Abstract
For decades, the river health of the Yellow River source region (YRSR) on the Qinghai-Tibetan Plateau has been a focal issue owing to its unique geographic location and ecological functions. This study investigated the ecological status of the headwater streams, the main stem, and the tributaries of the Yellow River in the YRSR using the tolerance values of macroinvertebrates and those related to biotic indices. The macroinvertebrate assemblages of the headwater streams were characterized by lower biodiversity than the tributaries downstream, based on comparisons of taxonomical composition, functional feeding group composition, and the pollution-tolerant capacity of taxa. The headwater streams had a lower ratio (16%) of pollution-sensitive macroinvertebrate taxa than that of the tributaries downstream (30%). The biotic indices (family- and genus-level biotic indices) indicated that the ecological health of the headwater streams was comparably poorer than that of the downstream tributaries. The combined effect of vulnerable natural conditions and increasing human disturbance is likely the main cause of eco-environmental degradation in the Yellow River headwater streams.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The ecological condition of the Yellow River source region (YRSR) on the Qinghai-Tibetan Plateau has been a focus of attention for decades because of its unique geographical location and ecological functions including water conservation, biodiversity protection, and ecological safeguards (Brierley et al. 2016). Under the increasing influences of global warming and human disturbance, the plateau river ecosystems appear to be suffering from decreasing surface runoff, shrinking lakes and wetlands, and conflicts between ecological protection and socioeconomic development (Chang et al. 2007). The ecological and environmental features of the riparian and terrestrial ecosystem in the YRSR were significantly influenced by climate change and anthropogenic activities during the last half century, especially over the last 30 years (Feng et al. 2006). Temperature and precipitation variations are considered to be among the main driving forces for the ecological and environmental changes in the YRSR (McGregor 2016).
However, the way in which the river ecosystems, particularly the aquatic communities, respond to the changes is yet to be clearly studied. Owing to logistic difficulties, previous eco-environmental surveys in the YRSR have primarily been carried out around the two large lakes, Erling Lake and Zhaling Lake, and the stem and tributaries of the Yellow River downstream of these lakes (Pan et al. 2012; Xu et al. 2012; Zhao et al. 2017). Field investigations have rarely been conducted in the headwaters of the YRSR. This study thus attempted to expand the investigations in the YRSR and to test the hypothesis that changes in climate and anthropological disturbances have also caused changes in the aquatic ecosystems of the YRSR.
Local biota may adapt to environmental modification by changing their community composition, so exploring the variations of the biological communities could reveal a comprehensive picture of the eco-environmental status (Fu et al. 2016; Duka et al. 2017). Bioassessment methods based on macroinvertebrate communities have been developed and widely used in river ecology assessment since the 1900s, as they are suitable for evaluating the effects of environmental quality and cumulative responses to ecological stresses as well as for providing historical information on water quality (Poikane et al. 2016; Wang and Tan 2017). Therefore, macroinvertebrate communities served as the biological indicators in this study.
Biomonitoring is a vital and rapidly growing field in which benthic macroinvertebrates (aquatic insects, mollusks, crustaceans, and worms) are used for the biological assessment for water quality in lakes and streams (Rosenberg and Resh 1993). Many rapid bioassessment methods directly rely on macroinvertebrate taxa richness or diversity indices based on the number of taxa to evaluate the eco-environmental status, or focus on sensitive taxa such as Ephemeroptera, Plecoptera, and Trichoptera (EPT) to evaluate pollution levels (Rosenberg and Resh 1993; Kitchin 2005). However, these methods may overlook the traits of each individual taxon and treat different taxa with different tolerance levels in the same way. In addition, taxa composition and richness are usually affected by many factors, especially the background conditions in different regions, so the assessment of eco-environment status merely based on rapid bioassessment methods could be misleading (Klemm et al. 2002).
To improve the reliability of eco-environmental assessment and to reveal the regional variation in eco-environmental status, we attempted to use the pollution tolerance abilities of macroinvertebrates as a supplementary biological indicator. The pollution tolerance abilities, clearly represented by tolerance values (TVs) (Bressler et al. 2006; Raburu et al. 2017), are critically important components reflecting the ecological condition of streams (Ferreira et al. 2017). The family-level biotic index (FBI) and biotic index (BI), which are based on the averages of tolerance values of all taxa in a sample (Hilsenhoff 1988) and regarded as valuable for river health assessment (Carrie et al. 2017), were also used in this study.
Therefore, the ecological condition of the headwater streams, main stem, and tributaries of the Yellow River in the YRSR was explored based on comparisons of the indicators of water quality variables and the structural and functional traits of macroinvertebrate communities. The main objectives of this study were (1) to explore the traits of macroinvertebrate communities in the rivers of the YRSR, (2) to evaluate the ecological condition of the headwater streams based on the TVs of the macroinvertebrates and the TV-related biotic indices, and (3) to reveal the main factors causing changes in the ecological condition and community structure and functional composition of macroinvertebrates in the YRSR.
Materials and methods
Study area and sampling sites
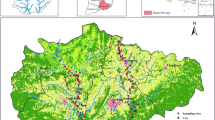
The YRSR is located in the hinterland of the Qinghai-Tibetan Plateau and has elevations of 4200–4600 m above sea level. The plateau landscape features many swamps, lakes, wetlands, and glaciers (Zhang et al. 2012). Due to its high altitude, the YRSR mainly possesses a cold and arid climate, long periods of sunshine, and strong irradiation (Brierley et al. 2016). With rising global temperatures, increasing evaporation rates are leading to a decrease of flow discharges in the source region (Chang et al. 2007; Wang et al. 2015). In addition, precipitation in this region is monsoonal and distributed unevenly over the year, with the majority of rainfall occurring from May to September (Zhang et al. 2012). The YRSR is the major water resource region of the northwest and the north of China; therefore, runoff changes in the region will affect the stability of ecosystems and the environment in northern China (Wang et al. 2015). Field investigations were carried out in three groups of rivers in the YRSR: the headwater streams in Maduo County (H group), the main stem of the Yellow River (M group), and its major downstream tributaries the Bai River and Hei River near Jiuzhi County (T group). On the headwater streams, including the Yueguzonglie, Kariqu, and Duoqu rivers, six representative sampling sections were set. Four representative sampling sections were set on each of the Bai and Hei rivers in the YRSR. Three representative sampling sections were set on the main stem. All the sampling sections are shown in Fig. 1.
