Abstract
The present work describes the successful functionalization/magnetization of bio-polymeric spores of Lycopodium clavatum (sporopollenin) with 1-(2-hydroxyethyl) piperazine. Analytical techniques, i.e., Fourier transform infrared (FT-IR), field emission scanning electron microscope (FESEM), energy-dispersive X-ray spectroscopy (EDS), and vibrating sample magnetometer (VSM), were used to confirm the formation of 1-(2-hydroxyethyl) piperazine-functionalized magnetic sporopollenin (MNPs-Sp-HEP). The proposed adsorbent (MNPs-Sp-HEP) was used for the removal of noxious Pb(II) and As(III) metal ions from aqueous media through a batch-wise method. Different experimental parameters were optimized for the effective removal of selected noxious metal ions. Maximum adsorption capacity (q m ) 13.36 and 69.85 mg g−1 for Pb(II) and As(III), respectively, were obtained. Thermodynamic parameters such as free energy (ΔG°), entropy (ΔS°), and enthalpy (ΔH°) were also studied from the adsorption results and were used to elaborate the mechanism of their confiscation. The obtained results indicated that newly adsorbent can be successfully applied for the decontamination of noxious Pb(II) and As(III) from the aqueous environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, industrial sectors discharge a huge amount of effluents which contains variety of highly toxic as well as persistent pollutants such as dyes, phenols, pesticides, and heavy metals (Liu et al. 2010; Sáez et al. 2014; He 2015; Meenakumari and Philip 2015; Vymazal and Brezinova 2015; Bakhshaei et al. 2016; Shahabuddin et al. 2016). The release of the untreated effluents to the natural stream is of great anxiety because due to rapidly growing urbanization and industrialization, the scale of contaminated effluents is escalating day by day. Industrial units like battery manufacturing and metal pleating and finishing are renowned for the Pb(II) and As(III) contamination (Gupta and Rastogi 2008). Exposure of Pb(II) to the natural aqueous environment as a result of different anthropogenic activities such as industrialization, mining, and agriculture is becoming alarming form last few decades (Cheng et al. 2015; He 2015). The toxicity of Pb(II) can triggered to brain damage, mental deficiency, anemia, and behavioral problem if inhaled or swallowed (Gupta and Rastogi 2008). Due to the high toxicity of the Pb(II), the World Health Organization (WHO) has set maximum residual level 10 ng mL−1 in water samples (WHO 2008; Edition 2011). As(III) is also an omnipresent eco-toxin; its presence in aqueous media victimizes the huge amount of masses throughout the world (Tyrovola et al. 2007; Ali 2012). Its short-term exposure brings about dermal, respiratory, gastrointestinal, cardiovascular, mutagenic, and carcinogenic effects while chronically lead to hyper- and hypo-pigmentation, lungs, bladder and kidney cancer, nausea, neurological disorders, muscular weakness, and loss of appetite (Gohari et al. 2013; Tang et al. 2013). In this regard, European Union (EU) and WHO set 0.001 μg L−1 as an extreme contamination level for As(III) in drinking water (Community 1998; WHO 2008). Thus, precise monitoring, determination, and remediation of noxious Pb(II) and As(III) metal ions from aqueous environment is extremely important for human health.
Therefore, it is very important to develop a new technique that is able to remove and reduce the concentration of Pb(II) and As(III) in aqueous environment to permitted levels. There are numerous technologies have been studied for low-cost and more effective to remove heavy metals such as chemical precipitation, ion exchange, adsorption, and membrane separation (Dabrowski et al. 2004; Fadzil et al. 2016; Swarnalatha and Ayoob 2016; Vilela et al. 2016). Among these methods, adsorption has become one of the alternative treatments in current years, since it can effectively remove various toxic metals as the course of convenient design and operation (Swarnalatha and Ayoob 2016; Vilela et al. 2016). Furthermore, adsorption process is simple, easy, and convenient approach to deal with metal-contaminated effluents (Barakat 2011).
Different types of materials have been used for adsorption approaches such as activated carbon, silica-based materials, polymers, and biomass (Aguado et al. 2009; Ucar et al. 2014; Jain et al. 2015; Lapwanit et al. 2016). For adsorption process, structure of adsorbent plays an important role in order to enhance mechanical, chemical, and thermal stability. Bio-polymeric spores of Lycopodium clavatum (sporopollenin) possess tremendous mechanical, thermal, and chemical stability. Additionally sporopollenin due the high content of functional groups can be easily functionalized/magnetized (Paunov and Stoyanov 2007). Numerous modifications on the surface of sporopollenin have been stated by various researchers (Çimen et al. 2014; Kamboh et al. 2016; Şener et al. 2016).
