Abstract
This study reports the eco-friendly preparation of a novel composite material consisting of red mud and carbon spheres, denoted as red mud@C composite, and its application for the removal of 2,4-dichlorophenoxyacetic acid herbicide (2,4-D) from aqueous solution. The preparation route has a green approach because it follows the low-energy consuming one-step hydrothermal process by using starch as a renewable carbon precursor and red mud as a waste from aluminum production industry. Characterization of the red mud@C composite was performed by FT-IR, TGA, SEM, TEM, BET, XRD, and Raman microscopy analyses. The batch adsorption studies revealed that the red mud@C composite has higher 2,4-D adsorption efficiency than those of the red mud and the naked carbon spheres. The maximum removal at initial pH of 3.0 is explained by considering the pKa of 2,4-D and pH of point of zero charge (pHpzc) of the composite material. The adsorption equilibrium time was 60 min, which followed the pseudo-second-order kinetic model together with intra-particle diffusion model. The isotherm analysis indicated that Freundlich isotherm model better represented the adsorption data, with isotherm parameters of k [15.849 (mg/g) (mg/L)−1/n] and n (2.985). The prepared composite is reusable at least 5 cycles of adsorption-desorption with no significant decrease in the adsorption capacity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
2,4-Dichlorophenoxyacetic acid (2,4-D) is a widely used herbicide against broad-leaved weeds and plants in agriculture and aquatic weeds due to its good selectivity (Ruiz de Arcaute et al. 2016). Therefore, it has been frequently found in water bodies all over the world (Hameed et al. 2009; Tang et al. 2015). The World Health Organization (WHO) has identified 2,4-D as moderately toxic (class II) to human beings and animals (WHO 2009) and United States Environmental Protection Agency (US EPA) regulated the limit concentration of 2,4-D for drinking water as 70 μg/L (US EPA 2012). Thus, the studies on the removal of 2,4-D from water is important for environmental health.
Various techniques, such as a combined photo-Fenton and biological oxidation (Ballesteros Martín et al. 2009), electrocatalytic dechlorination (Xu et al. 2013), photocatalytic degradation (López-Ayala et al. 2015), combined microwave-assisted degradation and UV irradiation (Horikoshi et al. 2003), chemical oxidation with hydrogen peroxide (Badellino et al. 2006), and adsorption (Aksu and Kabasakal 2005; Gupta et al. 2006; Pirsaheb et al. 2014), have been reported for the removal of 2,4-D from water. Among these techniques, adsorption is considered to be an effective, versatile, and economical technique for the treatment of polluted water due to its simplicity, high efficiency, and low-capital investment (Dupont and Guillon 2003; Gerçel et al. 2007; Rafatullah et al. 2010; Ai and Li 2013).
In recent studies on the adsorption, the composite materials containing carbonaceous materials with other solid adsorbents have emerged as a new type of adsorbents. The advantages, such as high adsorption efficiency, simple preparation process, and low operational cost, motivate researchers to focus on this type of composite material in the adsorption-based water treatment applications. For instance, Ai and Li (2013) reported the efficient removal of organic dyes from aqueous solution by using eco-friendly biomass-derived carbon@montmorillonite nanocomposites by one-step hydrothermal process. Zhang and Kong (2011) studied the removal of methylene blue from aqueous solution by using the solvothermal-synthesized Fe3O4@C nanoparticles. Gong et al. (2011) indicated that double-layered hydroxide nanocrystals@carbon nanospheres had high efficiency for removing metal ions from aqueous solution. Chen et al. (2011) showed that attapulgite@carbon nanocomposite could be efficiently used for the removal of Cr(VI) and Pb(II) ions from aqueous solutions. Zhou et al. (2012) produced the rattle-type carbon-alumina core-shell spheres, and they investigated the adsorption efficiency of this adsorbent for dye-stuff in water. Banerjee et al. (2012) reported that metal organic framework-derived carbon-Fe3O4 nanocomposite could be employed as a high-efficient adsorbent for the removal of pollutants, including oil, hydrocarbon, dye, and phenol. Such composite adsorbents have been frequently prepared via hydrothermal carbonization process. Because glucose, sucrose, and cellulose have been often used as a renewable carbon precursor and carbonization occurs at low temperature in this process, it has been considered as a green and effective technique for the preparation of carbon-based composite adsorbent (Chen et al. 2011; Li et al. 2012; Zhang et al. 2014).
In the presented study, an environmentally benign red mud-carbon composite adsorbent (red mud@C) was prepared for the first time by using one-step hydrothermal process. The preparation of red mud@C has a green approach because of the fact that it follows the one-step hydrothermal process by using starch as a renewable carbon precursor and waste red mud. Red mud, which is a bauxite waste of aluminum production, has been efficiently used in many adsorption studies as unconventional adsorbent since it is comprised of metal oxides and hydroxides, which are able to remove various pollutants including dye (Tor and Cengeloglu 2006), organochlorine pesticides (Ozcan et al. 2011), phenol (Tor et al. 2006), boron (Cengeloglu et al. 2007), phosphate (Pradhan et al. 1998), nitrate (Cengeloglu et al. 2006), heavy metals (Apak et al. 1998), and arsenic (Altundoğan et al. 2002; Genç-Fuhrman et al. 2004) from aqueous solutions. The other advantages of using red mud in adsorption studies are that none of the trace elements are released from the red mud in aqueous solutions and spent red mud is not toxic because it satisfied the toxicity characteristic leaching procedure (Genç et al. 2003). Based on this background, the aims of this study are (i) preparation of an original composite adsorbent (red mud@C) using red mud and starch through one-step hydrothermal process, (ii) characterization of the product, and (iii) investigation on the adsorption performance of red mud@C composite for 2,4-D in aqueous solutions.