River basin and sampling sites in the study area. Sites 1–3 indicate the sampling sections on the Yueguzonglie River; 4 and 5 indicate the sampling sections on the Kariqu River; 6 indicates the sampling section on the Duoqu River. Sites 1–6 belong to the H group. Sites 7, 8, and 9 indicate the sampling sections on the main stem in the YRSR, and they were the M group. Sites 10, 11, 12, and 13 are on the tributary Bai River; 14, 15, 16, and 17 are on the Hei River. Sites 10–17 were the T group
Field investigation
Field investigations were carried out in July 2014 and July 2016. Geographical locations and altitudes were measured using an iHand differential GPS (GPS 72H, China). The general features of land use, river patterns, and riparian vegetation were recorded and photographed. River width was measured with a laser rangefinder (TruPulse-200L, USA). Water depth and velocity were measured using a propeller-type current meter (Model LS 1206B, China). The in situ measured variables are listed in Table 1. Flow discharge for each measured section was conventionally calculated as the product of water depth, flow velocity, and river width. All of the sampling sections of macroinvertebrates were generally characterized by cobble-sand substrate covered by aquatic macrophytes. It is noteworthy that, owing to higher altitudes, the aquatic macrophytes and riparian vegetation of the headwater streams were generally sparser than those of the tributaries downstream from the headwater streams. Except for altitude and vegetation condition, other physical environmental variables such as discharge, water depth, stream velocity, and substrate composition were similar among the sampling sections of the three groups.
An EXO Sondes and EXO Handheld System (Xylem, USA) were used to determine water quality parameters in situ, including water temperature (WT), dissolved oxygen (DO), conductivity (Cond), and turbidity. In addition, water samples (500 ml each, consisting of 250 ml of water from near the surface and 250 ml water from near the stream bed) were taken for laboratory analyses. The concentrations of total nitrogen (TN), total phosphorus (TP), and total organic carbon (TOC) in the mixed water samples were analyzed in the laboratory according to the Standard Methods for Water and Wastewater Monitoring and Analysis (The State Environmental Protection Administration 2002; Zhao et al. 2017).
Macroinvertebrate sampling and identification
At each sampling section, macroinvertebrate organisms were collected from three subsamples (1/3 m2 of each) covering all representative substrata using a kick-net (area: 1 m2, mesh opening: 420 μm). The organisms were manually picked out, placed in 25% and then 50% ethanol to help maintain their shape, and finally preserved in 75% ethanol. Macroinvertebrates were identified mostly to genus level (Morse et al. 1994; Epler 2001; He 2011; Wiggins 2015) under a stereoscopic microscope (XYH-3A, China) and an optical microscope (XSP-8CA, China). Photographs of the specimens were captured using a SmartV Camera image acquisition system (YH5001-3, China), and then the body length of each individual was measured to the nearest 0.01 mm. The individuals were counted for density estimations (ind. m−2), and their wet weight was determined to the nearest 0.1 mg, using an analytical balance, for biomass (g m−2) calculations.
All specimens were divided into five taxonomical groups (TGs): the four commonly seen fauna (Chironomidae, EPT taxa, Mollusca, Oligochaeta), and the other taxa. Chironomidae are one of the most widely distributed groups across all water bodies (Garcia et al. 2007). EPT taxa are indicators of clean running water (Kitchin 2005; Duka et al. 2017). Mollusca usually have a larger size and biomass than the other taxa. Oligochaeta are generally the dominant taxa in streams with poor water quality (Raburu et al. 2017). In addition, the macroinvertebrates were categorized into eight functional feeding groups (FFGs): filter collector (FC); gatherer collector (GC); omnivore (OM); parasite (PA); piercer (PI); predator (PR); scraper (SC); or shredder (SH); and also into seven behavior groups (BGs): burrower (bu); climber (cb); clinger (cn); diver (dv); skater (sk); sprawler (sp); or swimmer (sw). The taxa that possess multiple possible feeding or behavior strategies were assigned to a single FFG and a single BG which described their major feeding habits or behaviors (Palmer et al. 1996).
Assignment of tolerance values (TVs) for macroinvertebrates
In this study, we attempted to use TVs to represent the relative pollution tolerance abilities of macroinvertebrates. TVs ranging from 0 to 10 are typically assigned to different taxa, with water quality degrading as values increase (Hilsenhoff 1988). Taxa with TVs lower than 3 are regarded as pollution-sensitive taxa, whereas those with TVs higher than 7 are categorized as pollution-tolerant taxa; meanwhile, taxa with TVs from 3 to 7 are considered intermediate pollution-tolerant taxa (Maxted et al. 2000). The family-, or genus-level TVs of macroinvertebrate taxa, which were collected from published papers, are shown in Fig. 2, and were referred to in this study (Wang 2003; Wang and Yang 2004; Duan et al. 2010; Qin et al. 2014). To be noticed, even though the TV of an individual taxon varies to some extent among the different studies for the different regions, the mean TV increases as the taxon’s pollution tolerance ability increases. Therefore, the mean TVs (dots in Fig. 2) were used in the assignment of TVs for the macroinvertebrate taxa in this study. After normality tests of TVs were conducted for the macroinvertebrate assemblages in the H, M, and T groups, the TVs were drawn in a statistical histogram, and the distribution curve of TVs for each group was fitted in the histogram using OriginPro 2016.