To obtain selective adsorption of Pb(II) and As(III), amine functionalized adsorbents are considered as eminent and was previously reported (Georgiou et al. 2016; Nonkumwong et al. 2016). According to Pearson acid base concept (HSAB), this functional groups show higher selectivity toward both selected metals. In addition, iron oxide nanoparticles are capable to treat enormous amounts of wastewater in a short period and also can be easily separated from wastewater (Ibrahim et al. 2015a, b). The specific characteristics of iron oxide nanoparticles provide a convenient tool for exploring magnetic separation techniques (Ibrahim et al. 2015a). The higher surface area offered by magnetic particles has improved the adsorption behavior compared to that of the raw bio-sorbent (Sureshkumar and Daniel 2016). Magnetically modified bio-sorbent reveal promising adsorption capacity toward mixed wastewaters (Sivashankar et al. 2014).
In current study, the sporopollenin was modified with 3-cyanopropyltrimethoxysilane (CPTS) and functionalized with 1-(2-hydroxyethyl) piperazine (Sp-HEP). The functionalized sporopollenin was magnetized with iron oxide nanoparticles (MNPs-Sp-HEP). The combination of amine ligand and iron oxide nanoparticles with sporopollenin enhanced the adsorption properties of sporopollenin and resulting in strong binding affinities toward selected target pollutants. The synthesized of MNPs-Sp-HEP was successfully used for the removal of Pb(II) and As(III) ions from aqueous solution. The adsorption kinetics was examined using the pseudo-first-order, pseudo-second-order, and intraparticle diffusion equations and the equilibrium data fitted with two isotherm equations (Langmuir and Freundlich). The thermodynamic parameters (ΔG°, ΔH°, and ΔS°) were also calculated.
Experiment
Chemicals
All chemicals used were analytical grade and were used as received. 1-(2-Hydroxyethyl) piperazine (HEP), 3-cyanopropyltriethoxysilane (CPTS), and sporopollenin (Sp) were purchased from Sigma-Aldrich (Steinheim, Germany). FeCl3.6H2O, FeCl2.4H2O, 30% ammonia solution, and toluene were purchased from R&M Chemicals.
Instruments
An inductively coupled plasma–mass spectrometer (ICP-MS; Agilent 7500A series, Agilent Technologies, Palo Alto, CA, USA) was applied for determination of the heavy metals in this work. The surface morphology of the MNPs-Sp-HEP was determined at 2.0 kV using a JSM-6390 field emission-scanning electron microscope (FE-SEM) from JEOL (Tokyo, Japan). The FT-IR spectrum was obtained using ATR technique on a Spectrum 400 PerkinElmer in the range of 4000–450 cm−1 with diamond as a detector. The magnetic property was tested using a vibration sample magnetometer (VSM) model 9600 (Quantum Design Inc., San Diego, USA). Magnetization measurements were carried out in an external field of up to 15 kOe at room temperature.
Synthesis of functionalized magnetic sporopollenin
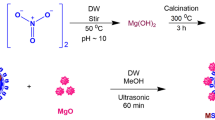
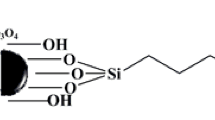
3-Cyanopropyltriethoxysilane-modified sporopollenin (CPTS-Sp) was prepared according to the previously reported method (Çimen et al. 2014). Ten-gram sporopollenin was suspended in 67 mL dry toluene and 6 mL of CPTS was added. The mixture was then refluxed for 72 h and dried under vacuum. Herein, the functionalization as well as magnetization of CPTS-Sp with HEP and Fe3O4, respectively, in order to obtain a new MNPs-Sp-HEP (Fig. 1) is reported for the first time.
Functionalization of CPTS-modified Sp with HEP
Five grams of freshly prepared Sp-CPTS was added to 25% (v/v) of HEP solution and stirred at room temperature for 15 h. The residue was filtered and washed with deionized water. The Sp-HEP was then dried under vacuum at 80 °C for 20 h.
Magnetization of Sp-HEP (MNPs-Sp-HEP)
Five grams of FeCl3.6H2O, 7.5 g of FeCl2.4H2O, 2 mL 5 M HCl, 15 mL distilled water, and 2 mL ethanol was mixed in a 100-mL flask. The mixture was heated until the salt was complete dissolved. In 10 mL of solution, 0.3 g Sp-HEP was dispersed and stirred for 2 h at room temperature. Then, Sp-HEP was filtered. Immediately, it was washed with distilled water and hurriedly transferred into 1 M ammonia solution. Magnetic sporopollenin-functionalized HEP (Fig. 1) was stirred for 2 h at room temperature before collected by external magnet and washed thoroughly with distilled water (Kamboh and Yilmaz 2013).
Adsorption experiment
The adsorption efficiency of synthesized MNPs-Sp-HEP for Pb(II) and As(III) metal ions has been examined through adsorption procedure. The experiments were conducted into Erlenmeyer flasks (50 mL) which contain specific dosage (10–60 mg) of the adsorbent at different concentrations, i.e., 5–100 mg L−1 of adsorbate (aqueous solution of Pb(II) and As(III) metal ions). Finally, following the magnetic separation, residual concentration of Pb(II) and As(III) metal ions in the solution was measured by inductively coupled plasma mass spectrometry (ICP-MS) (Fig. 2). The adsorption capacity of synthesized MNPs-Sp-HEP and % extraction of Pb(II) and As(III) ions was calculated using Eqs. (1) and (2), respectively.
where q e is the adsorption capacity (mg g−1), V is the aqueous volume (L), m is the adsorption dosage (mg), and C e (mol L−1) and C o (mol L−1) are the residual and initial concentrations of the Pb(II) and As(III) ions in the solution, respectively.