Experiment
Chemical reagents and apparatus
All reagents were analytical grade and used without further purification. 2,4-D was obtained from Merck Co. (İstanbul, Turkey). 2,4-D given in Fig. 1 is a plane molecule due to the presence of aromatic cycle and Csp2 in the carboxylic acid group. HCl, NaOH, and NaCl were purchased from Merck Co. (Darmstadt, Germany). (NH4)2Fe(SO4)2 was purchased from Sigma-Aldrich (Steinheim, Germany). Maize starch was obtained from a local market. The red mud was supplied by an aluminum production factory (Seydişehir, Konya, Turkey). Because the red mud has alkaline property (pH ≈ 12–13), prior to use in this study, it was neutralized (until equilibrium pH ≈ 7.5) and dried by following the procedure described in our previous study (Tor et al. 2006). The neutralized red mud is comprised of particles with size less than 10 μm (Tor et al. 2006). Elemental compositions of the neutralized red mud determined by using a X-ray fluorescence (XRF) spectrometer (Nexcg, Rigaku) were as follows (wt.%): Fe (25.9%), Na (11.8%), Al (11.3%), Si (8.1%), Ti (4.3%), Ca (2.4%), K (0.3%), Mg (0.2%), P (%0.1), and others.
A PerkinElmer 1600 FT-IR spectrometer (USA) was used in order to obtain FT-IR spectrum of the materials (red mud, carbon spheres, and red mud@C composite) between 500 and 4000 cm−1. Thermal gravimetric analysis (TGA) of the materials was achieved using a thermogravimetric analyzer (Setaram, Setsys Evolution, France) in the temperature range between 40 and 900 °C with a heating rate of 10 °C/min in argon atmosphere with a flow rate of 20 mL/min. X-ray diffractometer (GNR APD 2000 PRO, Italy) was used to determine the crystallographic nature of the studied materials. The specific surface area of the materials was determined by N2 adsorption isotherms at 77 K with a (Quantachrome Quadrasorb Evo BET analyzer, USA). The surface images of the materials were obtained via a SEM (JEOL 5600-LU, UK) after covering the materials with 10–20-nm layer of Au. The materials were also examined by using a TEM (JEOL TEM 2100 UHR-TEM) equipped with an EDX detector. Raman spectroscopy (Renishaw inVia-UK) was used in order to obtain Raman images (3D in a size of 3.0 × 6.0 × 6.0 μm; x, y, z). A layer of the red mud@C composite was deposited onto a glass slide, and spectrums were recorded with an objective (×100) creating a 532-nm DPSS laser. The surface charge of the red mud@C composite was measured in terms of zeta potential at each studied pH value in the adsorption experiments by using a zeta potential analyzer (Malvern Zetasizer Nano Z, UK).
Preparation of red mud@C composite
Red mud@C composite adsorbent was prepared through a modified hydrothermal process described by Feng et al. (2016). One gram of neutralized red mud was dispersed in 20 mL of distilled water using ultrasonication for 10 min. One gram of starch was added into the mixture, and it was heated at 95 °C for 25 min for gelatinization of starch. After 10 mL of (NH4)2Fe(SO4)2 solution (0.5 mM) was added into the mixture, it was placed in a Teflon-lined stainless steel autoclave with capacity of 50 mL and heated at 180 °C for 12 h. The resulting product was centrifuged, washed with water, and dried at 100 °C in order to obtain red mud@C composite. Carbon spheres were also prepared by following the same route with no addition of red mud for the control of their adsorption performance.
Batch adsorption experiments
The desired concentration of 2,4-D solutions was obtained through diluting 500 mg/L of stock solution. pH adjustment of the solutions was carried out by using 1 M HCl and/or 1 M NaOH solutions on a pH meter (Hach, HQ411d, USA) with a combined glass-Ag/AgCl electrode. Ionic strength of the solutions was adjusted to 0.01 M with NaCl.
The adsorption of 2,4-D from aqueous solutions by prepared red mud@C composite was studied according to the batch experiments. For that, red mud@C composite was contacted with 20 mL of the corresponding 2,4-D solutions over a period of time on a temperature-controlled horizontal shaker (JSR, 300C, Korea) at 150 rpm. After red mud@C composite was separated from each corresponding solution by filtration, the remaining concentration of 2,4-D in each solution was determined by using a UV spectrophotometer (Hach Lange, DR 500, Germany) at wavelength of 284 nm (Hameed et al. 2009).
The amount of 2,4-D removed by red mud@C composite was determined according to Eq. (1).
where q is the amount of 2,4-D removed by per gram of red mud@C composite (mg/g), C o and C e are the initial and equilibrium concentrations of 2,4-D (mg/L), V is the solution volume (L), and m is the mass of red mud@C composite (g). Furthermore, the effects of following operating parameters on the adsorption were investigated: pH of solution (pH 3–11), contact time (2–150 min), initial concentration of 2,4-D (20–400 mg/L), and reusability of red mud@C composite. Whole experiments were performed in triplicate and their relative standard deviations were presented in the corresponding figures.
