Abstract
Seaweeds have been used as animal feed since a long time and are consumed as food in several cultures. In fact, macroalgae are a source of protein, fiber, polyunsaturated fat, and minerals. The concentration of trace elements was determined in dominant macroalga species from three sites of the northwestern Mediterranean Sea. A high interspecies variability was observed, with higher metal levels in brown and green than those in red seaweeds. The maximum values set by European regulations for arsenic, mercury, and cadmium in food and feed were never exceeded, but a few samples were very close to limits set for mercury. Conversely, the maximum limit for lead in feed was exceeded in all species from one of the considered sites. Analogously, lead in seaweeds could constitute a potential risk for human health, due to the exceeding of the maximum value set for food supplements.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In European countries, there is a notable interest in marine macroalgae as alternative food and feed components, due to the increasing demand of sustainable protein source of non-animal origin. Seaweeds are already part of the diet in many Asian countries since a long time, and in Western countries, their beneficial properties are now intriguing new markets.
The nutritional value of seaweeds is due to their content of essential amino acids, fiber, vitamins, minerals, and ω-3 fatty acids (Astorga-Espanã et al. 2015; Hamid et al. 2015). In particular, in human nutrition, the use of macroalgae as food supplements is recommended to increase the contents of essential trace elements (Subba Rao et al. 2007; Bocanegra et al. 2009).
Algae have been used as a feed supplement for animals since ancient times, especially in shortage and wars times, and are increasingly recognized as valuable alternative feeds for livestock and aquaculture (Evans and Critchley 2014) as they were found to supply several bioactive compounds improving animal health (Makkar et al. 2016). It is also well known that seaweeds contain higher concentrations of trace elements than plants (Rupérez 2002; Rohani-Ghadikolaei et al. 2012) and that this content is strictly related to macroalga families, genera, and species, as well to geographical provenience and environmental conditions in which macroalgae live (Rupérez 2002; Akcali and Kucuksezgin 2011). However, the ability to bioaccumulate metals at levels thousands of times higher than in seawater constitutes a potential threat to human and animal health.
Thirteen metals and metalloids have been recognized as potentially hazardous to men and aquatic biota and have been included on the Priority Pollutants List by environmental control agencies such as the US Environmental Protection Agency (EPA 2014). Between them, arsenic (As), cadmium (Cd), mercury (Hg), and lead (Pb) could pose a serious risk to human and animal due to their recognized toxicity and were classified as probable human carcinogens according to the International Agency for Research on Cancer (IARC 1987). Maximum limits were then set for these elements worldwide and by the European Community (Commission Regulations 186/2015 EU, 1005/2015 EU, 1275/2013 EU, 488/2014 EU, 629/2008 EU) for their content in food and feed.
Moreover, the majority of essential trace elements such as chromium (Cr), cobalt (Co), copper (Cu), iron (Fe), manganese (Mn), and zinc (Zn) are also known to exert toxic effects if assumed above the recommended levels (EFSA 2012, 2013a, b, 2014a, b). Cobalt maximum level was recently set for animal nutrition (Commission Regulations 134/2014).
A few studies assessing the levels of trace elements in seaweeds have been performed in Europe and in particular in the Mediterranean Sea, and they mostly focused on few metals and few types of seaweed species.
Llorente-Mirandes and coauthors (2010) focused on As compounds in littoral zone algae from the western Mediterranean Sea (Catalan coast, Spain), while Akcali and Kucuksezgin (2011) analyzed Fe, Zn, Cu, Cr, Cd, Hg, and Pb in macroalgae from eastern Aegean coastal areas. Malea and Kevrekidis (2014) investigated the presence of 15 trace elements in macroalgae from the Gulf of Thessaloniki, Aegean Sea; moreover, Malea and coauthors (2015) analyzed trace element seasonality in four green algae (Ulva intestinalis, Ulva rigida, Codium fragile, Gracilaria gracilis) from the same area. Khaled and coauthors analyzed the distribution of Cd, Cu, Fe, Ni, Pb, and Zn concentrations in six species of marine macroalgae from Marsa-Matrouh beaches, Egyptian Mediterranean Sea.
Italian Mediterranean coasts were scarcely investigated with regard to inorganic contaminants in macroalgae. Campanella et al. (2003) quantified Cd, Cr, Cu, Pb, and Zn levels in the brown alga Padina pavonica from Sicily Island while Conti and Cecchetti (2003) measured Cd, Cr, Cu, Pb, and Zn concentrations in the green alga Ulva lactuca and in the brown alga Padina pavonica from Tyrrhenian coastal areas. Finally, Conti and coauthors investigated the presence of Cd, Cr, Cu, Pb, and Zn in the brown alga Cystoseira sp. (2015) and the same trace elements in the brown alga Padina pavonica (2010) from the Tyrrhenian Sea.
Mediterranean seaweed resources are still underexploited, in spite of their potential use for animal feed and human nutrition. However, it is necessary to assess the levels of contaminants in order to evaluate if macroalga consumption is safe. We choose to investigate seaweeds from three sites located in northwestern Mediterranean coastal areas, representative to Chlorophyta (green algae), Ochrophyta (brown algae), and Rhodophyta (red algae) phylum, easily available and abundant then of potential use for feed and food.
The aims of this investigation were as follows:
-
i.
To assess the concentrations of non-essential trace elements (aluminum, antimony, arsenic, beryllium, cadmium, mercury, lead, tin, thallium, and vanadium) and essential trace elements (cobalt, chromium, copper, iron, manganese, molybdenum, nickel, selenium, and zinc) in 13 macroalga species widespread in northwestern Mediterranean Sea coastal areas, and then of potential use in human and animal nutrition
-
ii.