The percent efficiency (%E) of Pb(II) and As(III) ions were calculated using Eq. (2):
Desorption and regeneration studies
To evaluate the reusability of the adsorbent, adsorption of analytes (Pb(II) and As(III)) and regeneration of Pb-loaded MNPs-Sp-HEP and As-loaded MNPs-Sp-HEP were performed in seven consecutive cycles. In each cycle, 10 mg L−1 analytes (Pb(II) and As(III)) in 10 mL of solution was mixed with 15 mg MNPs-Sp-HEP for 15 min. The MNPs-Sp-HEP was separated magnetically, and the supernatant was subjected to Pb(II) and As(III) measurements. The resultant Pb- and As-loaded sorbent was mixed with 10 mL of 0.1 M HNO3 solution for 5 min. Prior to the next adsorption-desorption cycle, regeneration of MNPs-Sp-HEP was washed thoroughly with deionized water.
Results and discussion
Synthesis
The main objective of this study was to design a new organic-inorganic hybrid sporopollenin-based magnetic material with having heterocyclic core as well as amino functional groups and exploration of its extraction properties toward the selected Pb(II) and As(III) ions. To achieve the desired goal, sporopollenin was modified with 3-cyanopropyltriethoxysilane. In second step, heterocyclic core/amino functionality was achieved by the functionalization of CPTS-Sp with HEP in the presence of dry toluene. In order to aquire the hybrid/magnetic nature, the Sp-HEP was functionalized with Fe3O4 and the resultant hybrid MNPs-Sp-HEP was chosen as precursor.
Characterization
FT-IR spectral analysis
The FT-IR spectra of the functionalized sporopollenin are shown in Fig. 3a–d. The spectrum of raw sporopollenin (Fig. 3a) shows the characteristic adsorption bands of hydroxyl groups (OH), a broadband ranging from 3200 to 3400 cm−1. The peaks at 2924 and 2854 cm−1 were due to C–H symmetric and a symmetric stretching, respectively. The peak shown at 1516 cm−1 was due to the C = C stretching vibration of the aromatic rings, and the one at 1710 cm−1 was due to the C = O stretching vibration of a carboxylic group (Dyab et al. 2016). The spectrum of CPTS-modified sporopollenin (Fig. 3b) shows the characteristic adsorption bands of cyano group (C ≡ N) and siloxane (Si–O–Si) group stretching at 2245 and 1110 cm−1, respectively. The lower intensity of carboxylic group stretching in CPTS-modified sporopollenin (Fig. 3b) as compared to the raw sporopollenin (Fig. 3a) might be due to the modification process, and it is due to the attachment of CPTS on hydroxyl and carboxylic group (Fig. 3b). Frequency of OH stretching vibration in Sp-CPTS is shifted to 3358 from 3249 cm−1 (Sp) (Çimen et al. 2014). The successful functionalization of Sp-CPTS with HEP can be explain on the bases of disappearance as well as shifting of some diagnostic stretching bands in IR spectrum of Sp-HEP (Fig. 3c). Disappearance of cyano group (C ≡ N) in Sp-HEP spectrum (Fig. 3c) confirms that HEP was functionalized with the cyano group (C ≡ N), and it is a qualitative evidence for the conversion of cyano group (C ≡ N) into (C = N) group. The frequency of secondary amine from HEP was observed at range of 3313–3318 cm−1 overlapping with OH functional group form sporopollenin (Fig. 3c). The peaks at 3249 and 1710 cm−1 in sporopollenin (Fig. 3a) decreased drastically after immobilization of CPTS shows that the attachment of CPTS on hydroxyl and carboxylic group (Fig. 3b) and after functionalized with HEP shift to 1647 cm−1 due to presence of amine (Fig. 3c). Moreover, the sharp peak at 564 cm−1 may be attributed to nanoparticles (Fe3O4) groups obtained when Sp-HEP was coated with Fe3O4 (Fig. 3d) (Arora et al. 2016).
Field emission scanning electron microscopy
The morphology images of pure sporopollenin and MNPs-Sp-HEP (Fig. 4) revealed that raw sporopollenin consists of an interconnected uniform hexagonal shape with pore structure (in the form of round microcapsule). The open and uniform pore structure of modified sporopollenin showed an irregular pattern due to the functionalization/magnetization with HEP and Fe3O4, respectively, non-uniformly deposited inside the openings and on the walls. It can be seen from Fig. 4 that each pore of sporopollenin remained hollow even after modification, functionalization, and magnetization process.
Energy-dispersive spectroscopy
The pureness and elemental composition of sporopollenin and functionalized sporopollenin were investigating using EDS. The functionalized sporopollenin was compared with raw sporopollenin. Table 1 shows the elemental composition for raw sporopollenin, Sp-CPTS, Sp-HEP, and MNPs-Sp-HEP. The presence of additional nitrogen, silica, and iron on functionalized sporopollenin show that they are successfully functionalized/magnetize onto sporopollenin as the natural composition of sporopollenin spores are only form hydrogen, carbon, and oxygen.