Results and discussion
Characterization
Structural analysis
FT-IR spectrums of the red mud, carbon spheres, and red mud@C composite are shown in Fig. 2. In the spectrum of red mud, the main bands obtained at 974 and 530 cm−1 can be assigned to the stretching of Si–O–Si bond (Sarkar et al. 2015). For carbon spheres obtained through the hydrothermal process of starch in the absence of red mud, the bands obtained at 1691 and 1590 cm−1 can be attributed to C = O (carbonyl, ester, or carboxyl) and C = C vibrations, respectively (Sevilla and Fuertes 2009). For red mud@C composite material, the broad band between 3000 and 3700 cm−1 corresponds to the –OH stretching (Nath and Sahoo 2014). Additionally, the spectrum observed for the red mud@C composite material has the same characteristic bands (at 1691, 1600, 1006, and 530 cm−1) as red mud and carbon spheres. The red shift in Si–O–Si stretching band from 974 to 1006 cm−1 can be attributed to an increase of O ratio in the environment of SiOx (Landreau et al. 2012).
TGA curves of the red mud, carbon spheres, and red mud@C composite are shown in Fig. 3. For red mud, the mass loss (approximately 2%) at temperature up to 200 °C is due to the loss of physically adsorbed water on the red mud particles (Sglavo et al. 2000). The mass loss (approximately 5%) at temperature range of 200–600 °C is due to the dehydroxylation of FeO(OH) (Atasoy 2005; Song and Boily 2016) and AlO(OH) (Atasoy 2005; Liu et al. 2016) phases of the red mud. Further loss at temperature up to 800 °C can be attributed to the decomposition of sodalite group of the red mud (Atasoy 2011). For carbon spheres, the mass loss (5%) at temperature up to 150 °C can be assigned to the physically adsorbed water on the carbon spheres (Szymański et al. 2002). The considerable loss (45%) at temperature range of 150–800 °C can be due to the degradation of carbon species especially including oxygen-containing functional groups (Feng et al. 2016). This mass loss also means that the carbon spheres obtained through the hydrothermal carbonization process have an important amount of oxygen-containing functional groups (Tang et al. 2016). This is in agreement with the results from FT-IR spectrum of carbon spheres, especially observed bands at 1691 and 1590 cm−1 due to C = O and C = C groups. For red mud@C composite, the mass loss observed up to 800 °C is less than that of carbon spheres. It is probably due to the fact that red mud prevents the loss of the covered carbon species in the structure of composite material.
XRD patterns of the red mud, carbon spheres, and red mud@C composite material are shown in Fig. S1. For red mud, the characteristic diffraction peaks at 2θ = (14.0°, 18.8°, and 35.6°) correspond to boehmite (AlOOH); 2θ = 20.4° corresponds to goethite (FeOOH); 2θ = (24.3°, 33.1°, 40.9°, 49.4°, and 54.3°) indicate hematite (Fe2O3); and peaks at 2θ = 27.4° and 27.6° assign to rutile (TiO2) and quartz (SiO2), respectively (Lim et al. 2014). In case of carbon spheres, a broad peak observed at 2θ between 10° and 30° is due to its amorphous structure (Liu et al. 2013). Because red mud@C composite has the same characteristic diffraction peaks as red mud, it can be inferred that composite material was successfully prepared. Moreover, a decrease in the peak intensity at 2θ = 10° to 30° for red mud@C composite may also prove that surface of the carbon spheres was covered by red mud particles.
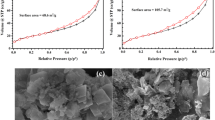
BET surface area and total pore volume of the red mud, carbon spheres, and red mud@C composite are listed in Table 1, which shows that composite material has the highest surface area (89.512 m2/g). This result can be attributed to the presence of carbon spheres during the preparation of composite material under the hydrothermal process. The carbon spheres can act as suitable support materials for the red mud particles, which provide a composite material having increased morphological properties. In other words, the carbon spheres not only help in the formation of homogenous dispersion of the red mud onto their surfaces but also prevent agglomeration of the red mud particles, which leads to an increase in the surface area of the red mud@C composite material (Wu et al. 2014; Sarkar et al. 2015). Moreover, Fig. 4 shows that the red mud@C composite has more micropores (<2 nm) and mesopores (2–50 nm) (Lee et al. 2006) than the red mud and carbon spheres, which resulted in the highest total pore volume for the composite material (0.198 cm3/g). The micropores are considered to be responsible for adsorption. While, mesopores and macropores have a key function in transportation of adsorbate to the adsorption sites (Unur 2013). Based on the above results, it is expected that in adsorption studies, red mud@C composite will provide higher removal efficiency than red mud and carbon spheres. In fact, the pore diameters in the range of 10–30 Å (1–3 nm) fit well with size of 2,4-D (~0.5 × 1 nm), which makes red mud@C composite potential adsorbent for the removal of 2,4-D from aqueous solution.