Tolerance values (TVs) of macroinvertebrates indicated in different studies in China. In a, F01, Leuctridae; F02, Brachycentridae; F03, Nemouridae; F04, Perlodidae; F05, Ephemerellidae; F06, Corydalidae; F07, Gomphidae; F08, Tipulidae; F09, Heptageniidae; F10, Limnephilidae; F11, Gammaridae; F12, Siphlonuridae; F13, Baetidae; F14, Hydropsychidae; F15, Naucoridae; F16, Simuliidae; F17, Athericidae; F18, Ceratopogonidae; F19, Tabanidae; F20, Hydrachnidae; F21, Lebertiidae; F22, Planorbidae; F23, Lymnaeidae; F24, Hydrophilidae; F25, Chironomidae; F26, Naididae. In b, sp01, Leuctridae (ud); sp02, Brachycentrus sp.; sp03, Drunella sp.; sp04, Ephemerella sp.; sp05, Podmosta sp.; sp06, Nemoura sp.; sp07, Isoperla sp.; sp08, Perlodidae (ud); sp09, Heptagenia sp.; sp10, Neochauliodes sp.; sp11, Serratella sp.; sp12, Tipula sp.; sp13, Gomphidae (ud); sp14, Limonia sp.; sp15, Ormosia sp.; sp16, Cinygmula sp.; sp17, Ironodes sp.; sp18, Hydropsyche sp.; sp19, Pseudostenophylax sp.; sp20, Gammarus sp.; sp21, Siphluriscus sp.; sp22, Baetis sp.; sp23, Leptonema sp.; sp24, Simulium sp.; sp25, Naucoridae (ud); sp26, Atrichops sp.; sp27, Ceratopogonidae (ud); sp28, Eukiefferiella sp.; sp29, Tanytarsus sp.; sp30, Hemerodromia sp.; sp31, Hydrachnidae (ud); sp32, Lebertia sp.; sp33, Planorbidae (ud); sp34, Ablabesmyia sp.; sp35, Polypedilum sp.; sp36, Rheopelopia sp.; sp37, Trissopelopia sp.; sp38, Orthocladius sp.; sp39, Chaetocladius sp.; sp40, Dicrotendipes sp.; sp41, Diplocladius sp.; sp42, Hydrobaenus sp.; sp43, Krenosmittia sp.; sp44, Limnophyes sp.; sp45, Nanocladius sp.; sp46, Parakiefferiella sp.; sp47, Paratanytarsus sp.; sp48, Psectrocladius sp.; sp49, Cladotanytarsus sp.; sp50, Neozavrelia sp.; sp51, Parachironomus sp.; sp52, Paracladopelma sp.; sp53, Rheocricotopus sp.; sp54, Clinotanypus sp.; sp55, Pseudodiamesa sp.; sp56, Thalassomya sp.; sp57, Hydrophilidae (ud); sp58, Radix sp.; sp59, Cricotopus sp.; sp60, Rhyacodrilus sp.; sp61, Limnodrilus sp.; sp62, Tubifex sp.
Calculation of biotic indices
The EPT families index (EPT-Fa) was the number of families observed belonging to the orders Ephemeroptera, Plecoptera, and Trichoptera (Kitchin 2005). The Shannon-Wiener index is a commonly used diversity index calculated with Eq. (1) (Shannon 1948).
where N is the total number of individuals in a unit sampling area (ind. m−2), S is the taxa richness, and Ni is the number of individuals of the ith taxon.
The family-level biotic index (FBI) and the genera-level biotic index (BI) were calculated with Eqs. (2) and (3) respectively, in which N is the total number of individuals in the sample, Ni is the number of individuals of the ith family, ni is the number of individuals of the ith genus, and ti is the family- or genus-level TVs (Hilsenhoff 1988; Fierro et al. 2017).
Data analyses
Firstly, the Kolmogorov-Smirnov (K–S) test was used to test for normality of the data (environmental variables, macroinvertebrate bio-indices, and TVs). If the data followed a normal distribution, a one-way analysis of variance (one-way ANOVA) was performed to test for a statistically significant difference (p < 0.05) among the groups. An analysis of the Pearson correlation coefficient was then performed to describe the relationships between the biotic indices and environmental variables. All of the aforementioned analyses were carried out using the IBM SPSS Statistics 23 software package.
The abundance values of macroinvertebrate assemblages were standardized and square root transformed prior to calculating Bray-Curtis similarity values for group-averaged cluster analysis. Using the Bray-Curtis similarity matrix, samples were first clustered by applying a hierarchical agglomerative method on group average-linking. Then, a two-dimensional nonmetric multidimensional scaling (NMDS) ordination was derived by applying an iterative procedure to refine point positions. To identify the best possible solution, a minimum of ten random restarts was set for the analysis to select the solution with the lowest stress coefficient. The stress coefficient is a measure of how well the two-dimensional plot represents the n-dimensional similarity matrix. Stress values below 0.2 indicate an acceptable representation of the underlying similarity matrix in the nMDS diagram. A closer distance between two dots in the NMDS figure indicates that the two samples are more similar. Based on the Bray-Curtis similarity matrix, differences in macroinvertebrate assemblage structures or water quality variables among groups were examined using analysis of similarity (ANOSIM). A similarity percentage procedure (SIMPER) was applied to find the percentage contribution for each taxon to the Bray-Curtis similarity, and to determine the representative taxa for each group. These analyses were carried out using the Primer v5.2 statistical package.
Equation (4) was applied to calculate the dominance (Y) of each family in the macroinvertebrate assemblages (Lampitt et al. 1993). For each group, si is the number of individuals of the ith family in all the sampling sites, S is the number of all individuals in the group, and fi is the occurrence frequency of the ith family in each group.
Canonical correspondence analysis (CCA) was applied to explore the relationships between the sample sites or the TVs and the water quality parameters, to determine the key parameters responsible for the changes in taxa composition, and to elucidate the relationship between the TVs or scores of biotic indices and gradients of water quality parameters. Prior to CCA, detrended correspondence analysis (DCA) was used to choose a model for constrained ordination. Based on the threshold value of the longest gradient in the DCA ordination, unimodal methods (CCA) should be used when the value is over 4.0—otherwise, linear methods (e.g., RDA) should be used (Lepš and Šmilauer 2003). In CCA, we applied different symbols in the figure plots to indicate the different traits of sample sites or macroinvertebrate taxa. The analyses were carried out using CANOCO 4.5 (Micro-computer Power, USA).