VSM analysis
Magnetic properties were characterized by measuring the hysteresis and remanence curves by means of VSM. Figure 5 shows the magnetization curves of Fe3O4 and MNPs-Sp-HEP . The two curves show similar symmetrical about the origin shape which exhibits the characteristic feature of superparamagnetism. The Fe3O4 achieved a saturation magnetization value of 63.3 emu g−1 similar to that obtained from literature (Baharin et al. 2016). After functionalized with Sp-HEP, the saturation magnetization value dropped significantly (15.64 amu g−1). This was predicted due to the coating contribution from a non-magnetic functionalized sporopollenin. However, the decreases of saturation magnetization give no significant difference between the two superparamagnetic properties of MNPs which is sufficient for magnetic separation with a conventional magnet (Zheng et al. 2014).
Optimization of effective parameters on removal of Pb(II) and As(III) ions
Adsorbent dosage
Figure 6a displays the influence of the adsorbent mass on the removal of target analytes. This is due to the importance of adsorbent amount in adsorption efficiency removal from water samples. Five different amounts of MNPs-Sp-HEP (10–50 mg) were used for the adsorption of 10 ppm of Pb(II) from 10 mL deionized water samples. The adsorption efficiencies of Pb(II) and As(III) are directly proportional to the mass of adsorbent due to an increase in adsorption sites, and thus, more analyte can occupy these sites. Beyond the 25 mg of the adsorbent dosage, there is no significant increase and adsorbent becomes almost constant. The result indicated that MNPs-Sp-HEP has reached the adsorption equilibrium, where the maximum capacity of the adsorbent was reached. Consequently, 25 mg was selected for the subsequent experiments.
Effect of pH
The pH of the solution plays an important role in the adsorption characteristics of Pb(II) and As(III). Figure 6c displays the removal of Pb(II) and As(III) at different pH values. The increase of the pH of the solution up to pH 6.5 increased the percentage removal of Pb(II) ions due to the electrostatic interaction between Pb(II) and negatively charged surfaces (Fig. 6d). Significant 95% was acquired at pH 6.5. The lower percentage removal of Pb(II) at low pH might be due to the competition between Pb2+ and H+ with active sites and repulsion of Pb2+ and protonated amine. Meanwhile, the percentage removal of As(III) is optimum from pH 3.5 to 5.5. After this point, based on zeta potential result (Fig. 6d) at higher pH, surface of the adsorbent become more negatively charged due to deprotonation of C = N and acidic group of particle surfaces (Binks et al. 2011). Hence, the percentage removal of As(III) was decreased due to repulsion between dominant species of As(III), H2AsO3 −, and negatively charged surfaces.
Effect of contact time
Figure 6b shows the effect of the adsorption time on the removal of Pb(II) and As(III) ions. As predicted, as the contact time increase, the percent of removal for the both metal ions also increase until reached steady state values. In this study, the rapid uptake kinetics can be attributed to the hydroxyl group and nitrogen unit of nitrile and amine group from HEP. Thus, 15 min was enough to remove both metal ions and selected for further analysis of Pb(II) and As(III).
Adsorption isotherm
Optimization the design of an adsorption process is importance to study the interaction of adsorbate with adsorbents (adsorption isotherms). Adsorption study was carried out using different concentrations of Pb(II) and As(III) (5–100 mg L−1) at pH 5.5 (Fig. 7). When the concentrations of Pb(II) and As(III) were increased, q e also increased until it reached equilibrium at higher concentrations since at high concentration adsorption sites were saturated. The adsorption mechanism of Pb(II) and As(III) on MNPs-Sp-HEP was studied by using adsorption models, namely, Langmuir and Freundlich.
The Langmuir model was established to represent chemisorption which assumes a monolayer deposition on a surface with a finite number of identical sites. The equation is valid for a homogenous surface (Chou et al. 2011). Therefore, at equilibrium, a saturation point is reached where no further adsorption can occur (Fig. 7). The non-linear expression of Langmuir isotherm is represented by the following equation:
where q e is the corresponding adsorption capacity, C e is the equilibrium ion concentration in the solution, q m is the adsorption capacity, and K L is the energy or net enthalpy. The plot of q e vs. C e for the sorption is plotted (Fig. 7).
The non-linear expression of Freundlich isotherm model is an empirical equation that describes the heterogonous adsorption energies on the adsorbent surface (Fadzil et al. 2016), and multilayer adsorption is controlled by physisorption (i.e., van der Waal’s interaction), which can be written as
where K F and n are the Freundlich constants related to the adsorption capacity and adsorption intensity, respectively; q e is the equilibrium solute concentration on adsorbent; and C e is the equilibrium concentration of the solute. According to Eq. (4), the plot of q e vs. C e is plotted (Fig. 7).