Morphological analysis
SEM image of red mud (Fig. 5a) indicates that it has an aggregated morphology. Figure 5b, b′ shows that the carbon spheres have smooth surfaces. Interestingly, as it is seen in Fig. 5c, c′, the red mud@C composite has different morphology in comparison to the red mud and carbon spheres. Because the red mud particles were adhered to the carbon spheres, surface of the obtained red mud@C composite became rougher than that of the carbon spheres.
Further evidence concerning the successful preparation of the composite is shown on the TEM images (Fig. 5d, e). Meanwhile, the corresponding EDX mapping images of the red mud@C composite material in Fig. 5f, f′ qualitatively indicate that composite material has mostly the same elements (i.e., Fe, Na, Al, Si) as red mud. EDX analysis also confirms that red mud particles were deposited on the carbon spheres.
In order to illustrate the inner properties revealing the distribution of chemical compounds of the composite material, 3D confocal volume imaging was obtained with an excitation wavelength of 532 nm. Figure 5g displays the resulting 3D Raman image of red mud@C composite, which reveals the carbon spheres in red color, all surrounded homogenously by red mud with green/blue color. This imaging result confirms that the composite material was successfully obtained.
Adsorption experiments
Effect of solution pH
The effect of solution pH on the adsorption of 2,4-D by suggested red mud@C composite was examined in the pH range of 3–11 with initial 2,4-D concentration of 100 mg/L at 25 °C. The result in Fig. 6a indicates that adsorption was maximum at pH 3. Further increase in the solution pH caused a decrease in the adsorption efficiency, which can be attributed to the electrostatic interaction between 2,4-D and surface of the red mud@C composite material. Considering pKa of 2,4-D (2.64) (Aksu and Kabasakal 2005), it exists as an anionic molecular form in the aqueous solution when pH is above 2.64. However, it is present in the non-ionic molecular form when pH of solution is less than 2.64. In addition, pH of point of zero charge (pHpzc) for the red mud@C composite material was estimated as 3.5 (Fig. 6b). In other words, the surface of red mud@C composite material is positively charged at pH below 3.5 and negatively charged at pH values above 3.5. Therefore, the reason for maximum adsorption at pH 3 is due to the electrostatic attraction between the anionic molecular form of 2,4-D and positively charged of red mud@C composite surface. At pH 3, adsorption efficiency of the red mud@C composite was higher than those of the carbon spheres and red mud (Fig. S2). This result can be attributed to the higher BET surface area of the red mud@C composite as described in the “Structural analysis” section.
Effect of contact time and kinetic analysis
The effect of contact time on the adsorption of 2,4-D by red mud@C composite is presented in Fig. 7, showing that equilibrium time was 60 min and it was independent of initial concentration of 2,4-D. This result can be attributed to that the red mud@C composite material has limited number of active sites, which become saturated at equilibrium time (60 min) for each initial concentration of 2,4-D (Hema and Arivoli 2007; Nidheesh et al. 2012).
The experimental adsorption data was analyzed by employing the pseudo-first-order and pseudo-second-order models and intra-particle diffusion model. The linearized pseudo-first-order model is shown in Eq. (2) (Lagergren 1898).
where q e and q t are the amounts of adsorbed 2,4-D (mg/g) at equilibrium and at time t (min), respectively, and k 1 (1/min) is the adsorption rate constant of pseudo-first-order kinetic model.
Linearized form of the pseudo-second-order kinetic model described by Ho and McKay (Mckay 1984; Ho and McKay 1999) is shown in Eq. (3).
where k 2 is the rate constant of pseudo-second-order model (g/(mg min)) and q e and q t are the same as described above.
By using 20 and 100 mg/L of 2,4-D solutions, the fit of the kinetic models was tested through linear plots of (log (q e − q t ) versus t) and (t/q t versus t) for pseudo-first-order and pseudo-second-order models, respectively (Figs. S3 and S4). The constants of the models determined from the slope and intercept of the plots are given in Table 2, which shows that the pseudo-second-order model is of higher R 2 value than the pseudo-first-order model. Besides, the pseudo-second-order model provided q e values, which were in good agreement with the experimental q e values for 2,4-D concentrations of 20 and 100 mg/L. As a consequence, it can be reported that the adsorption of 2,4-D by using red mud@C composite followed the pseudo-second-order kinetic model.
The intra-particle diffusion model given in Eq. (4) (Weber and Morris 1963) was also applied to the experimental data.
where k intra is a constant of the intra-particle diffusion rate [mg/(g min1/2)] and C is a constant about the boundary layer (mg/g).
The values of k intra were determined via the slope of the plot of Eq. (4). For concentrations of 20 and 100 mg/L, the plots of q t versus t 1/2 have three linear parts for the adsorption (Fig. S5). Based on the study by dos Santos et al. (2014), the first linear stage is because of the diffusion of 2,4-D molecules to the surface of composite material. The second linear stage is because of the intra-particle diffusion of 2,4-D, which is followed by the third linear stage concerning the equilibrium. From the first point of the third stage, the equilibrium time was 60 min.
Effect of initial concentration and isotherm analysis
It is observed that increasing initial concentration of 2,4-D improved the amount of adsorbed 2,4-D by red mud@C composite material. For instance, the amount of adsorbed 2,4-D was found as 18.047(±0.284), 36.198(±1.774), and 52.586(±0.902) mg/g, for initial concentrations of 20, 50, and 100 mg/L, respectively. This result can be linked to the driving force for mass transfer (Ünlü and Ersoz 2006).