Results
Water quality variables of the YRSR
The similarity analysis of water quality variables (Fig. 3) indicated that the water quality of the headwater streams (H), the main stem (M), and the tributaries (T) in the YRSR were significantly different (global R = 0.756, p = 0.001, ANOSIM). In pairwise tests, water quality variables of the H group were significantly different from those of the T group (R = 1, p = 0.001, ANOSIM), while the variables of the M group were not significantly different from the other two groups (M group vs. H group: R = 0.25, p = 0.286; M group vs. T group: R = 0.362, p = 0.133). From the H group to the M group through to the T group, the concentrations of TN decreased significantly (p = 0.0002, one-way ANOVA) from 1.28 ± 0.23 mg/L to 0.34 ± 0.06 mg/L to 0.22 ± 0.03 mg/L, respectively. Conductivity (Cond) decreased significantly (p = 0.001, one-way ANOVA) with a similar trend as TN. In contrast to TN and Cond, the concentrations of TOC, TP, and WT increased significantly (p = 0.002 for TOC, p = 0.073 for TP, and p = 0.020 for WT, respectively) from the H group to the M group to the T group. Meanwhile, there were no significant differences among the DO (one-way ANOVA: p = 0.699) or pH (one-way ANOVA: p = 0.945) values for the three groups.
Macroinvertebrate community traits of the YRSR
A total of 30 genera and 15 families were obtained in the H group, 31 genera and 17 families in the M group, and 31 genera and 21 families in the T group. Figure 4 shows the community composition of the TGs in each sampling site. The macroinvertebrate communities mostly consisted of insects in all of the sampling sites. The Shannon-Wiener diversity indices were 1.095 ± 0.409, 1.381 ± 0.346, and 1.293 ± 0.518 for the H, M, and T groups, respectively, which were not significantly different (p = 0.619, one-way ANOVA). Pielou’s evenness indices of them were very similar (p = 0.896, one-way ANOVA), with the value as 0.703 ± 0.039, 0.711 ± 0.141, and 0.700 ± 0.057 for the H, M, and T groups. The most dominant families were different among the three groups. The H group was dominated by Chironomidae and Oligochaeta (totaling 62.75 ± 10.41%) and had a low ratio of EPT taxa (19.78 ± 7.60%). In contrast, the proportion of EPT taxa in the T group was up to 32.56 ± 6.02%. The most dominant family for the H group was Chironomidae (35.54%), while it was Naididae (22.75%) and Gammaridae (17.44%) for the M and T groups, respectively.
In addition to the composition of TGs, Fig. 4 also shows the compositions of FFGs and BGs of macroinvertebrate communities. The sites in the H group possessed less diverse and uneven compositions of FFGs than those of the M and T groups. One-way ANOSIM on individual abundance showed significant differences in feeding functional compositions among the three groups (global R = 0.581, p = 0.001). To be specific, the H group was significantly different from the M and T groups (H group vs. M group: R = 0.593, p = 0.024; H group vs. T group: R = 0.756, p = 0.001, respectively), while there was a nonsignificant difference between the M group and the T group (R = 0.237, p = 0.121). From the H to the M to the T group, the number of FFGs increased significantly from 2.7 ± 0.9 to 5.0 ± 1.0 to 5.0 ± 0.6, respectively (p = 0.018, one-way ANOVA). The GCs, the most dominant FFG, differed significantly among the three groups, accounting for 68.82 ± 7.25%, 49.17 ± 6.51%, and 30.10 ± 4.26%, respectively in the H, M, and T groups (p = 0.001, one-way ANOVA). Ratios of the SH taxa observed in the H, M, T groups were relatively low and not significantly different, with values of 6.71 ± 3.09, 8.33 ± 5.51, and 11.15 ± 3.68, respectively (p = 0.68, one-way ANOVA). Ratios of the SC taxa in the H, M, and T groups were significantly different with values of 2.08 ± 2.08, 4.17 ± 2.08, and 11.07 ± 2.32, respectively (p = 0.03, one-way ANOVA); a significant difference was noted between the H and the T groups according to multiple comparisons tests (p = 0.012, LSD). In all of the three groups, BGs were mainly composed of sp., cn, and cb. A one-way ANOSIM of BGs indicated that the community structures of BGs were not significantly different among the H, M, and T groups (global R = 0.07, p = 0.262). In summary, based on either taxa richness or individual abundance, GCs were the dominant FFGs in the headwater streams while the other FFGs, such as SC and SH, were rare or even absent.
Hierarchical cluster analysis of the macroinvertebrate communities shown in Fig. 5a also revealed that all the samples from the headwater streams (H group) were clustered in one group at a similarity level of 27.17%, and all the samples from the tributaries (T group) were gathered in another group at a similarity level of 31.07%. Based on Bray-Curtis similarity indices, the NMDS ordination (stress = 0.14) indicated that clear-cut dissimilarities existed in the community structures of the H group and the M group, and in the H and T groups (Fig. 5b). An overlap distribution was detected between the M and the T groups as seen in the cluster analysis. One-way ANOSIM analyses of all the samples revealed significant differences among the macroinvertebrate communities of the different groups (global R = 0.662, p = 0.001). Specifically, the H group was significantly different from the M and the T groups (H group vs. M group: R = 0.728 > global R, p = 0.012; H group vs. T group: R = 0.814 > global R, p = 0.010; respectively), while no significant difference was detected between the M and the T groups (R = 0.328 < global R, p = 0.048). Analysis of SIMPER showed that the average dissimilarity between the H and the T groups was as high as 88.18% with dominant contributions by Gammarus sp. (14.82%) and Limnodrilus sp. (13.08%).