Linear forms of the isotherms models are also widely adopted to determine the isotherm parameters or the most fitted model for the adsorption system due to the mathematical simplicity. The linear form of Langmuir is expressed as
where q e is the amount of solute sorbed on the surface of the sorbent, C e is the equilibrium ion concentration in the solution, q m is the maximum surface density at monolayer coverage, and b is the Langmuir adsorption constant. The plot of \( \frac{C_e}{q_e} \) vs. C e for the sorption gives a straight line of slope \( \frac{1}{q_m} \) (Fig. 8).
The linear form of Freundlich isotherm model is represented as
where K F and n are the Freundlich constants related to the adsorption capacity and adsorption intensity, respectively; q e is the equilibrium solute concentration on adsorbent; and C e is the equilibrium concentration of the solute. According to Eq. (6), the plot of ln q e vs. ln C e gives a straight line, and K F and n values can be calculated from the intercept and slope of this straight line (Fig. 9).
Analysis of the experimental data using non-linear equation and linear of Langmuir and Freundlich isotherm showed an opposite result. R 2 was used to measure the strength of the model by determining the closeness of R 2 value to the unity. In this study, the R 2 values of linear analysis for Pb(II) exhibit higher than R 2 values of non-linear analysis for both isotherms. However, a contradicting result is shown for As(III). R 2 values for non-linear analyses for both Langmuir and Freundlich isotherms are higher than linear analysis, hence implying that the Pb(II) is best fitted to linear analysis and As(III) is best fitted to non-linear analysis for both isotherms. In addition, the R 2 values for both isotherms showed that Pb(II) and As(III) fitted well with both isotherms.
The constant parameters (K F , 1/n, q m , and K L ) of isotherms estimated by linear and non-linear expressions were presented in Table 2. Based on the calculation, Freundlich model showed that theoretical q e was comparable with experimental q e because the exponent is between 1 < n < 10. This trend showed favorable adsorption for Pb(II) and As(III) onto MNPs-Sp-HEP adsorbent.
Thermodynamic studies
Thermodynamic parameters including enthalpy changes (ΔH°) and entropy changes (ΔS°) were obtained from Eq. (8), and Gibbs free energy changes (ΔG°) was calculated according to Eq. (7) to study the adsorption behaviors:
where K (L/mol) is from Langmuir equation, R is the gas constant (8.314 J/mol K), and T is the temperature in Kelvin. In the plot of ln K against 1/T, the ΔH° and ΔS° values are calculated from the slope and intercept of the plot.
The thermodynamic parameters are listed in Table 3. Negative ΔG° values are obtained at different temperature, showing that the adsorption process is possible and spontaneous and required energy from outside of the system. As the temperature increases, the value of ΔG° is decreasing, suggesting that the spontaneous nature of adsorption increases as the temperature goes down.
The negative values of ΔH°, demonstrating the exothermic nature of adsorption and the positive values of ΔS°, suggest that the organization of the adsorbate at the solid-solution interface becomes more random as ion replacement reaction occurred, while negative values suggest the opposite fact (Daraei et al. 2015; ALOthman et al. 2016).
Adsorption kinetic
Different kinetic models were carried out to study the time required for the adsorption equilibrium to be reached and condition under which there is no variation in adsorption capacity. In this study, the pseudo-first-order, pseudo-second-order kinetic and intraparticle diffusion models were applied to the kinetic adsorption data to find the best model for the experimental data.
The pseudo-first-order model proposed that as time progress, the sorbate ions are accumulating over the surface of sorbent (Ho 2006; Daraei et al. 2015).
The pseudo-first-order equation is expressed as
In equation above, q e and q t are the amounts of Pb(II) and As(III) ions (mg g−1) absorbed at equilibrium and at time, t, respectively, and k 1 is the first-order rate constant (min−1). The values of q e and k 1 were obtained from the linearity of pseudo-first-order rate by plotting ln (q e − q t ) vs. time (Fig. 10).
Pseudo-second-order kinetic model which has been applied for analyzing chemisorption kinetics from liquid solutions can be represented by Eq. (10) as follows:
where k 2 is the rate constant of pseudo-second-order adsorption (g/mg min). The values of k 2 and q e can be described from the slope and the intercept of the graph t/q t vs. t, respectively.
By comparing the correlation coefficient (R 2) value from both first-order and second-order kinetics (Table 4), it can be concluded that the experimental data followed pseudo-second-order kinetic and involves chemisorption mechanism (Journal et al. 2010), which revealed that the adsorption process is driven by a chemical reaction occurring at the exposed surface.
Sporopollenin has porous structure; therefore, the adsorption process on porous sorbents was described by intraparticle diffusion, wherein mass transfer within the sorbent particles may involve a short-range diffusion in both the fluid and adsorbed phase (Unlü and Ersoz 2006; Nair et al. 2012). The model is expressed with the equation given by Weber and Morris.
where q t and k id are the amount of metal ions adsorbed at time t and intraparticle diffusion rate constant, respectively, whereas C i is the intercept which gives the idea about the thickness of the boundary layer. Intraparticle diffusion values (Table 4) were obtained from linearity of q t vs. t 1/2 plot. Figure 10c shows two-part linearity; i.e., mass transfer (first stage) and equilibrium part (second stage) for intraparticle diffusion model revealed that Pb(II) and As(III) ion adsorption was conducted in two separate regions with two straight lines. First part with a sharp slope showed that the adsorption rate is fast and adsorption is favorable on the surface. The second part with slow slope shows that the adsorption rate is slow and intraparticle diffusion is not involved in adsorption (Nodeh et al. 2015).