To assess the compliance with the maximum levels established by the European Commission Regulations for arsenic, cadmium, mercury, and lead in food and feed
-
iii.
To analyze site-specific and/or inter-species variation in trace element accumulation, in order to identify the species potentially suitable for food and feed
Materials and methods
Study area and collection of samples
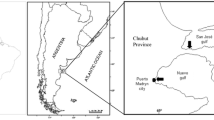
The sampling area was located in the northwestern Mediterranean, Ligurian Sea (Fig. 1).
The algae were collected during the summer 2016, in scuba diving, along a bathymetric transects between 0 and 42 m of depth, on photophilous and sciaphilous sea floor (sea bottom), in three sites with different environmental protections in the Ligurian and Northern Tyrrhenian Sea, NW Mediterranean area. The species of seaweed collected for this study are more abundant and more widespread in all three sampling sites.
The first sampling site was Bergeggi (SV), along the coast of the Ligurian Sea, in a marine protected area (MPA “Isola di Bergeggi”—44° 14′ 26. 94″ N, 8° 26′ 50.98″ E). The area of sampling is located in General Reserve named B zone of the MPA, in which the human activities are restricted and regulated by the Italian law (L. 979/82 and L. 394/91). Nevertheless, the marine protected area is close to a densely and populated district (Vado Ligure) with high shipping traffic and with an important industrial and commercial harbor.
The second site was Remaiolo rock (Elba Island, LI, Tuscan Archipelago: 42° 42′ 35.17″ N, 10° 24′ 44.97″ E), in the Tyrrhenian Sea, NW Mediterranean, 5 nmi off Tuscan coast. This site is an unprotected area in the most populated island of the Tuscan Archipelago, mainly in summer holidays.
The third one was located in Capraia Island (LI) 43° 4′ 26.90″ N, 9° 49′ 39.63″ E, in the Ligurian Sea, NW Mediterranean, about 30 nmi off Tuscan coast, in the National Park of Tuscan Archipelago (PNAT). This site is protected and has the same degree of protection of the B zone in the MPA “Isola di Bergeggi.” The island is sparsely populated without industrial activities.
These sites were the same of a previous investigation focused on the rare earth element levels in seaweeds (Squadrone et al. 2017).
Samples collected were preserved in seawater, stored on board in polyethylene bags, and transported and refrigerated to the laboratory. The specimens were examined at the stereomicroscope, and after cutting the seaweed in thin sections, the specific identification was performed.
In the chemical laboratory, seaweeds were rinsed with distilled water, freeze-dried, and homogenized. Five samples for each species from each sampling location, of approximately 1–1.5 g each, were subjected to trace element determination.
Detection of trace elements
Instrumentation
Sample mineralization was performed using a microwave digestion lab station (Ethos 1, Milestone, Shelton, CT, USA), equipped with a 10-position rotor for high-pressure polytetrafluoroethylene (PTFE) digestion tubes.
Trace element determination, with the exception of mercury, was performed by a quadrupole inductively coupled plasma mass spectrometer (ICP-MS Xseries II, Thermo Scientific, Bremen, Germany) equipped with a multi-vial autosampler (ASX 520, CETAC Technologies, Omaha, NE, USA). Instrument was tuned daily before each analytical trial; operating conditions are listed in Table S1. Direct Mercury Analyzer (DMA80, Milestone, Shelton, CT, USA), that does not require sample pre-treatment, was instead utilized to perform total mercury determination. This instrument features a circular, stainless steel, interchangeable 40-position autosampler and requires regular grade oxygen as a carrier and decomposition gas. DMA80 is equipped with a Hollow Cathode Lamp (kHg = 253.7 nm) and a Si-photodiode sensor.
Reagents
Analytical quality grade HNO3 and H2O2 were provided by Sigma-Aldrich (Merck, Saint Louis, USA). Argon, used for plasma generation and maintenance, and 5% hydrogen and 95% helium mixture, used in the collision cell, were all of high purity grade (SIAD, Turin, Italy). All metal standard stock solutions used for calibration curves were purchased from Ultra Scientific (Bologna, Italy). A water purification system (Arium611VF, Sartorius Stedim, Italy S.p.A.) was used to obtain ultrapure water.
Sample digestion and analysis
Before each analytical session, all digestion tubes were cleaned with concentrated acid, rinsed with ultrapure water, and dried at room temperature under a chemical hood. Disposable polypropylene tubes were used to storage mineralized samples. All samples were rinsed with ultrapure water and freeze-dried before analyses. Freeze-dried samples (1.0–1.5 g) were directly weighed into PTFE digestion tubes. Seven milliliters of HNO3 (70% v/v) and 1.5 mL of H2O2 (30% v/v) were then added before the microwave digestion process and programmed as follows: heating to 130 °C in 8 min, hold for 2 min; heating to 200 °C in 8 min, hold for 5 min; and cooling for 30 min. Digested samples were then quantitatively transferred to 50-mL polypropylene tubes and gravimetrically diluted to a final weight of 50 g with ultrapure water. Multi-elemental quantification was performed using an external standard calibration curve and rhodium and germanium as internal standards. Certified Reference Materials (Oyster Tissue—SRM 1566b and CRM-279 Sea lettuce from the National Institute of Standard and Technology) were processed during each analytical session to verify performances of the methods. Limit of quantification (LOQ), reference material values, and recovery percentages are shown in Table S2. The analytical method was validated according to ISO/IEC17025 (general requirements for the competence of testing and calibration laboratories).
Statistical analysis
All the analyses were performed using five replicates, and results were reported for each element for each species as mean ± standard error and for each site as mean ± standard deviation (Table 1). The one-way analysis of variance (ANOVA) was used to compare average contents of the trace elements in macroalgae between the three sampling sites (Table 2). Results were considered statistically significant at P values of < 0.05. Statistical calculations were performed using Graph Pad Statistics Software Version 6.0 (GraphPad Software, Inc., USA).