a Hierarchical clustering plot based on Bray-Curtis similarity and b no-metric multidimensional scaling (NMDS) ordination (stress = 0.14) of macroinvertebrate communities of the Yellow River source region. The colored labels in (b): H1–H6 represent the headwaters (red), M1–M3 represent main stem (blue), and T1–T8 represent tributaries (green)
River health bioassessment and influencing factors
The TVs of the H, M, and T groups were further assessed, and the distributions of the TVs were shown following normal distributions (K–S test, p = 0.088 for the H group, p = 0.293 for the M group, and p = 0.387 for the T group, respectively). Gaussian curves were used to demonstrate the distributions of the TVs with fitting functions indicated by Eqs. 5, 6, and 7 (Fig. 6). Through the Gaussian curves of the TVs, we could clearly figure out the composition of pollution-sensitive, intermediate pollution-tolerant, and pollution-tolerant taxa. In all the three groups, the taxa with the intermediate TVs had the highest relative frequencies, while the taxa with very low or very high TVs had lower relative frequencies. The Gaussian curve of the T group had a higher standard deviation and lower mean value than those of the curve of the H group. The mean TV was the highest in the H group (5.8 ± 0.46), followed by the M group (5 ± 0.4), and then the T group (4.7 ± 0.44). The ratio of pollution-tolerant taxa (with TVs > 7) to all taxa in the H group was 16%, higher than that of the M group (6.9%) and the T group (10%), while the ratio of pollution-sensitive taxa (with TVs < 3) in the H group was 16%, lower than that of the T group (30%). In short, there were more pollution-tolerant taxa and less pollution-sensitive taxa in the H group than that in the M and the T groups. Furthermore, the shapes of the three Gaussian curves indicated the highest diversification of pollution-tolerant capacity in the T group.
The scores of the four biotic indices (FBI, BI, EPT-Fa, and H′) for the H, M, and T groups are shown in Fig. 7. One-way ANOVAs showed that the FBI and BI scores were significantly different among the three groups (p = 0.048, p = 0.028, respectively), while H′ and EPT-Fa were not significantly different (p = 0.619, p = 0.226, respectively). The FBI and BI scores for the H group were significantly higher than that for the T group (p = 0.027 for FBI, p = 0.014 for BI, LSD). The average scores of FBI for the H, M, and T groups were 5.4 ± 0.51, 5.5 ± 0.74, and 4.1 ± 0.23, respectively, while the average scores of BI were 6.1 ± 0.73, 5.9 ± 1.31, and 3.9 ± 0.25 for the corresponding groups. There was a positive relationship between FBI scores and BI scores (r = 0.956, p < 0.001, Pearson’s correlation), and they consistently indicated that the ecological conditions of the tributaries were significantly better than those of the headwater streams. Even though the differences were not significant, the EPT-Fa scores of most sample sites of the tributaries were higher than those of the headwater streams, indicating that the tributaries had healthier ecological conditions. There were no obvious variations in Shannon-Wiener index scores among the H, M, and T groups.
To explore the factors influencing the macroinvertebrate communities, the distributions of sample sites and macroinvertebrate assemblages along the primary environmental gradients were plotted in Fig. 8 based on CCA results (the longest gradient in the DCA results was 4.61, indicating that unimodal methods worked reasonably well). A Monte-Carlo test (499 permutations) indicated that the first canonical axis was significantly related (p = 0.004). Figure 8a shows the relationships between the sampling sites and their BI scores with the environmental variables. Figure 8b shows the relationships between composition of taxa and their TVs with the environmental variables. TN was one of the most important determinant water quality parameters affecting BI scores and TVs. With TN increased, the BI scores of most sampling sites increased and the TVs of most taxa were increased. Most sites with high concentrations of TN had the characteristics of high BI scores (higher than 5.0) and high TVs (higher than 5.0). In particular, 83.3% of the sampling sites of the H group had BI scores higher than 5.0, while all the sampling sites of the T group had BI scores lower than 5.0. There was a negative correlation between flow discharge (Q) and TN concentration. Therefore, as Q increased, the ecological conditions of the river improved in the H group. TOC, WT, and TP gradients were also among the important parameters affecting macroinvertebrate assemblages and their pollution tolerance; however, no clear tendencies were seen between the BI scores/TVs and the TOC, WT, and TP gradients.
Canonical correspondence analysis (CCA) ordination plots (axis 1 explained 27.5% of the variation in taxa-environment relations; axes 1 and 2 together explained 46.7% of the overall variation). a The ordination of genera-level biotic index (BI) scores based on changes along the environmental gradients; b the ordination of TVs of taxa along the environmental gradients. In a, different symbols indicate different groups of the sample sites; different colors indicate different grades of BI scores. In b, the green x-mark indicates TVs from 0 to 2.5; the light-green cross indicates TVs from 2.6 to 5.0; the light-red cross indicates TVs from 5.1 to 7.5; the red x-mark indicates TVs from 7.6 to 10. Q, discharge; WT, water temperature; DO, dissolved oxygen; Cond, conductivity; TN, total nitrogen; TP, total phosphorus; TOC, total organic carbon
Discussion
As creatures adapted to their environments, macroinvertebrate communities evolved to reflect the changes in the aquatic ecological conditions (Sola et al. 2004; Ali et al. 2017). As the river source, the headwaters of the Yellow River were presumed to be minimally impacted by human activities and were thus thought to have good water quality (Ali et al. 2017). However, this study indicated that as a result of climate change and anthropological disturbance, the ecological conditions of the YRSR’s headwater streams have deteriorated, altering the macroinvertebrate communities.