Regeneration of MNPs-Sp-HEP
The restoration of adsorption capability is a crucial factor in practical application of the adsorbent. The adsorption of heavy metals observed on MNPs-Sp-HEP at low pH implies that acid treatment is a feasible approach to regenerating the heavy metal-loaded adsorbent.
Due to the stability of MNPs-Sp-HEP under acidic conditions, Pb- and As-loaded adsorbent was regenerated using 0.1 M HNO3 solution and the regeneration adsorbent was reused in adsorption in seven consecutive cycles. Adsorption efficiency of the regenerated adsorbents was shown in Fig. 11. The adsorption capacity had decreased slightly by first five adsorption-regeneration capacity cycles, and drastically dropped at cycles 5 and 6 for As(III) and Pb(II), respectively. This is might be due to the partial breakup of outer MNPs-Sp-HEP shell and cell walls at high concentration of nitric acid (Şener et al. 2014).
Mechanism
The adsorption mechanism of Pb(II) and As(III) metal ions on MNPs-Sp-HEP was explained in Fig. 9. Pb(II) and As(III) metal ion adsorption mechanism for the MNPs-Sp-HEP adsorbent is highly pH dependent and can be explain as follows: since MNPs-Sp-HEP contains nitrogen and oxygen groups, these N and O groups are highly electronegative due to the abundance of free lone pair of electrons. These lone pair electrons shows binding abilities toward the Pb(II) and As(III) cation as represented in Fig. 12.
Conclusion
The magnetic sporopollenin-functionalized 1-(2-hydroxyethyl) piperazine was successfully synthesized, characterized, and employed as an promising adsorbent for the removal of Pb(II) and As(III) ions from aqueous solution prior to ICP determination. The MNPs-Sp-HEP is suitable for extraction operation as it provides outstanding magnetically separation, stabilization in aqueous sample, and quick process. Adsorption experimental data of Pb(II) and As(III) fitted well with both isotherms. Kinetic model performed that the adsorption rate was controlled by pseudo-second-order. Thermodynamic study showed that Pb(II) and As(III) adsorption on MNPs-Sp-HEP are spontaneous and exothermic process due to negative energy of −ΔG and −ΔH, respectively. The positive value of ΔS shows that the organization of adsorbate at the solid/solution interfaces becomes more random. The chelating effect of MNPs-Sp-HEP increased to the sorption of heavy metal ions compared to raw sporopollenin. Overall, it can be concluded that the proposed sorbent system is practical and efficient for the removal of heavy metals from aqueous environment with advantages of being inexpensive and largely available.
References
Aguado J, Arsuaga JM, Arencibia A et al (2009) Aqueous heavy metals removal by adsorption on amine-functionalized mesoporous silica. J Hazard Mater 163:213–221. doi:10.1016/j.jhazmat.2008.06.080
Ali I (2012) New generation adsorbents for water treatment. Chem Rev 112:5073–5091
ALOthman ZA, Habila MA, Al-Shalan NH et al (2016) Adsorptive removal of Cu(II) and Pb(II) onto mixed-waste activated carbon: kinetic, thermodynamic, and competitive studies and application to real wastewater samples. Arab J Geosci 9:315. doi:10.1007/s12517-016-2350-9
Arora V, Sood A, Shah J, et al (2016) Synthesis and characterization of thiolated pectin stabilized gold coated magnetic nanoparticles. Mater Chem Phys 1–7. doi: 10.1016/j.matchemphys.2016.01.056
Baharin SNA, Muhamad Sarih N, Mohamad S et al (2016) Removal of endocrine disruptor di-(2-ethylhexyl)phthalate by modified polythiophene-coated magnetic nanoparticles: characterization, adsorption isotherm, kinetic study, thermodynamics. RSC Adv 6:44655–44667. doi:10.1039/C6RA04172H
Bakhshaei S, Kamboh MA, Nodeh HR et al (2016) RSC advances aromatic hydrocarbons and chlorophenols based nanoparticles and their determination by. RSC Adv 6:77047–77058. doi:10.1039/C6RA13702D
Barakat MA (2011) New trends in removing heavy metals from industrial wastewater. Arab J Chem 4:361–377. doi:10.1016/j.arabjc.2010.07.019
Binks BP, Boa AN, Kibble MA et al (2011) Sporopollenin capsules at fluid interfaces: particle-stabilised emulsions and liquid marbles. Soft Matter 7:4017. doi:10.1039/c0sm01516d
Cheng Y, Yang C, He H et al (2015) Biosorption of Pb(II) ions from aqueous solutions by waste biomass from biotrickling filters: kinetics, isotherms, and thermodynamics. J Environ Eng:1–7. doi:10.1061/(ASCE)EE.1943-7870.0000956