To verify the compliance to maximum limits for contaminants set by European Regulation for feed (Commission Regulations 186/2015 EU, 134/2014 EU, and 1275/2013 EU), results were expressed as requested in feed with a moisture content of 12% (Table S3). The uncertainty of measurement was also considered to establish the compliance of results, according to Regulation 333/2007 EU and amendments, laying down the methods of sampling and analysis for the official control for food and feed (Table S3).
Results and discussion
The concentrations of the 19 investigated trace elements in marine macroalgae from three collection sites of the northwestern Mediterranean Sea are shown in Table 1 and were expressed in milligrams per kilogram dry weight (mean ± standard error). Non-essential element levels were also graphically presented for the three sites in Fig. 2 and essential trace elements in Fig. 3 (mean ± standard deviation). The different metal concentrations in species from the same sampling site were graphically presented in Fig. 4. A high variability in metal levels between species and between sampling sites was recorded.
Inter-site variability
Metal levels in the analyzed seaweeds were greatly influenced by the different areas of collection.
In fact, macroalgae from site 1 (Bergeggi, SV), despite they were collected in a marine protected area, have shown the highest concentrations for most of all the analyzed trace elements. In particular, the highest values were recorded in the Ochrophyta Halopteris filicina (Al 9916 mg kg−1, Be 0.70 mg kg−1, Cr 35 mg kg−1, Ni 32 mg kg−1, Pb 40 mg kg−1, Se 2.0 mg kg−1, Tl 0.13 mg kg−1, V 43 mg kg−1, and Zn 53 mg kg−1) and in the Chlorophyta Codium bursa (As 37 mg kg−1, Cd 0.32 mg kg−1, Co 5.6 mg kg−1, Cu 73 mg kg−1, Fe 14,148 mg kg−1, and Sn 4.6 mg kg−1) as shown in Table 1 and Figs. 2 and 3. This site, in a marine protected area, is however affected by its proximity to an important industrial and touristic harbor.
In site 2 (Elba Island, LI), we detected the highest levels of Mn and Sb (379 mg kg−1 and 1.1 mg kg−1 respectively) in the Chlorophyta Flabellia petiolata, of Mo (4.7 mg kg−1) in the Chlorophyta Codium bursa, and of Hg (0.12 mg kg−1) in the Rhodophyta Peyssonnelia squamaria.
In site 3 (Capraia Island, LI), the lowest levels for all the trace elements were registered, with the exception of cadmium, mercury, and molybdenum which values were comparable with those of the other two sites (Table 1; Figs. 2 and 3). This marine protected area seems to be the less contaminated by trace elements of anthropogenic origin; in fact, population is scarce and industrial activities are absent.
In Table 2, the analysis of variance for the three sampling sites was shown. There were no statistical significant differences (P > 0.05) for Cd, Cu, Hg, Mo, and Sb levels in the three sites, while highly significant differences (P < 0.0001) were registered for Al, Cr, Pb, and Se. Moreover, Be, Co, Fe, Mn, Ni, Sn, Tl, and Zn were differently significant at the 0.01 probability level, and As and V were differently significant at the 0.05 probability level.
Inter-macroalga species variability
Site 1
In Bergeggi (SV), the highest values of Al, Be, Cr, Mn, Mo, Ni, Pb, Se, Tl, V, and Zn were found in the Ochrophyta Halopteris filicina (Table 1, Fig. 4), while in the Chlorophyta Codium bursa, the highest levels of As, Cd, Co, Cu, Fe, Sb, and Sn were recorded. Highest concentration of mercury was found in the Ochrophyta Padina pavonica; conversely, in the Rhodophyta Ganonema farinosum, the lowest values of Al, As, Be, Cd, Co, Hg, Fe, Mn, Mo, Ni, Pb, Se, and Zn were recorded.
The total metal content (sum of all the analyzed metals for each species) was in the following decreasing order: Halopteris filicina (20,172 mg kg−1 d.w.) > Codium bursa (19,731 mg kg−1 d.w.) > Flabellia petiolata (14,724 mg kg−1 d.w.) > Padina pavonica (10,357 mg kg−1 d.w.) > Ganonema farinosum (8292 mg kg−1 d.w.)
Site 2
In Elba Island (LI), the highest values of most metals were found in green algae (Table 1, Fig. 4) and precisely As, Be, Co, Fe, Mn, and Sb in Flabellia petiolata, Cr, Mo, and Sn in Codium bursa, and Tl in Caulerpa racemosa. The brown alga Padina pavonica had the highest values of Sn and Zn, Halopteris filicina of Hg, and Dictyota dichotoma of Al, Pb, and Se. In the brown alga Halopteris filicina, the lowest values of Al, Cd, Co, Cr, Cu, Fe, Mn, Pb, Sb, Se, Sn, Tl, and V were recorded, differently from site 1, where this species showed the highest value for the majority of metals.
The total metal content was in the following decreasing order: Flabellia petiolata (12,534 mg kg−1 d.w.) > Codium bursa (11,529 mg kg−1 d.w.) > Dictyota dichotoma (8818 mg kg−1 d.w.) > Peyssonnelia squamaria (8222 mg kg−1 d.w.) > Laurencia obtusa (5081 mg kg−1 d.w.) > Padina pavonica (4578 mg kg−1 d.w.) > Caulerpa racemosa C (3720) > Halopteris filicina O (1092 mg kg−1 d.w.).