The experimental adsorption data was also analyzed via Langmuir, Freundlich, and Redlich-Peterson isotherm models (Fig. 8). The Langmuir isotherm represents the monolayer adsorption on the adsorbent surfaces, and it is considered that adsorption takes place on a homogeneous surface. The Langmuir isotherm model is shown in Eq. (5) (Langmuir 1916).
where C e is the equilibrium concentration of 2,4-D (mg/L), Q o is the Langmuir adsorption capacity of the red mud@C composite (mg/g), and b is the adsorption constant of Langmuir isotherm (L/mg). The values of Q o and b were calculated through the slope and intercept of the linearized Langmuir model {C e /q e = 1/(Q o .b) + C e /Q o}, respectively (Fig. S6 and Table 3).
The Freundlich isotherm model indicated in Eq. (6) represents the multilayer adsorption on a heterogeneous surface (Freundlich 1906).
where k is an indicator of the adsorption capacity [(mg/g) (mg/L)−1/n] and n is an empirical parameter. k and n values were determined from the intercept and slope of the linearized Freundlich model {log q e = log k + 1/n.log C e }, respectively (Fig. S6 and Table 3).
The Redlich-Peterson isotherm is an empirical model involving three parameters as given in Eq. (7). This model can be employed for both homogenous or heterogeneous adsorption systems (Redlich and Peterson 1959).
where K R (L/mg) and a R (L/mg)β are the constants of Redlich-Peterson model and β is the exponent between zero and one. When value of β is 1, Eq. (7) reduces to Langmuir isotherm model. Whereas, in case of β = 0, Eq. (7) reduces to Henry’s equation. The constants were determined by using the intercept and slope of the linearized Redlich-Peterson model {ln[(K R .C e /q e ) − 1] = β.ln C e + ln a R }(Fig. S6 and Table 3).
Based on the R 2 values in Table 3, 2,4-D adsorption data was better described by Freundlich and Redlich-Peterson isotherm models than the Langmuir isotherm. The chi-squared (χ 2) test could also be applied to adsorption data in order to confirm the most-fit isotherm model. The chi-squared test is given in Eq. (8) (Ho 2004).
where q m is the adsorption capacity estimated from the model (mg/g) and q e is the experimental value of the adsorption capacity (mg/g). Small value of χ 2 means that data obtained from the model is close to the experimental one. However, high χ 2 value shows that there is a significant difference between data from the model and the experimental one. Based on the χ 2 values in Table 3, the most-fit isotherm model for the adsorption of 2,4-D was the Freundlich model. In fact, the Redlich-Peterson isotherm model promoted the Freundlich model. This finding can be also concluded from the value of β (0.684) in Table 3, which is less than unity (Ho 2004).
The adsorption capacity of red mud@C composite was compared with values in constants of Langmuir and Freundlich isotherm models for the adsorption of 2,4-D by using various adsorbents in the literature that were compared with those determined in this study (Table 4). It can be concluded that red mud@C composite material has considerable adsorption capacity. Although some adsorbents, such as iron oxide nanoparticle-doped carboxylic ordered mesoporous carbon (Tang et al. 2015), granular activated carbon (Aksu and Kabasakal 2004), and activated carbon derived from date stones (Hameed et al. 2009), can be employed to remove 2,4-D from water with higher adsorption capacity, the red mud@C composite material provides advantages over these adsorbents, i.e., use of waste red mud and renewable carbon source, easier and greener preparation approach.
Regeneration and reusability of red mud@C composite
In order to regenerate the red mud@C composite applied to 2,4-D solution (20 mg/L) under optimum experimental condition, it was treated with 20 mL of 0.001 M NaOH as a desorption solution for 30 min on a horizontal shaker (150 rpm) at 25 °C. After this treatment, the red mud@C composite was washed with distilled water several times for neutralization. The batch adsorption experiments were repeated using the regenerated red mud@C composite. It was found that after 5 cycles, there were no significant changes in its sorption capacity (Fig. S7), which means that red mud@C composite could be reused for at least five adsorption/desorption cycles for removing 2,4-D from aqueous solutions.
Conclusion
This study is the first that reports the preparation of an original red mud@C composite adsorbent using red mud and starch. This composite material has a green approach because it is based on the less energy-consuming, one-step hydrothermal carbonization process using a renewable carbon precursor (starch) and aluminum industry waste (red mud). The prepared red mud@C composite was characterized by FT-IR, TGA, SEM, TEM, BET, XRD, and Raman microscopy analyses and zeta potential measurements. The red mud@C composite was examined on the removal of 2,4-D from aqueous solution as an adsorbent material. In comparison to the red mud and the naked carbon spheres, the composite material has quite satisfactory adsorption capacity, which is also comparable with previously published adsorbent materials. The batch experiments indicated that adsorption of 2,4-D was maximum at initial pH of 3.0. The contact time required for equilibrium was 60 min and the pseudo-second-order kinetic model together with intra-particle diffusion model governed the adsorption behavior. Isotherm analysis showed that adsorption data was better described by the Freundlich isotherm model, confirmed by χ 2 statistical test. The composite is reusable at least five adsorption-desorption cycles with no significant changes in its sorption capacity.