Chou WL, Wang CT, Huang KY, Liu TC (2011) Electrochemical removal of salicylic acid from aqueous solutions using aluminum electrodes. Desalination 271:55–61. doi:10.1016/j.desal.2010.12.013
Çimen A, Bilgiç A, Kursunlu AN, Gübbük İH (2014) Adsorptive removal of Co(II), Ni(II), and Cu(II) ions from aqueous media using chemically modified sporopollenin of Lycopodium clavatum as novel biosorbent. Desalin Water Treat. doi:10.1080/19443994.2013.806228
Community E (1998) Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Off J Eur Communities L330:32–54
Dabrowski A, Hubicki Z, Podkoscielny P, Robens E (2004) Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 56:91–106. doi:10.1016/j.chemosphere.2004.03.006
Daraei H, Mittal A, Noorisepehr M, Mittal J (2015) Separation of chromium from water samples using eggshell powder as a low-cost sorbent: kinetic and thermodynamic studies. Desalin Water Treat 53:214–220. doi:10.1080/19443994.2013.837011
Dyab AKF, Abdallah EM, Ahmed SA, Rabee MM (2016) Fabrication and characterisation of novel natural Lycopodium clavatum sporopollenin microcapsules loaded in-situ with nano-magnetic humic acid-metal complexes Retrieved 6 April 2017, from http://file.scirp.org/pdf/JEAS_2016112417465025.pdf
Edition F (2011) Guidelines for drinking-water quality. WHO Chron 38:104–108
Fadzil F, Ibrahim S, Hanafiah MAKM (2016) Adsorption of lead(II) onto organic acid modified rubber leaf powder: batch and column studies. Process Saf Environ Prot 100:1–8. doi:10.1016/j.psep.2015.12.001
Georgiou Y, Mouzourakis E, Bourlinos AB et al (2016) Surface decoration of amine-rich carbon nitride with iron nanoparticles for arsenite (As III) uptake: the evolution of the Fe-phases under ambient conditions. J Hazard Mater 312:243–253
Gohari RJ, Lau WJ, Matsuura T, Ismail AF (2013) Fabrication and characterization of novel PES/Fe–Mn binary oxide UF mixed matrix membrane for adsorptive removal of As(III) from contaminated water solution. Sep Purif Technol 118:64–72
Gupta VK, Rastogi A (2008) Biosorption of lead from aqueous solutions by green algae spirogyra species: kinetics and equilibrium studies. J Hazard Mater 152:407–414. doi:10.1016/j.jhazmat.2007.07.028
He Z (2015) Heavy metal contamination of soils: sources, indicators, and assessment. J Environ Indic 9:17–18
Ho YS (2006) Isotherms for the sorption of lead onto peat: comparison of linear and non-linear methods. Polish J Environ Stud 15:81–86. doi:10.1016/j.watres.2005.10.040
Ibrahim WAW, Nodeha HR, Aboul-Enein HY, Sanagi MMS (2015a) Magnetic solid-phase extraction based on modified ferum oxides for enrichment, preconcentration, and isolation of pesticides and selected pollutants. Crit Rev Anal Chem 45:270–287. doi:10.1080/10408347.2014.938148
Ibrahim WA, Nodeh HR, Sanagi MM (2015b) Graphene-based materials as solid phase extraction sorbent for trace metal ions, organic compounds and biological sample preparation. Crit Rev Anal Chem 46:267–83. doi:10.1080/10408347.2015.1034354
Jain M, Garg V, Kadirvelu K, Sillanpää M (2015) Adsorption of heavy metals from multi-metal aqueous solution by sunflower plant biomass-based carbons. Int J Environ Sci doi. doi:10.1007/s13762-015-0855-5
Journal B, Kumar PS, Vincent C et al (2010) Kinetics and equilibrium studies of Pb2+ ion removal from aqueous solutions by use of nano-silver sol-coated activated carbon. Brazilian J Chem Eng 27:339–346
Kamboh MA, Yilmaz M (2013) Synthesis of N-methylglucamine functionalized calix[4]arene based magnetic sporopollenin for the removal of boron from aqueous environment. Desalination 310:67–74. doi:10.1016/j.desal.2012.10.034
Kamboh MA, Ibrahim WAW, Nodeh HR et al (2016) The removal of organophosphorus pesticides from water using a new amino-substituted calixarene-based magnetic sporopollenin. New J Chem 40:3130–3138. doi:10.1039/C5NJ02284C
Lapwanit S, Trakulsujaritchok T, Nongkhai PN (2016) Chelating magnetic copolymer composite modified by click reaction for removal of heavy metal ions from aqueous solution. Chem Eng J 289:286–295. doi:10.1016/j.cej.2015.12.073
Liu Q-S, Zheng T, Wang P et al (2010) Adsorption isotherm, kinetic and mechanism studies of some substituted phenols on activated carbon fibers. Chem Eng J 157:348–356. doi:10.1016/j.cej.2009.11.013
Meenakumari M, Philip D (2015) Degradation of environment pollutant dyes using phytosynthesized metal nanocatalysts. Spectrochim Acta - Part A Mol Biomol Spectrosc 135:632–638. doi:10.1016/j.saa.2014.07.037