Site 3
In Capraia Island (LI), the highest values of Al, Be, Co, Cr, Hg, Fe, Mn, Mo, Ni, Pb, Sb, Se, and V were found in the Ochrophyta Halopteris scoparia (Table 1, Fig. 4), of As in the Chlorophyta Codium bursa, and of Tl in Flabellia petiolata, while the highest value of Cd was found in the Ochrophyta Cystoseira spp. The red alga Dudresnaya verticillata had shown the lowest value of Al, Be, Cd, Fe, Ni, Sb, Sn, and Tl (Table 1, Fig. 4).
The total metal content was in the following decreasing order: Halopteris scoparia (4038 mg kg−1 d.w.) > Halimeda tuna (1682 mg kg−1 d.w.) > Peyssonnelia squamaria (1637 mg kg−1 d.w.) > Padina pavonica (1513 mg kg−1 d.w.) > Codium bursa (997 mg kg−1 d.w.) > Flabellia petiolata C (919 mg kg−1 d.w.) > Cystoseira spp. (916 mg kg−1 d.w.) > Dudresnaya verticillata (407 mg kg−1 d.w.).
In general, species belonging to green and brown macroalgae accumulated trace elements to a higher extent in comparison to red seaweeds, according to previous studies suggesting that metal accumulation is likely to be related to the phylogenetic origin of the species, which determines the biochemical composition of macroalgae (Stengel et al. 2004). Moreover, brown seaweeds seem to have higher accumulation capacity of some elements such as Al, As, Be, Co, Cr, Ni, Pb, Se, Tl, V, and Zn than red and green macroalgae, and this was particularly evident in site 1, the more affected by anthropogenic activities. This ability of brown seaweeds to concentrate some elements, irrespective of thallus morphology and growth strategy, was already suggested by Malea and Kevrekidis (2014) in examining Mediterranean macroalgae.
Conversely, Rubio and coauthors (2017), analyzing edible seaweed cultivated in Asia (China, Japan, and South Korea) and in European countries (Spain), found higher concentrations of trace and toxic elements in red seaweeds. Moreover, they found that the exposure to Al, Cd, and Pb through the consumption of these cultivated seaweeds was not of concern.
Compliance with European Regulations for feed and food
The Commission Regulation 2015/186 EU sets the maximum levels for arsenic, lead, and mercury as undesirable substances in animal feed (maximum content in milligrams per kilogram relative to a feed with a moisture content of 12%). The limits are the following: arsenic in seaweed meal and feed materials derived from seaweed, 40 mg kg−1; lead in feed materials, 10 mg kg−1; and mercury in feed materials, 0.1 mg kg−1. The Commission Regulation 1275/2013 EU sets the maximum level for cadmium as undesirable substance in animal feed (maximum content in milligrams per kilogram relative to a feed with a moisture content of 12%). The limit for cadmium in feed materials of vegetable origin is 1 mg kg−1.
The maximum limit for arsenic was never exceeded in the analyzed samples; the highest level of As was detected in the green alga Codium bursa from site 1 (33 mg kg−1 with a moisture content of 12%). In food, the maximum arsenic content is still not regulated, with the exception of rice.
Cadmium concentrations were an order of magnitude lower than the maximum limit set for feed in all samples; the highest level of Cd was detected in the green alga Codium bursa from site 1 (0.32 mg kg−1 with a moisture content of 12%).
Commission Regulation 488/2014 EU sets maximum levels of cadmium in food and the limit for food supplements “consisting exclusively or mainly of dried seaweed, products derived from seaweed, or of dried bivalve mollusks” is 3.0 mg kg−1. All the analyzed seaweeds were far from this level.
Mercury concentrations were close to the maximum limit for feed (0.1 mg kg−1) in the brown alga Halopteris filicina (site 2) and Halopteris scoparia (site 3), in the red alga Peyssonnelia squamaria from site 2 (0.09 mg kg−1 with a moisture content of 12% taking into account the measurement uncertainty according Regulation 333/2007 EU) as shown in Table S3.
In human nutrition, mercury maximum levels were set for several fishery products by the Commission Regulation 629/2008 EU and for food supplements. There is nowadays a wide use of alga-based dietary supplements; in these supplements, Hg level must be compliant with the maximum limit of 0.10 mg kg−1. Mercury level was close to this limit in the three species from site 2 and 3 cited above (H. filicina, H. scoparia, P. squamaria; Table S3).
The maximum limit of 10 mg kg−1 set for lead in animal nutrition (expressed with a moisture content of 12%, taking into account the measurement uncertainty according to Regulation 333/2007 EU) was exceeded in four out of five species from site 1, with levels ranging from 12 mg kg−1 in the alga Padina pavonica to 30 mg kg−1 in the alga Halopteris filicina (Table S3).
As intended for human consumption, analogously to mercury, the limit was set for lead in food supplements by Commission Regulation 1005/2015 EU, and the concentration not to be exceeded is 3 mg kg−1. In this case, lead levels exceeded the maximum level in all five species from site 1, in three out of eight analyzed species in site 2 (in the green alga Flabellia petiolata and Codium bursa (respectively 6 and 4 mg kg−1) and in the brown alga Dictyota dichotoma (7 mg kg−1)), and in five out of eight sampled species from site 3 (the green macroalga Flabellia petiolata and Halimeda tuna (5 mg kg−1), the brown alga Halopteris scoparia and Cystoseira spp. (respectively 10 and 4 mg kg−1), and the red alga Peyssonnelia squamaria (5 mg kg−1)).
Cobalt is a component of vitamin B12 (cobalamin) that exerts essential biological functions in human and animal; moreover, cobalt compounds are employed as nutritional additives in animal nutrition (EFSA 2012), and the maximum cobalt content in complete feed was set by EU Regulation 134/2014 at 1 mg kg−1 (relative to a feed with a moisture content of 12%). This level was set in order to reduce an excessive cobalt intake in animals and consequently in humans consuming food of animal origin. Cobalt was found higher in algae from site 1 (3.3 mg kg−1 mean value, 4.7 highest value in H. filicina) than that from sites 2 and 3 (Fig. 3, Table 1). In particular, levels exceeding the maximum limit were found in green and brown alga, precisely in F. petiolata, C. bursa, and H. filicina from site 1, in F. petiolata and C. bursa from site 2, and in H. scoparia from site 3 (Table S3).