References
Ai L, Li L (2013) Efficient removal of organic dyes from aqueous solution with ecofriendly biomass-derived carbon@montmorillonite nanocomposites by one-step hydrothermal process. Chem Eng J 223:688–695. doi:10.1016/j.cej.2013.03.015
Aksu Z, Kabasakal E (2004) Batch adsorption of 2,4-dichlorophenoxy-acetic acid (2,4-D) from aqueous solution by granular activated carbon. Sep Purif Technol 35:223–240. doi:10.1016/S1383-5866(03)00144-8
Aksu Z, Kabasakal E (2005) Adsorption characteristics of 2,4-dichlorophenoxyacetic acid (2,4-D) from aqueous solution on powdered activated carbon. J Environ Sci Heal Part B 40:545–570. doi:10.1081/PFC-200061533
Altundoğan HS, Altundoğan S, Tümen F, Bildik M (2002) Arsenic adsorption from aqueous solutions by activated red mud. Waste Manag 22:357–363. doi:10.1016/S0956-053X(01)00041-1
Apak R, Güçlü K, Turgut MH (1998) Modeling of copper(II), cadmium(II), and lead(II) adsorption on red mud. J Colloid Interface Sci 203:122–130. doi:10.1006/jcis.1998.5457
Atasoy A (2005) An investigation on characterization and thermal analysis of the Aughinish red mud. J Therm Anal Calorim 81:357–361. doi:10.1007/s10973-005-0792-5
Atasoy A (2011) Reduction of ferric oxides in the red mud by the aluminıothermic process. In: 6th international advanced technologies symposium. pp 16–18
Badellino C, Rodrigues CA, Bertazzoli R (2006) Oxidation of pesticides by in situ electrogenerated hydrogen peroxide: study for the degradation of 2,4-dichlorophenoxyacetic acid. J Hazard Mater 137:856–864. doi:10.1016/j.jhazmat.2006.03.035
Ballesteros Martín MM, Sánchez Pérez JA, Casas López JL et al (2009) Degradation of a four-pesticide mixture by combined photo-Fenton and biological oxidation. Water Res 43:653–660. doi:10.1016/j.watres.2008.11.020
Banerjee A, Gokhale R, Bhatnagar S et al (2012) MOF derived porous carbon–Fe3O4 nanocomposite as a high performance, recyclable environmental superadsorbent. J Mater Chem 22:19694. doi:10.1039/c2jm33798c
Cengeloglu Y, Tor A, Ersoz M, Arslan G (2006) Removal of nitrate from aqueous solution by using red mud. Sep Purif Technol 51:374–378. doi:10.1016/j.seppur.2006.02.020
Cengeloglu Y, Tor A, Arslan G et al (2007) Removal of boron from aqueous solution by using neutralized red mud. J Hazard Mater 142:412–417. doi:10.1016/j.jhazmat.2006.08.037
Chen L-F, Liang H-W, Lu Y et al (2011) Synthesis of an attapulgite clay@carbon nanocomposite adsorbent by a hydrothermal carbonization process and their application in the removal of toxic metal ions from water. Langmuir 27:8998–9004. doi:10.1021/la2017165
Deng S, Ma R, Yu Q et al (2009) Enhanced removal of pentachlorophenol and 2,4-D from aqueous solution by an aminated biosorbent. J Hazard Mater 165:408–414. doi:10.1016/j.jhazmat.2008.10.029
Deokar SK, Mandavgane SA (2015) Rice husk ash for fast removal of 2,4-dichlorophenoxyacetic acid from aqueous solution. Adsorpt Sci Technol 33:429–440. doi:10.1260/0263-6174.33.5.429
dos Santos DC, Adebayo MA, de Fátima Pinheiro Pereira S et al (2014) New carbon composite adsorbents for the removal of textile dyes from aqueous solutions: kinetic, equilibrium, and thermodynamic studies. Korean J Chem Eng 31:1470–1479. doi:10.1007/s11814-014-0086-3
Dupont L, Guillon E (2003) Removal of hexavalent chromium with a lignocellulosic substrate extracted from wheat bran. Environ Sci Technol 37:4235–4241. doi:10.1021/ES0342345
Feng Z, Liu D, Ma X (2016) The rectorite/carbon composites: fabrication, modification and adsorption. Chemosphere 144:621–627. doi:j.chemosphere.2015.09.032
Freundlich HMF (1906) Über die Adsorption in Lösungen. Z Phys Chem 385–470
Genç H, Tjell JC, McConchie D, Schuiling O (2003) Adsorption of arsenate from water using neutralized red mud. J Colloid Interface Sci 264:327–334. doi:10.1016/S0021-9797(03)00447-8
Genç-Fuhrman H, Tjell JC, McConchie D (2004) Increasing the arsenate adsorption capacity of neutralized red mud (Bauxsol). J Colloid Interface Sci 271:313–320. doi:10.1016/j.jcis.2003.10.011
Gerçel Ö, Özcan A, Özcan AS, Gerçel HF (2007) Preparation of activated carbon from a renewable bio-plant of Euphorbia rigida by H2SO4 activation and its adsorption behavior in aqueous solutions. Appl Surf Sci 253:4843–4852. doi:10.1016/j.apsusc.2006.10.053
Gong J, Liu T, Wang X et al (2011) Efficient removal of heavy metal ions from aqueous systems with the assembly of anisotropic layered double hydroxide nanocrystals@carbon nanosphere. Environ Sci Technol 45:6181–6187. doi:10.1021/es200668q