The variations in the dominant families from the headwater streams to the tributaries in the YRSR suggest a degeneration in the water quality of the headwater streams. The dominant family in the tributaries was Gammaridae (TV = 3.8), while in the headwater streams it was Chironomidae (TV = 6.9). This could indicate that the ecological conditions of the tributaries are more pristine than those of the headwater streams (Xu et al. 2014). Analyses of taxa richness and taxa evenness in the macroinvertebrate communities indicate that the biodiversity of the headwater streams was lower than that of the tributaries, which might imply that the macroinvertebrate communities in the headwater streams were negatively affected by ecological stressors (Duan et al. 2011; Svensson et al. 2018). The TVs of macroinvertebrates and TVs related to biotic indices were suitable supplementary indicators and proved advantageous in the assessment of the river’s ecological conditions. Compared with traditional taxonomy-based rapid descriptors, assessments based on taxa traits provide quantitative statistical comparisons for aquatic biomonitoring scenarios (Menezes et al. 2010). In this study, TVs and TV-related metrics also indicated that the ecological conditions in the headwater streams were inferior to those of the tributaries and might be constrained by habitat stresses (Tomanova et al. 2006; Tomanova and Usseglio-Polatera 2007; Oguma and Klerks 2017). In terms of TV-related metrics, there were significant differences (p < 0.05) between the macroinvertebrate communities in the headwater streams and those in the tributaries of the YRSR. Meanwhile, there were no significant differences (p > 0.05) in taxa richness and Shannon-Wiener diversity indices. The Shannon-Wiener diversity index and the EPT-Fa index had only considered the number of taxa, which rarely varied in this study area, so the differentiation of these indices among the three groups was not significant. In contrast, with a view to pollution tolerance abilities of each taxon, TVs and TV-related biotic indices might be better indicators for describing the ecological conditions in the YRSR. However, the chemical water quality measurements indicated that the pollution of headwater streams was not too bad, although the ecological conditions were less pristine than those of the tributaries. This situation might result from the combined influence of anthropogenic disturbances and the local fragile eco-environment.
In this study, the average TN concentration of the headwater streams was 1.247 ± 0.227 mg/L, while the average TN concentration of the downstream tributaries was only 0.223 ± 0.031 mg/L. Therefore, the headwater streams were considered to be nitrogen enriched relative to the tributaries. In addition, the CCA analyses indicated a close correlation between the TVs of taxa and TN concentration, as well as an obvious relationship between the BI scores and TN concentration. Xu et al. (2014) also pointed out that TN concentrations above 0.5 mg/L affected the diversity and structure of macroinvertebrate communities. Therefore, a high TN concentration was regarded as one of the main ecological stressors for macroinvertebrate communities in the headwater streams of the YRSR. Nevertheless, it could also be associated with the riparian soil runoff due to the high conductivity in the upper headstreams. High TN concentrations might be the byproduct of human activities. There were increasing human activities in those regions. For example, Madoi, the headwater county used purely for animal husbandry, has been negatively impacted by overgrazing for years (Zhou et al. 2003). Compared with the theoretical carrying capacity of domestic animals, the grasslands of Madoi have been 141.5% overstocked, and the other counties in the YRSR, such as Dari, Maqen, and Gade, have been overstocked by four or five times (Wang et al. 2002). These overstocked domestic animals yield large amounts of livestock waste, which provides a plentiful source of nitrogen in the streams through leaching and runoff (Teira-Esmatges and Flotats 2003; Martinez et al. 2009; González et al. 2014).
Stream self-purification could reduce pollutants through physical, chemical, and biological processes. However, if streamflow is too low to meet the pollutant dilution demand, the stream may not be able to self-purify thoroughly (González et al. 2014). As a result of global warming, the YRSR has shown steady rises in annual average temperature and evapotranspiration, leading to a decrease in runoff (Chang et al. 2007; Zhang et al. 2012; Qin et al. 2017), which might further reduce the self-purification capacities of the headwater streams in the YRSR. In particular, streams could efficiently remove nitrogen through denitrification (Galloway et al. 2003). Denitrification rates increase with warming when temperatures are below optimum (Gödde and Conrad 1999; de Klein et al. 2017).
Generally, denitrifiers in cold regions have an optimum temperature of 20 °C, which is much higher than the mean water temperature (10 °C) in the warmest period in the YRSR (Gödde and Conrad 1999). Therefore, the capacity of denitrifiers in the headwater streams might be restrained (Christensen and Sørensen 1986). A lack of aquatic macrophytes might be another important reason for river health degradation in the YRSR headwaters. Aquatic macrophytes could decrease nutrient loads in streams through various mechanisms (Tyler et al. 2012). They might play an important role in reducing nitrogen in streams through the direct uptake of nutrients (Brix 1997; Tyler et al. 2012). The root metabolism of aquatic macrophytes could improve the number of rhizosphere microorganisms and adjust the community structure to enhance its purification abilities (Christensen and Sørensen 1986). The existence of aquatic macrophytes could also improve macroinvertebrate communities such that they can increase the nitrogen-cycling rate in streams (Grimm 1988; Grutters et al. 2016). However, the headwater streams have the characteristic of sparse aquatic macrophytes for shallow water and long periods of freezing. Above all, because of the weak self-purification abilities of the headwater streams, the relative lighter disturbance might cause degradation.
Conclusions
Based on taxonomical composition, FFG composition, and the pollution tolerance capacities of taxa, the biodiversity of YRSR headwater streams was lower than that of the downstream tributaries. There were less pollution-sensitive macroinvertebrate taxa in the headwater streams. Quantitative bioassessments based on the TVs of macroinvertebrates and the TV-related biotic indices consistently indicated that the headwater streams may be more ecologically degraded than the tributaries. This might be the result of the combined influence of the local vulnerable eco-environment and increased human disturbance. Because of the weak self-purification abilities of headwater streams, too much livestock and livestock waste likely caused the degradation of river health of headwater streams.