Inorganic priority pollutants EPA listed
In addition to the metals discussed above, antimony, beryllium, chromium, copper, nickel, selenium, thallium, and zinc were indicated as priority pollutant by the United States Environmental Protection Agency (EPA).
Antimony, beryllium, and thallium are non-essential trace elements, and their presence in the marine environment derives from natural processes and human activities. Concentrations of antimony in natural waters are usually low (less than 1 μg L−1), and in seaweeds from different world areas, values were generally found to be in the range 0.1–0.2 mg kg−1 dry weight and up to 1.5 mg kg−1 (Filella et al. 2007). Differences between the three sampling sites were not statistical significant for Sb (Table 2, Fig. 2), and levels ranged from 0.045 mg kg−1 in the red alga P. squamaria (site 3) to 1.1 mg kg−1 in the green alga F. petiolata (site 2) (Table 1).
Be and Tl had shown significant statistical differences between sites (Table 2), with highest beryllium mean value (0.38 mg kg−1) and upper value (0.70 mg kg−1) in the brown alga H. filicina from site 1 and the lowest in site 3 (mean value 0.053 and lower value 0.010 mg kg−1 in the Rhodophyta Dudresnaya verticillata). The same trend was registered for thallium; the highest mean concentration (0.071 mg kg−1) and upper value (0.13 mg kg−1) were found in the brown alga H. filicina from site 1.
Our findings were consistent with the low concentrations of Be and Tl that are usually found in natural environments (Edmunds 2011; Viraraghavan and Srinivasan 2011).
Aluminum was the non-essential trace elements for which we registered the highest levels in seaweeds, in particular from site 1 (Table 1 and Fig. 2) with a mean value of 6089 mg kg−1; the difference in concentrations between sites was highly statistical significant (P < 0.0001; Table 2). The highest value (9916 mg kg−1) was found in the brown alga H. filicina (Table 1, Fig. 3). In our knowledge, there are no other studies regarding aluminum level in marine seaweeds, in particular from the Mediterranean Sea. In a previous study (Battuello et al. 2016), we found that Al levels in Mediterranean zooplankton (Tyrrhenian Sea) from superficial waters ranged from 1200 to 3500 mg kg−1 dry weight, while Al levels in seawater were in the range 1.50–1.80 μg L−1.
Aluminum is the most abundant metal in the earth’s crust, but notable levels in the environment may be due to mining and processing of Al ores (ASTDR 2008). The concentration of dissolved Al in the upper water column of the Mediterranean Sea was found to be higher than that in the Atlantic Ocean (Rolison et al. 2015). Moreover, Caschetto and Wollast (1979) observed in the Mediterranean Sea the highest Al concentrations of any marine basin, while Chou and Wollast (1997) suggested that in the western Mediterranean basin, the atmospheric depositions are a significant external source of Al. In our study, site 1 Bergeggi (SV) has shown a significant level of Al in seaweeds while site 3, Capraia (LI), appeared to be the less contaminated. There were no limits for Al in food and feed, but EFSA (2011) recommended a provisional tolerable weekly intake (PTWI) of 2 mg Al kg−1 body weight.
Vanadium mean concentration in site 1 was twice the values found in sites 2 and 3 (Table 1, Fig. 2); surprisingly, the highest values were registered in the brown alga H. filicina. The levels we found in macroalgae from site 1 (up to 43 mg kg−1) were higher than those reported by Malea and Kevrekidis (2014) in seaweeds from Mediterranean Sea (Gulf of Thessaloniki), where the highest level was 26.21 mg kg−1. Vanadium was suggested to be an essential element for marine macroalgae (Fries 1982).
Chromium, copper, nickel, selenium, and zinc are known to be essential trace elements and to exert several biological functions in humans and animals.
Cr levels were highly statistical significant different between the three sites (Tables 1 and 2). In fact, in site 1, the mean concentration was 21 mg kg−1, and the highest value was registered in the brown alga H. filicina (35 mg kg−1); in sites 2 and 3, Cr mean value was 3.3 mg kg−1.
Recently, EFSA proposed a tolerable daily intake (TDI) corresponding to 0.3 mg kg−1 b.w. of trivalent Cr (EFSA 2014a, b). Nickel mean value was highest in site 1 (18 mg kg−1), the maximum concentration was found in H. filicina (32 mg kg−1), and in sites 2 and 3, Ni values were lower (4.7 and 5.7 mg kg−1 respectively). Data of Ni in seaweeds are scarce, but both Ni and Cr had higher levels in the most industrialized site (Bergeggi, SV) than their concentrations may be related to anthropogenic origins.
The essential trace element copper did not show significant differences between sites (Tables 1 and 2), and the highest value (73 mg kg−1) was found in the green alga C. bursa from site 1 and the lowest (4.1 mg kg−1) in H. filicina from site 2 (Table 1). Seaweeds are able to bioconcentrate Cu from seawaters (Ho 1988), and Cu levels up to 300 mg kg−1 have been registered in macroalga species collected in polluted areas (Hawk et al. 1974). Our samples are well below this concentration. Established a tolerable upper intake of 5 mg day−1 of copper for adults.
Se and Zn concentrations were found highly statistical different between site (Tables 1 and 2, Fig. 4), and the highest values of both elements were found in H. filicina from site 1 (2 and 52 mg kg−1 respectively) while the mean values were 1.4 and 34 mg kg−1.