Gupta VK, Ali I, Suhas SVK (2006) Adsorption of 2,4-D and carbofuran pesticides using fertilizer and steel industry wastes. J Colloid Interface Sci 299:556–563. doi:10.1016/j.jcis.2006.02.017
Hameed BH, Salman JM, Ahmad AL (2009) Adsorption isotherm and kinetic modeling of 2,4-D pesticide on activated carbon derived from date stones. J Hazard Mater 163:121–126. doi:10.1016/j.jhazmat.2008.06.069
Hema M, Arivoli S (2007) International journal of physical sciences. Int J Phys Sci 2:10–17
Ho Y-S (2004) Selection of optimum sorption isotherm. Carbon N Y 42:2115–2116
Ho Y-S, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465. doi:10.1016/S0032-9592(98)00112-5
Horikoshi S, Hidaka H, Serpone N (2003) Environmental remediation by an integrated microwave/UV-illumination technique: IV. Non-thermal effects in the microwave-assisted degradation of 2,4-dichlorophenoxyacetic acid in UV-irradiated TiO2/H2O dispersions. J Photochem Photobiol A Chem 159:289–300. doi:10.1016/S1010-6030(03)00185-0
Kuśmierek K, Szala M, Świątkowski A (2016) Adsorption of 2,4-dichlorophenol and 2,4-dichlorophenoxyacetic acid from aqueous solutions on carbonaceous materials obtained by combustion synthesis. J Taiwan Inst Chem Eng 63:371–378. doi:10.1016/j.jtice.2016.03.036
Lagergren S (1898) Zur theorie der sogenannten absorption... - Google Akademik. K K Sven Vetenskapsad Handl 24:1–39
Landreau X, Lanfant B, Merle T et al (2012) A thorough FT-IR spectroscopy study on micrometric silicon oxide films deposited by atmospheric pressure microwave plasma torch. Eur Phys J D 66:160. doi:10.1140/epjd/e2012-20647-x
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I Solids J Am Chem Soc 38:2221–2295. doi:10.1021/ja02268a002
Lee J, Kim J, Hyeon T (2006) Recent progress in the synthesis of porous carbon materials. Adv Mater 18:2073–2094. doi:10.1002/adma.200501576
Li T, Shen J, Li N, Ye M (2012) Facile and novel hydrothermal preparation of functionalised carbon microspheres from glucose by using graphene sheets as a substrate. Mater Lett 89:202–205. doi:10.1016/j.matlet.2012.08.132
Lim X, Sanna A, Andrésen JM (2014) Influence of red mud impregnation on the pyrolysis of oil palm biomass-EFB. Fuel 119:259–265. doi:10.1016/j.fuel.2013.11.057
Liu Y, Cai Q, Li H, Zhang J (2013) Fabrication and characterization of mesoporous carbon nanosheets using halloysite nanotubes and polypyrrole via a template-like method. J Appl Polym Sci 128:517–522. doi:10.1002/app.38208
Liu Y, Naidu R, Ming H et al (2016) Effects of thermal treatments on the characterisation and utilisation of red mud with sawdust additive. Waste Manag Res 34:518–526. doi:10.1177/0734242X16634197
López-Ayala S, Rincón ME, Quiroz Alfaro MA et al (2015) Nanocrystalline titania xerogels doped by metal precursors in the photocatalytic degradation of 2,4-D sodium salts. J Photochem Photobiol A Chem 311:166–175. doi:10.1016/j.jphotochem.2015.06.019
Mckay G (1984) The adsorption of basic dye onto silica from aqueous solution-solid diffusion model. Chem Eng Sci 39:129–138. doi:10.1016/0009-2509(84)80138-4
Nath H, Sahoo A (2014) A study on the characterization of red mud. Int J Appl bio-engineering 8:1–4. doi:10.18000/ijabeg.10118
Nidheesh PV, Gandhimathi R, Ramesh ST, Amatha Singh TS (2012) Kinetic analysis of crystal violet adsorption on to bottom ash. Turkish J Eng Env Sci 36:249–262. doi:10.3906/muh-1110-3
Ozcan S, Tor A, Aydin ME (2011) Removal of organochlorine pesticides from aqueous solution by using neutralized red mud. CLEAN - Soil, Air, Water 39:972–979. doi:10.1002/clen.201000596
Pirsaheb M, Dargahi A, Hazrati S, Fazlzadehdavil M (2014) Removal of diazinon and 2,4-dichlorophenoxyacetic acid (2,4-D) from aqueous solutions by granular-activated carbon. Desalin Water Treat 52:4350–4355. doi:10.1080/19443994.2013.801787
Pradhan J, Das J, Das S, Thakur RS (1998) Adsorption of phosphate from aqueous solution using activated red mud. J Colloid Interface Sci 204:169–172. doi:10.1006/jcis.1998.5594
Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2010) Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater 177:70–80. doi:10.1016/j.jhazmat.2009.12.047
Redlich O, Peterson DL (1959) A useful adsorption isotherm. J Phys Chem 63:1024–1024. doi:10.1021/j150576a611
Ruiz de Arcaute C, Soloneski S, Larramendy ML (2016) Toxic and genotoxic effects of the 2,4-dichlorophenoxyacetic acid (2,4-D)-based herbicide on the Neotropical fish Cnesterodon decemmaculatus. Ecotoxicol Environ Saf 128:222–229. doi:10.1016/j.ecoenv.2016.02.027