Nair DG, Hansdah K, Dhal B et al (2012) Bioremoval of chromium(III) from model tanning effluent by novel microbial isolate. Int J Metall Eng 1:12–16. doi:10.5923/j.ijmee.20120102.01
Nodeh H, Wan Ibrahim W, Sanagi M (2015) Magnetic graphene oxide as adsorbent for the removal of lead(II) from water samples. Jurnal Teknologi 78(3–2):25–30
Nonkumwong J, Ananta S, Srisombat L (2016) Effective removal of lead(II) from wastewater by amine-functionalized magnesium ferrite nanoparticles. RSC Adv 6:47382–47393
Paunov VN, Stoyanov SD (2007) Sporopollenin micro-reactors for in-situ preparation , encapsulation and targeted delivery of active components. J Mater Chem 17:609–612. doi:10.1039/b615865j
Sáez C, de Vidales MJM, Cañizares P et al (2014) Irradiated electrochemical processes for the removal of persistent organic pollutants from waters and wastewaters. Chem Eng Trans 41:103–108. doi:10.3303/CET1441018
Şener M, Reddy DHK, Kayan B (2014) Biosorption properties of pretreated sporopollenin biomass for lead(II) and copper(II): application of response surface methodology. Ecol Eng 68:200–208. doi:10.1016/j.ecoleng.2014.03.024
Şener M, Kayan B, Akay S, Gözmen B (2016) Fe-modified sporopollenin as a composite biosorbent for the removal of Pb2+ from aqueous solutions. Desalin Water Treat. doi:10.1080/19443994.2016.1182449
Shahabuddin S, Sarih NM, Afzal Kamboh M et al (2016) Synthesis of polyaniline coated graphene oxide @ SrTiO3 nanocube nanocomposites for enhanced removal of carcinogenic dyes from aqueous solution. Sciprints. doi:10.20944/preprints201608.0128.v1
Sivashankar R, Sathya AB, Vasantharaj K, Sivasubramanian V (2014) Magnetic composite an environmental super adsorbent for dye sequestration—a review. Environ Nanotechnol, Monit Manag 1–2:36–49. doi:10.1016/j.enmm.2014.06.001
Sureshkumar V, Daniel SCGK (2016) Fabrication of chitosan–magnetite nanocomposite strip for chromium removal. Appl Nanosci:277–285. doi:10.1007/s13204-015-0429-3
Swarnalatha K, Ayoob S (2016) Adsorption studies on coir pith for heavy metal removal. Int J Sustain Eng 7038:1–7. doi:10.1080/19397038.2016.1152323
Tang W, Su Y, Li Q et al (2013) Superparamagnetic magnesium ferrite nanoadsorbent for effective arsenic (III, V) removal and easy magnetic separation. Water Res 47:3624–3634. doi:10.1016/j.watres.2013.04.023
Tyrovola K, Peroulaki E, Nikolaidis NP (2007) Modeling of arsenic immobilization by zero valent iron. Eur J Soil Biol 43:356–367
Ucar S, Erdem M, Tay T, Karagöz S (2014) Removal of lead(II) and nickel(II) ions from aqueous solution using activated carbon prepared from rapeseed oil cake by Na2CO3 activation. Clean Technol Environ Policy 747–756. doi: 10.1007/s10098-014-0830-8
Unlü N, Ersoz M (2006) Adsorption characteristics of heavy metal ions onto a low cost biopolymeric sorbent from aqueous solutions. J Hazard Mater 136:272–280. doi:10.1016/j.jhazmat.2005.12.013
Vilela D, Parmar J, Zeng Y, et al (2016) Graphene based microbots for toxic heavy metal removal and recovery from water. Nano Lett acs. Nanolett. 6b00768. doi:10.1021/acs.nanolett.6b00768
Vymazal J, Brezinova T (2015) The use of constructed wetlands for removal of pesticides from agricultural runoff and drainage: a review. Environ Int 75:11–20. doi:10.1016/j.envint.2014.10.026
WHO (2008) Guidelines for drinking-water quality: incorporating first and second addenda to third edition. Theatr Rec 1:1–494
Zheng X, He L, Duan Y et al (2014) Poly(ionic liquid) immobilized magnetic nanoparticles as new adsorbent for extraction and enrichment of organophosphorus pesticides from tea drinks. J Chromatogr A 1358:39–45. doi:10.1016/j.chroma.2014.06.078
Acknowledgments
The authors would like to thank the University of Malaya for the financial support through the grants PG049-2015B, RP011B-14SUS, and RP020A-16SUS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Guilherme L. Dotto
Rights and permissions
About this article
Cite this article
Ahmad, N.F., Kamboh, M.A., Nodeh, H.R. et al. Synthesis of piperazine functionalized magnetic sporopollenin: a new organic-inorganic hybrid material for the removal of lead(II) and arsenic(III) from aqueous solution. Environ Sci Pollut Res 24, 21846–21858 (2017). https://doi.org/10.1007/s11356-017-9820-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9820-9