Moore and Ramamurti (1987) suggested a Zn background level up to of 100 mg kg−1 for areas not impacted by anthropogenic pollution that is double the highest concentration we found, while the tolerable upper intake level of 25 mg day−1 for adults was suggested by EFSA (2014a, b).
Iron, manganese, molybdenum, and tin
These trace elements were not considered in the list of EPA inorganic priority pollutants and are essential trace elements with the exception of tin.
Iron was the essential trace elements for which we registered the highest values (Table 1), up to 14,148 mg kg−1 in the green alga C. bursa from site 1. In the three sites, concentrations were quite different, decreasing from site 1 (mean value 8156 mg kg−1) to site 3 (mean value 666 mg kg−1) as shown in Fig. 3. The high Fe concentration encountered in seaweeds could be related to necessity of this element and involved in photosynthetic and respiratory electron transport, in marine alga growth (Storelli et al. 2001), and with the recognized capacity of seaweeds to bioaccumulate Fe from the surrounding marine environment (Eisler 1981). A tolerable upper level for iron in human nutrition has still not settled, but the Institute of Medicine (2001) suggested an intake of 45 mg day−1 in adults.
Manganese was the second trace element for concentrations (Table 1); similarly to Fe, levels decreased in algae from site 1 (229 mg kg−1) to site 3 (48 mg kg−1), but the highest level was found in the green alga F. petiolata from site 2. As well as iron, manganese is involved in photosynthetic functions, and it is necessary for autotrophic growth (Tanner et al. 1960). EFSA (2013a, b) proposed for adult population an adequate intake (AI) of 3 mg day−1 of Mn.
Molybdenum concentrations were not statistically different in alga from the three sites (Table 2), and the highest concentration was found in C. bursa and the lowest level in H. filicina from site 2.
Tin was found higher in site 1 (Bergeggi, SV) (mean value 1.8 mg kg−1, highest value 4.7 in C. bursa) than that in sites 2 and 3 (Table 1, Fig. 2), and its presence could be related to the proximity of this site to an important industrial and commercial harbor.
Comparison with previous findings in Mediterranean Sea
Due to the high inter-species variability in metal accumulation in the analyzed seaweeds, comparison with previous findings in the Mediterranean Sea was performed only with studies that analyzed the same macroalga species of our investigation.
Between the analyzed seaweed species, the brown alga P. pavonica from the Gulf of Gaeta (Central Italy, Tyrrhenian Sea) was tested for Cd, Cr, Cu, Pb, and Zn contents by Conti and Cecchetti (2003).
Cadmium was found in the range 0.39–0.66 mg kg−1, slightly higher than the values we found in our investigation in the three sampling sites (0.25, 0.29, and 0.26 mg kg−1 respectively; Table 1). Copper levels were found in the range 11.8–12.4, as well as we estimated in sites 1 and 2, while in site 3 (Capraia, LI), Cu concentration was lower (5.4 mg kg−1). Zinc in P. pavonica from the Gulf of Gaeta (Conti and Cecchetti 2003) was in the range 45–65 mg kg−1, slightly higher than the values we register in our three sampling sites (31, 25, and 22 mg kg−1 respectively; Table 1). Chromium and lead concentrations were comparable with those we recorded in P. pavonica from sites 2 and 3, while in site 1 (Bergeggi, SV), the levels were an order of magnitude higher (Cr 18 mg kg−1 and Pb 17 mg kg−1) than those registered in Gaeta Gulf (Conti and Cecchetti 2003).
In the Mediterranean Sea, Gulf of Thessaloniki, Malea and Kevrekidis (2014) investigated metal concentrations in P. pavonica; Cd and Mo levels were comparable to the concentrations found in our study; As concentration was comparable to sites 1 and 2, but higher than the level we found in site 3 (Capraia, LI). Co, Cr, Mn, Ni, Se, V, and Zn concentrations were generally higher in P. pavonica from the Gulf of Thessaloniki while Pb was lower than the concentration we found in site 1 (Bergeggi, SV).
Akcali and Kucuksezgin (2011) analyzed seaweeds from the eastern Aegean coast, eastern Mediterranean Sea, such as P. pavonica and Cystoseira spp.
P. pavonica samples from Aegean coast has shown Pb, Cr, Cu, and Zn comparable with those we registered in sites 2 and 3, but lower than site 1. Mercury concentration was in the same range of our samples, while iron was an order of magnitude lower than the levels we registered in P. pavonica from the three sampling sites.
In Cystoseira spp. samples from site 3 (Capraia, LI), we found levels of Cu, Fe, Pb, and Zn comparable with those recorded in the same species from Aegean coasts. Cadmium, chromium, and mercury concentrations were higher in Cystoseira from eastern Aegean coast. In the other two sites (1 and 2) of our study, Cystoseira was not available and then comparison was not possible.
In general, the comparison with other Mediterranean areas (Conti and Cecchetti 2003; Akcali and Kucuksezgin 2011; Malea and Kevrekidis 2014) has shown that lead, chromium, iron, and zinc were higher in samples of P. pavonica collected in site 1 (Bergeggi, SV).
Conclusions
Different species of seaweeds differently accumulated metals from seawaters, and this could be related to their particular morphology, growth rates, and affinity to certain types of metals rather than for other. In order to evaluate the use of Mediterranean macroalgae for human and animal nutrition in the point of view of food and feed safety, we found higher metal levels in brown and green macroalgae than those in red seaweeds. Significant inter-site differences were registered for the majority of metals, emphasizing the fact that the provenience of seaweeds has a significant influence in metal bioaccumulation. Toxic elements such as cadmium and mercury had comparable levels in the three examined sites, and while cadmium levels were of no concern, mercury concentrations in few samples were close to limit set for feed and food supplements.
