosphere.2015.11.011
Lu A, Zhang S, Shan X (2005) Time effect on the fractionation of heavy metals in soils. Geoderma 125(3-4):225–234. https://doi.org/10.1016/j.geoderma.2004.08.002
Lu A, Zhang S, Qin X, Wu W, Liu H (2009) Aging effect on the mobility and bioavailability of copper in soils. J Environ Sci 21(2):173–178. https://doi.org/10.1016/S1001-0742(08)62247-0
Lu R (2000) Analytical methods for soils and agricultural chemistry. China Agricultural Science and Technology Press, Beijing, China (in Chinese)
Maderova L, Watson M, Paton GI (2011) Bioavailability and toxicity of copper in soils: integrating chemical approaches with responses of microbial biosensors. Soil Biol Biochem 43:1162–1168
Mesquita M, Carranca C (2005) Effect of dissolved organic matter on copper-zinc competitive adsorption by a sandy soil at different pH values. Environ Technol 26(9):1065–1072. https://doi.org/10.1080/09593332608618493
Mousa W, Soloman S, El-Bialy A, Shier HA (2013) Removal of some heavy metals from aqueous solution using rice straw. J Appl Sci Res 9:1696–1701
Narwal RP, Salbu B (1999) Association of cadmium, zinc, copper, and nickel with components in naturally heavy metal rich soils studied by parallel and sequential extractions. Commun Soil Sci Plan 30(7-8):1209–1230. https://doi.org/10.1080/00103629909370279
Qi Y, Zhu J, Fu Q, Hu H, Rong X, Huang Q (2017) Characterization and Cu sorption properties of humic acid from the decomposition of rice straw. Environ Sci Pollut R 24(30):23744–23752. https://doi.org/10.1007/s11356-017-9999-9
Qu C, Ma M, Chen W, Cai P, Huang Q (2017) Surface complexation modeling of Cu (II) sorption to montmorillonite-bacteria composites. Sci Total Environ 607-608:1408–1418. https://doi.org/10.1016/j.scitotenv.2017.07.068
Qu C, Ma M, Chen W, Cai P, Yu X, Feng X, Huang Q (2018) Modeling of Cd adsorption to goethite-bacteria composites. Chemosphere 193:943–950. https://doi.org/10.1016/j.chemosphere.2017.11.100
Ramos MC (2005) Metals in vineyard soils of the Penedès area (NE Spain) after compost application. J Environ Manag 72:1–7
Rizwan MS, Imtiaz M, Huang G, Chhajro MA, Liu Y, Fu Q, Zhu J, Ashraf M, Zafar M, Bashir S, Hu H (2016) Immobilization of Pb and Cu in polluted soil by superphosphate, multi-walled carbon nanotube, rice straw and its derived biochar. Environ Sci Pollut R 23(15):15532–15543. https://doi.org/10.1007/s11356-016-6695-0
Sablu B, Krekelig T, Qughton DH (1998) Characterization of radioactive particles in the environment. Analyst 123:843–849
Sparks DL (2002) Environmental soil chemistry. Soil Conservation Service. U.S. Academic Press, San Diego, CA
Tang X, Zhu Y, Cui Y, Duan J, Tang L (2006) The effect of ageing on the bioaccessibility and fractionation of cadmium in some typical soils of China. Environ Int 32(5):682–689. https://doi.org/10.1016/j.envint.2006.03.003
Tessier A, Campbell PGG, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51(7):844–851. https://doi.org/10.1021/ac50043a017
Yu H, Liu C, Zhu J, Li F, Deng D, Wang Q, Liu C (2016) Cadmium availability in rice paddy fields from a mining area: the effects of soil properties highlighting iron fractions and pH values. Environ Pollut 209:38–45. https://doi.org/10.1016/j.envpol.2015.11.021
Zeng F, Ali S, Zhang H, Ouyang Y, Qiu B, Wu F, Zhang G (2011) The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ Pollut 159(1):84–91. https://doi.org/10.1016/j.envpol.2010.09.019
Funding
This work was financed by the National Natural Science Foundation of China (NSFC) (No. 41230854), National Key Research and Development Program (2017YFA0605001), and the Fundamental Research Funds for the Central Universities (2662015PY016, 2662015PY116).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhihong Xu
Electronic supplementary material
ESM 1
(DOCX 24.7 kb)
Rights and permissions
About this article
Cite this article
Shi, H., Li, Q., Chen, W. et al. Distribution and mobility of exogenous copper as influenced by aging and components interactions in three Chinese soils. Environ Sci Pollut Res 25, 10771–10781 (2018). https://doi.org/10.1007/s11356-018-1288-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1288-8