References
Ali S, Gao JF, Begum F, Rasool A, Ismail M, Cai YJ, Ali S, Ali S (2017) Health assessment using aqua-equality indicators of alpine streams (Khunjerab National Park), Gilgit, Pakistan. Environ Sci Pollut Res 24:4685–4698
Bressler DW, Stribling JB, Paul MJ, Hicks MB (2006) Stressor tolerance values for benthic macroinvertebrates in Mississippi. Hydrobiologia 573:155–172
Brierley GJ, Li XL, Cullum C, Gao J (2016) Introduction: landscape and ecosystem diversity in the Yellow River Source Zone. In: Brierley GJ, Li X, Cullum C, Gao J (eds) Landscape and ecosystem diversity, dynamics and management in the Yellow River Source Zone. Springer, New York, pp 1–34
Brix H (1997) Do macrophytes play a role in constructed treatment wetlands? Water Sci Technol 35:11–17
Carrie R, Dobson M, Barlow J (2017) Challenges using extrapolated family-level macroinvertebrate metrics in moderately disturbed tropical streams: a case-study from Belize. Hydrobiologia 794:257–271
Chang GG, Li L, Zhu XD, Wang ZY, Xiao JS, Li FX (2007) Influencing factors of water resources in the source region of the Yellow River. J Geogr Sci 17:131–140
Christensen PB, Sørensen J (1986) Temporal variation of denitrification activity in plant-covered, littoral sediment from lake Hampen, Denmark. Appl Environ Microbiol 51:1174–1179
Duan XH, Wang ZY, Xu MZ (2010) Benthic macroinvertebrate and application in the assessment of stream ecology. Tsinghua University Press, Beijing (in Chinese with English abstract)
Duan XH, Wang ZY, Xu MZ (2011) Effects of fluvial processes and human activities on stream macro-invertebrates. Int J Sediment Res 26:416–430
Duka S, Pepa B, Keci E, Paparisto A, Lazo P (2017) Biomonitoring of water quality of the Osumi, Devolli, and Shkumbini rivers through benthic macroinvertebrates and chemical parameters. J Environ Sci Health A Tox Hazard Subst Environ Eng 52:471–478
Epler JH (2001) Identification manual for the larval Chironomidae (Diptera) of North and South Carolina: a guide to the taxonomy of the midges of the southeastern United States, including Florida. Special Publication SH2001-SP13. North Carolina Department of Environment and Natural Resources, Raleigh, North Carolina
Feng JM, Wang T, Xie CW (2006) Eco-environmental degradation in the source region of the Yellow River, Northeast Qinghai-Xizang Plateau. Environ Monit Assess 122:125–143
Ferreira WR, Hepp LU, Ligeiro R, Macedo DR, Hughes RM, Kaufmann PR, Callisto M (2017) Partitioning taxonomic diversity of aquatic insect assemblages and functional feeding groups in neotropical savanna headwater streams. Ecol Indic 72:365–373
Fierro P, Bertran C, Tapia J, Hauenstein E, Pena-Cortes F, Vergara C, Cerna C, Vargas-Chacoff L (2017) Effects of local land-use on riparian vegetation, water quality, and the functional organization of macroinvertebrate assemblages. Sci Total Environ 609:724–734
Fu L, Jiang Y, Ding J, Liu Q, Peng Q-Z, Kang M-Y (2016) Impacts of land use and environmental factors on macroinvertebrate functional feeding groups in the Dongjiang River basin, southeast China. J Freshw Ecol 31:21–35
Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth RW, Cowling EB, Cosby BJ (2003) The nitrogen cascade. BioScience 53:341–356
Garcia E, Suarez PA, Alejandro D (2007) Community structure and phenology of chironomids (Insecta : Chironomidae) in a Patagonian Andean stream. Limnologica 37:109–117
Gödde M, Conrad R (1999) Immediate and adaptational temperature effects on nitric: oxide production and nitrous oxide release from nitrification and denitrification in two soils. Biol Fertil Soils 30:33–40
González OS, Almeida CA, Calderon M, Mallea MA, Gonzalez P (2014) Assessment of the water self-purification capacity on a river affected by organic pollution: application of chemometrics in spatial and temporal variations. Environ Sci Pollut Res 21:10583–10593
Grimm NB (1988) Role of macroinvertebrates in nitrogen dynamics of a desert stream. Ecology 69:1884–1893
Grutters BMC, Gross EM, Bakker ES (2016) Insect herbivory on native and exotic aquatic plants: phosphorus and nitrogen drive insect growth and nutrient release. Hydrobiologia 778:209–220
He XB (2011) Studies on faunae of aquatic Oligochaeta (Annelida) in Tibet and four large rivers of China. Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan (in Chinese with English abstract)
Hilsenhoff WL (1988) Rapid field assessment of organic pollution with a family-level biotic index. J N Am Benthol Soc 7:65–68
Kitchin PL (2005) Measuring the amount of statistical information in the EPT index. Environmetrics 16:51–59
de Klein JJM, Overbeek CC, Jorgensen CJ, Veraart AJ (2017) Effect of temperature on oxygen profiles and denitrification rates in freshwater sediments. Wetlands 37:975–983
Klemm DJ, Blocksom KA, Thoeny WT, Fulk FA, Herlihy AT, Kaufmann PR, Cormier SM (2002) Methods development and use of macroinvertebrates as indicators of ecological conditions for streams in the Mid-Atlantic Highlands Region. Environ Monit Assess 78:169–212
Lampitt RS, Wishner KF, Turley CM, Angel MV (1993) Marine snow studies in the Northeast Atlantic Ocean: distribution, composition and role as a food source for migrating plankton. Mar Biol 116:689–702
Lepš J, Šmilauer P (2003) Multivariate analysis of ecological data using CANOCO. Cambridge University Press, Cambridge
Martinez J, Dabert P, Barrington S, Burton C (2009) Livestock waste treatment systems for environmental quality, food safety, and sustainability. Bioresour Technol 100:5527–5536
Maxted JR, Barbour MT, Gerritsen J, Poretti V, Primrose N, Silvia A, Penrose D, Renfrow R (2000) Assessment framework for mid-Atlantic coastal plain streams using benthic macroinvertebrates. J N Am Benthol Soc 19:128–144
McGregor GR (2016) Climate variability and change in the Sanjiangyuan region. In Landscape and Ecosystem Diversity, Dynamics and Management in the Yellow River Source Zone. Springer, Cham, pp 35–57
Menezes S, Baird DJ, Soares AMVM (2010) Beyond taxonomy: a review of macroinvertebrate trait-based community descriptors as tools for freshwater biomonitoring. J Appl Ecol 47:711–719