Lead is the element that could constitute the major limitation in the use of the examined algae for animal nutrition, in particular in green and brown seaweeds from site 1, because of the exceeding of maximum allowed level. Analogously, cobalt content could constitute a limiting factor in C. bursa, F. petiolata, and H. filicina from the three considered locations.
However, we should take into account that only in rare cases, animals survive entirely only eating seaweed (e.g., the OrKney sheep in North Ronaldsay, Scotland; the white-tailed deer in coastal Maine, USA). The high metal content in seaweeds has harmful effects on animal health, and in fact, these ruminants suffer from severe dental disease and present an extensive mineralization of the kidney medulla (Britt and Baker 1990). In most cases, seaweeds constitute a component of variable percentage in feeding stuffs, and then the trace elements are subjected to dilution in the complete feed. With this premise, most of the investigated macroalgae could constitute a potential feed ingredient, in particular Mediterranean seaweeds collected from sites 2 and 3, where trace elements registered lower values.
A different conclusion can be drawn about the use of marine macroalgae in human food supplements, because alga-based products are usually constituted in toto by seaweeds. A few investigations already suggested that alga-based supplements might result in a daily intake of some elements, such iron and chromium exceeding the daily safe level (Raab et al. 2016). In our study, seaweeds from site 1 have shown to contain a considerable amount of aluminum and iron; moreover, the maximum levels for lead in food supplements were exceeded in all species from site 1, in C. bursa, F. petiolata, D. dichotoma from site 2, and in F. petiolata, H. tuna, H. scoparia, Cystoseira spp., and P. squamaria from site 3.
We conclude that lead concentration in seaweeds could constitute a potential risk for human health and that the sampling site and the macroalga species should be carefully identified in order to prevent potentially toxic effects on consumers.
References
Agency for Toxic Substances and Disease Registry, ATSDR (2008). Toxicological profile for aluminum, pp 357
Akcali I, Kucuksezgin F (2011) A biomonitoring study: heavy metals in macroalgae from eastern Aegean coastal areas. Mar Pollut Bull 62(2011):637–645. https://doi.org/10.1016/j.marpolbul.2010.12.021
Astorga-Espanã MS, Rodriguez Galdon B, Rodrıguez Rodrıguez EM, Dıaz Romero C (2015) Mineral and trace element concentrations in seaweeds from the sub-Antarctic ecoregion of Magallanes (Chile). J Food Compos Anal 39:69–76. https://doi.org/10.1016/j.jfca.2014.11.010
Battuello M, Brizio P, Mussat Sartor R, Nurra N, Pessani D, Abete MC, Squadrone S (2016) Zooplankton from a North Western Mediterranean Area as a model of metal transfer in a marine environment. Ecol Indic 66:440–451. https://doi.org/10.1016/j.ecolind.2016.02.018
Bocanegra A, Bastida S, Benedí J, Ródenas S, Sánchez-Muniz FJ (2009) Characteristics and nutritional and cardiovascular-health properties of seaweeds. J Med Food 12(2):236–258. https://doi.org/10.1089/jmf.2008.0151
Britt DP, Baker JR (1990) Causes of death and illness in the native sheep of North Ronaldsay, Orkney. I. Adult sheep. Br Vet J 146(2):129–142. https://doi.org/10.1016/0007-1935(90)90005-N
Campanella L, Conti ME, Cubadda F, Sucapane C (2003) Trace metals in seagrass, algae and molluscs from an uncontaminated area in the Mediterranean. Environ Pollut 111:117–126
Caschetto S, Wollast R (1979) Vertical distribution of dissolved aluminium in the mediterranean sea. Mar Chem 7(2):141–155
Chou L, Wollast R (1997) Biogeochemical behavior and mass balance of dissolved aluminium in the western Mediterranean Sea. Deep-Sea Res II Top Stud Oceanogr 44(3):741–768. https://doi.org/10.1016/S0967-0645(96)00092-6
Conti ME, Cecchetti G (2003) A biomonitoring study: trace metals in algae and molluscs from Tyrrhenian coastal areas. Environ Res 93:99–112
Conti ME, Mecozzi M, Finoia MG (2015) Determination of trace metal baseline values in Posidonia oceanica, Cystoseira sp. and other marine environmental biomonitors: a quality control method for a study in South Tyrrhenian coastal areas. Environ Sci Pollut Res 22(5):3640–3651. https://doi.org/10.1007/s11356-014-3603-3
Edmunds WM (2011) Beryllium: environmental geochemistry and health effects, Reference Module in Earth Systems and Environmental Sciences, Encyclopedia of Environmental Health, 293–301. https://doi.org/10.1016/B978-0-444-52272-6.00358-5
EFSA (2011) Statement on the evaluation of a new study related to the bioavailability of aluminium in food. EFSA J 9:2157
EFSA (2012) Scientific opinion on safety and efficacy of cobalt carbonate as feed additive for ruminants, horses and rabbits. EFSA J 10(6):2727 27 pp
EFSA (2013a) Scientific opinion on dietary reference values for manganese. EFSA J 3419:11
EFSA (2013b) Scientific opinion on nutrient requirements and dietary intakes of infants and young children in the European Union. EFSA J 11(10):3408 103 pp
EFSA (2014a) Scientific opinion on dietary reference values for chromium. EFSA J 12(10):3845, 25 pp
EFSA (2014b) Scientific opinion on dietary reference values for zinc. EFSA J 12(10):3844, 76 pp