Sarkar B, Liu E, McClure S et al (2015) Biomass derived palygorskite–carbon nanocomposites: synthesis, characterisation and affinity to dye compounds. Appl Clay Sci 114:617–626. doi:10.1016/j.clay.2015.07.001
Sevilla M, Fuertes AB (2009) Chemical and structural properties of carbonaceous products obtained by hydrothermal carbonization of saccharides. Chem - A Eur J 15:4195–4203. doi:10.1002/chem.200802097
Sglavo VM, Campostrini R, Maurina S et al (2000) Bauxite “red mud” in the ceramic industry. Part 1: thermal behaviour. J Eur Ceram Soc 20:235–244. doi:10.1016/S0955-2219(99)00088-6
Song X, Boily J-F (2016) Surface and bulk thermal dehydroxylxation of FeOOH polymorphs. J Phys Chem A 120:6249–6257. doi:10.1021/acs.jpca.6b04294
Szymański GS, Karpiński Z, Biniak S, Świątkowski A (2002) The effect of the gradual thermal decomposition of surface oxygen species on the chemical and catalytic properties of oxidized activated carbon. Carbon N Y 40:2627–2639. doi:10.1016/S0008-6223(02)00188-4
Tang L, Zhang S, Zeng G-M et al (2015) Rapid adsorption of 2,4-dichlorophenoxyacetic acid by iron oxide nanoparticles-doped carboxylic ordered mesoporous carbon. J Colloid Interface Sci 445:1–8. doi:10.1016/j.jcis.2014.12.074
Tang J, Mu B, Wang W et al (2016) Fabrication of manganese dioxide/carbon/attapulgite composites derived from spent bleaching earth for adsorption of Pb(II) and brilliant green. RSC Adv 6:36534–36543. doi:10.1039/C5RA26362J
Tor A, Cengeloglu Y (2006) Removal of Congo red from aqueous solution by adsorption onto acid activated red mud. J Hazard Mater 138:409–415. doi:10.1016/j.jhazmat.2006.04.063
Tor A, Cengeloglu Y, Aydin ME, Ersoz M (2006) Removal of phenol from aqueous phase by using neutralized red mud. J Colloid Interface Sci 300:498–503. doi:10.1016/j.jcis.2006.04.054
Ünlü N, Ersoz M (2006) Adsorption characteristics of heavy metal ions onto a low cost biopolymeric sorbent from aqueous solutions. J Hazard Mater 136:272–280. doi:10.1016/j.jhazmat.2005.12.013
Unur E (2013) Functional nanoporous carbons from hydrothermally treated biomass for environmental purification. Microporous Mesoporous Mater 168:92–101. doi:10.1016/j.micromeso.2012.09.027
US EPA (2012) 2012 Edition of the Drinking Water Standards and Health Advisories (EPA 822-S-12-001). pp 1–12
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng 89:31–59
WHO (2009) The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2009
Wu X, Gao P, Zhang X et al (2014) Synthesis of clay/carbon adsorbent through hydrothermal carbonization of cellulose on palygorskite. Appl Clay Sci 95:60–66. doi:10.1016/j.clay.2014.03.010
Xu YH, Cai QQ, Ma HX et al (2013) Optimisation of electrocatalytic dechlorination of 2,4-dichlorophenoxyacetic acid on a roughened silver–palladium cathode. Electrochim Acta 96:90–96. doi:10.1016/j.electacta.2013.02.068
Zhang Z, Kong J (2011) Novel magnetic Fe3O4@C nanoparticles as adsorbents for removal of organic dyes from aqueous solution. J Hazard Mater 193:325–329. doi:10.1016/j.jhazmat.2011.07.033
Zhang W, Mu B, Wang A, Shao S (2014) Attapulgite oriented carbon/polyaniline hybrid nanocomposites for electrochemical energy storage. Synth Met 192:87–92. doi:10.1016/j.synthmet.2014.03.021
Zhou J, Tang C, Cheng B et al (2012) Rattle-type carbon–alumina core–shell spheres: synthesis and application for adsorption of organic dyes. ACS Appl Mater Interfaces 4:2174–2179. doi:10.1021/am300176k
Acknowledgements
The authors are grateful to the Scientific Research Projects of Necmettin Erbakan University (151219003) for the financial support.
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. All authors contributed equally.
Corresponding author
Additional information
Responsible editor: Guilherme L. Dotto
Electronic supplementary material
ESM 1
Supporting Information. XRD patterns of the materials, the removal efficiencies, the graphs of kinetic model, diffusion models, Langmuir, Freundlich, and Redlich-Peterson isotherms, and the changes in adsorption capacity of regenerated red mud@C composite.(DOCX 1257 kb)
Rights and permissions
About this article
Cite this article
Kazak, O., Eker, Y.R., Akin, I. et al. Green preparation of a novel red mud@carbon composite and its application for adsorption of 2,4-dichlorophenoxyacetic acid from aqueous solution. Environ Sci Pollut Res 24, 23057–23068 (2017). https://doi.org/10.1007/s11356-017-9937-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9937-x