Morse JC, Yang LF, Tian LX (1994) Aquatic insects of China useful for monitoring water quality. Hohai University Press, Nanjing
Oguma AY, Klerks PL (2017) Pollution-induced community tolerance in benthic macroinvertebrates of a mildly lead-contaminated lake. Environ Sci Pollut Res 24:19076–19085
Palmer CG, Maart B, Palmer AR, Okeeffe JH (1996) An assessment of macroinvertebrate functional feeding groups as water quality indicators in the Buffalo River, eastern Cape Province, South Africa. Hydrobiologia 318:153–164
Pan B, Wang Z, Xu M, Xing L (2012) Relation between stream habitat conditions and macroinvertebrate assemblages in three Chinese rivers. Quat Int 282:178–183
Poikane S, Johnson RK, Sandin L, Schartau AK, Solimini AG, Urbanič G, Arbačiauskas K, Aroviita J, Gabriels W, Miler O, Pusch MT, Timm H, Böhmer J (2016) Benthic macroinvertebrates in lake ecological assessment: a review of methods, intercalibration and practical recommendations. Sci Total Environ 543:123–134
Qin C, Zhou J, Cao Y, Zhang Y, Hughes RM, Wang B (2014) Quantitative tolerance values for common stream benthic macroinvertebrates in the Yangtze River Delta, Eastern China. Environ Monit Assess 186:5883–5895
Qin Y, Yang D, Gao B, Wang T, Chen J, Chen Y, Wang Y, Zheng G (2017) Impacts of climate warming on the frozen ground and eco-hydrology in the Yellow River source region, China. Sci Total Environ 605:830–841
Raburu PO, Masese FO, Tonderski KS (2017) Use of macroinvertebrate assemblages for assessing performance of stabilization ponds treating effluents from sugarcane and molasses processing. Environ Monit Assess 189(2):79
Rosenberg DM, Resh VH (1993) Freshwater biomonitoring and benthic macroinvertebrates. Chapman & Hall, London
Shannon CE (1948) A mathematical theory of communication. Bell Syst Tech J 27:379–423
Sola C, Burgos M, Plazuelo A, Toja J, Plans M, Prat N (2004) Heavy metal bioaccumulation and macroinvertebrate community changes in a Mediterranean stream affected by acid mine drainage and an accidental spill (Guadiamar River, SW Spain). Sci Total Environ 333:109–126
Svensson O, Bellamy AS, Van den Brink PJ, Tedengren M, Gunnarsson JS (2018) Assessing the ecological impact of banana farms on water quality using aquatic macroinvertebrate community composition. Environ Sci Pollut Res 25:13373–13381
Teira-Esmatges MR, Flotats X (2003) A method for livestock waste management planning in NE Spain. Waste Manag 23:917–932
The State Environmental Protection Administration (2002) Water and wastewater monitoring and analysis method. China Environmental Science Press, Beijing
Tomanova S, Usseglio-Polatera P (2007) Patterns of benthic community traits in neotropical streams: relationship to mesoscale spatial variability. Fundam Appl Limnol / Archiv für Hydrobiologie 170:243–255
Tomanova S, Goitia E, Helešic J (2006) Trophic levels and functional feeding groups of macroinvertebrates in neotropical streams. Hydrobiologia 556:251–264
Tyler HL, Moore MT, Locke MA (2012) Influence of three aquatic macrophytes on mitigation of nitrogen species from agricultural runoff. Water Air Soil Pollut 223:3227–3236
Wang JG (2003) Tolerance values of benthic macroinvertebrates and bioassessment of water quality in the Lushan nature reserve. Chin J Appl Environ Biol 9:279–284 (in Chinese with English abstract)
Wang X, Tan X (2017) Macroinvertebrate community in relation to water quality and riparian land use in a substropical mountain stream, China. Environ Sci Pollut Res 24:14682–14689
Wang BX, Yang LF (2004) A study on tolerance values of benthic macroinvertebrate taxa in eastern China. Acta Ecol Sin 24:2768–2775 (in Chinese with English abstract)
Wang GX, Guo XY, Cheng GD (2002) Dynamic variations of landscape pattern and the landscape ecological functions in the source area of the Yellow River. Acta Ecol Sin 22:1587–1598 (in Chinese with English abstract)
Wang YL, Wang X, Li CH, Wu FF, Yang ZF (2015) Spatiotemporal analysis of temperature trends under climate change in the source region of the Yellow River, China. Theor Appl Climatol 119:123–133
Wiggins GB (2015) Larvae of the North American caddisfly genera (Trichoptera). University of Toronto Press, Canada
Xu M, Wang Z, Pan B, Zhao N (2012) Distribution and species composition of macroinvertebrates in the hyporheic zone of bed sediment. Int J Sediment Res 27:129–140
Xu M, Wang Z, Duan X, Pan B (2014) Effects of pollution on macroinvertebrates and water quality bio-assessment. Hydrobiologia 729:247–259
Zhang J, Li G, Liang S (2012) The response of river discharge to climate fluctuations in the source region of the Yellow River. Environ Earth Sci 66:1505–1512
Zhao N, Xu M, Li Z, Wang Z, Zhou H (2017) Macroinvertebrate distribution and aquatic ecology in the Ruoergai (Zoige) Wetland, the Yellow River source region. Front Earth Sci 11:554–564
Zhou HK, Zhou L, Liu W, Zhao XQ, Lai DZ (2003) Causes of grassland degradation and sustainable development of animal husbandry in Maduo County, Qinghai Province. Grassland China 25:63–67 (in Chinese)
Acknowledgements
We thank the editor Dr. Philippe Garrigues and the two anonymous reviewers for their review comments that significantly strengthened the paper.
Funding
The study was financially supported by the National Science Fund China (91547204, 51779120, 51622901), the National Key Research and Development Program of China (2016YFC0402407, 2016YFC0402406), Tsinghua University Project (2015THZ02-1), State Key Laboratory of Hydroscience and Engineering Project (2016-KY-04), and the Yellow River Institute of Hydraulic Research (HKY-JBYW-2016-03).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, W., Xu, M., Zhao, N. et al. River health assessment of the Yellow River source region, Qinghai-Tibetan Plateau, China, based on tolerance values of macroinvertebrates. Environ Sci Pollut Res 26, 10251–10262 (2019). https://doi.org/10.1007/s11356-018-04110-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-04110-0