Eisler RI (1981) Trace metal concentrations in marine organisms. Pergamon, Oxford
Evans FD, Critchley AT (2014) Seaweeds for animal production use. J Appl Phycol 26(2):891–899. https://doi.org/10.1007/s10811-013-0162-9
Filella M, Belzile N, Lett MC (2007) Antimony in the environment: a review focused on natural waters. III. Microbiota relevant interactions. Earth Sci Rev 80:195–217
Fries L (1982) Vanadium an essential element for some marine macroalgae. Planta 154(5):393–396. https://doi.org/10.1007/BF01267804
Hamid N, Ma Q, Boulom S, Liu T, Zheng Z, Balbas J, Robertson J (2015) Seaweed sustainability, food and non-food applications, Chapter 8—Seaweed minor constituents, pp 193–242
Hawk A, Melsom S, Omang S (1974) Estimation of heavy metal pollution in two Norwegian fjord areas by analysis of the brown alga Ascophyllum nodosum. Environ Pollut 7:179–192
Ho YB (1988) Metal levels in three intertidal macroalgae in Hong Kong waters. Aquat Bot 29(4):367–372. https://doi.org/10.1016/0304-3770(88)90080-0
Institute of Medicine (2001) Iron. In: Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; pp 290–2393
International Agency for Research on Cancer (IARC) (1987) Monographs on the evaluation of carcinogenic risks to humans. In: Overall evaluation of carcinogenicity: an updating of monographs, vol. 1–42. Lyons: IARC, p 230–232
Llorente-Mirandes T, Ruiz-Chancho MJ, Barbero M, Rubio R, López-Sánchez JF (2010) Measurement of arsenic compounds in littoral zone algae from the Western Mediterranean Sea. Occurrence of arsenobetaine. Chemosphere 81(7):867–875. https://doi.org/10.1016/j.chemosphere.2010.08.007
Makkar HPS, Tran G, Heuzé V, Giger-Reverdin S, Lessire M, Lebas F, Ankers P (2016) Seaweeds for livestock diets: A review. Anim Feed Sci Technol 212:1–17
Malea P, Kevrekidis T (2014) Trace element patterns in marine macroalgae. Sci Total Environ 494–495:144–157
Malea P, Chatziapostolou A, Kevrekidis T (2015) Trace element seasonality in marine macroalgae of different functional-form groups. Mar Environ Res 103:18–26. https://doi.org/10.1016/j.marenvres.2014.11.004
Moore JW, Ramamoorthy S (1984) Heavy metals in natural waters: applied monitoring and impact assessment. Springer, New York, pp 28–246
Raab A, Stiboller M, Gajdosechova Z, Nelso J, Feldmann J (2016) Element content and daily intake from dietary supplements (nutraceuticals) based on algae, garlic, yeast fish and krill oils—should consumers be worried? J Food Compos Anal 53:49–60. https://doi.org/10.1016/j.jfca.2016.09.008
Rohani-Ghadikolaei K, Abdulalian E, Ng W-K (2012) Evaluation of the proximate, fatty acid and mineral composition of representative green, brown and red seaweeds from the Persian Gulf of Iran as potential food and feed resources. J Food Sci Technol 49(6):774–780. https://doi.org/10.1007/s13197-010-0220-0
Rolison JM, Middag R, Stirling CH, Rijkenberg MJA, de Baar HJW (2015) Zonal distribution of dissolved aluminium in the Mediterranean Sea. Mar Chem 177:87–100. https://doi.org/10.1016/j.marchem.2015.05.001
Rubio C, Napoleone G, Luis-Gonzalez G, Gutierrez AJ, Gonzalez-Weller D, Hardisson A, Revert C (2017) Metals in edible seaweed. Chemosphere 173:572–579. https://doi.org/10.1016/j.chemosphere.2017.01.064
Rupérez P (2002) Mineral content of edible marine seaweeds. Food Chem 79(1):23–26. https://doi.org/10.1016/S0308-8146(02)00171-1
Squadrone S, Brizio P, Battuello M, Nurra N, Mussat Sartor R, Benedetto A, Pessani D, Abete MC (2017) A first report of rare earth elements in northwestern Mediterranean seaweeds. Mar Pollut Bull 122(1–2):236–242. https://doi.org/10.1016/j.marpolbul.2017.06.048
Stengel DB, Macken A, Morrison L, Morley N (2004) Zinc concentrations in marine macroalgae and a lichen from western Ireland in relation to phylogenetic grouping, habitat and morphology. Mar Pollut Bull 48(9–10):902–909. https://doi.org/10.1016/j.marpolbul.2003.11.014
Storelli MM, Storelli A, Marcotrigiano GO (2001) Heavy metals in the aquatic environment of the Southern Adriatic Sea, Italy macroalgae, sediments and benthic species. Environ Int 26(7–8):505–509. https://doi.org/10.1016/S0160-4120(01)00034-4
Subba Rao PV, Mantri VA, Ganesan K (2007) Mineral composition of edible seaweed Porphyra vietnamensis. Food Chem 102:215–218
Tanner HA, Brown TE, Eyster HC, Treharne RW (1960) The photosynthetic function of manganese and chloride. Ohio J Sci 60(4):231–234
United States Environmental Protection Agency (EPA) (2014) Priority Pollutant List. https://www.epa.gov/sites/production/files/2015-09/documents/priority-pollutant-list-epa.pdf
Viraraghavan T, Srinivasan A (2011) Thallium: environmental pollution and health effects, Reference Module in Earth Systems and Environmental Sciences, Encyclopedia of Environmental Health, pp 293–301
Funding
The research was funded through an Italian Health Ministry Research (project no. IZS PLV 14/14RC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Severine Le Faucheur
Rights and permissions
About this article
Cite this article
Squadrone, S., Brizio, P., Battuello, M. et al. Trace metal occurrence in Mediterranean seaweeds. Environ Sci Pollut Res 25, 9708–9721 (2018). https://doi.org/10.1007/s11356-018-1280-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1280-3