Abstract
Shuang’an in Ziyang is a typical high-selenium (Se) area in China, where human selenium (Se) poisoning was reported 30 years ago. To assess the risk of Se poisoning in the area, the Se content in agricultural soil, plant, and water in Naore, Shuang Hekou, and Lin Benhe villages of Ziyang was systematically investigated. The probable daily intake (PDI) was calculated on the basis of Se contents in food, water, and dietary habits to evaluate the risk of selenosis. Se content in hair from local habitants (32 men and 34 women) was determined to further verify the risk. Results showed that Se content in the soil ranged from 0.21 to 36.07 mg/kg, with a geometric means of 3.02 ± 5.16 mg/kg, respectively. Approximately 60% of soil reached the Se toxicity threshold (>3 mg/kg). The Se content in plants ranged from 0.02 to 17 mg/kg, with an average of 0.76 ± 2.51 mg/kg. Approximately 40% of the plant reached the toxicity standard (1 mg/kg). Se content in soil and plant of Naore village was significantly higher than that from two adjacent villages. The Se content in vegetables (on fresh weight basis) was higher than that in cereals. Se contents were 3.73 ± 9.08 and 1.32 ± 3.50 mg/kg in eggplant and pepper, respectively. The Se content in drinking water was 7.85 ± 6.04 μg/L, lower than the upper tolerable limit (40 μg/L) set by WHO. The Se content in stream water (18.5 μg/L) was significantly higher than that in drinking water because of soil erosion. The calculated PDI of habitants in Naore village (1801 μg/day) was significantly higher than that in Lin Benhe (666 μg/day) and Shuang Hekou (686 μg/day), all of which was higher than the tolerable upper Se intake (400 μg/day) set by WHO. The calculated blood Se content was relatively high, especially for habitants of Naore village (2783–2824 μg/L). Moreover, the Se content in the hair of local habitants reached as high as 12.72 ± 13.81 mg/kg, and 78.79% exceeded hair Se toxicity threshold (>3 mg/kg), which further verified the potential selenosis risk. Our study provides significant implications of the potential Se intoxication of local residents. Therefore, governments and other institutions should implement various measures to reduce the daily Se intake and to mitigate the associated health risks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is an essential element for human health, and the most of natural intake is usually from diet (Chilimba et al. 2011). In recent years, this element has been applied to treat and prevent severe pathologies, such as cancer, cardiovascular disorders, thyroid adenoma, and osteodystrophia, among residents living in Se-deficient areas (Burk 2002; Thiry et al. 2012). Se can also be toxic at high concentration, and its toxicity is characterized as acute and chronic (Yang et al. 2013). Vomiting and diarrhea are often present as acute toxicity, usually accompanied by rapid heartbeat (ATSDR 2003). Hair and nail lose are the prime symptoms of chronic toxicity (Yang et al. 1983), which may also be associated with skin lesions and disorders of the nervous system (Nuttall 2006). Severe selenosis can lead to weakness, paralysis, and even death (Fordyce 2007). Thus, local habitants may suffer from severe Se poisoning over time when high Se contents exist in the environment (Hira et al. 2004).
Toxic Se levels in food chain accumulate via soil and water that cause serious health problems (Fordyce 2007; Yang et al. 2013); this phenomenon was found in some countries, such as China, USA, India, Canada, Mexico, New Zealand, and Australia (Yang et al. 1989; Aro et al. 1995; Navarro-Alarcon and Cabrera-Vique 2008; Combs 2001; Fordyce 2013). For example, high Se content in water and soil results in local inhabitants facing a high risk of Se poisoning via having Se-rich foods in Enshi seleniferous area of China as reported by Qin et al. (2013). In addition, Ziyang seleniferous region is another notable Se-excessive or seleniferous regions in China. Se toxicity symptoms occurring in Enshi were also observed in Ziyang in 1984, and the most severely affected area was Naore village of Shuang’an County (Mei 1985; Fang and Wu 2004). The reported Se content in soil of Naore village was 15.74 mg/kg (Mei 1985), 16.9 mg/kg (Fang et al. 2003), and 26 mg/kg (Zhao et al. 1993), respectively. Mei (1985) attributed the high soil Se content to a parent compound, and Luo et al. (2003) also demonstrated that high Se content in soil is mainly due to Se-rich pyritic carbonaceous slate and volcanic substances under a thin soil layer.
Se content and availability in soil determine Se levels in plants and water, which determine the Se status in the human body. Besides, Se levels in various plants differ because of diverse geological conditions, growth conditions, absorptive capacities, growth periods, and protein contents (Levander 1987, 1982; Temmerman et al. 2014); Finglas 2000; Yu et al. 2015). Therefore, the dietary Se intake of humans worldwide considerably varies because of different Se contents in food and various dietary structures (Mcnaughton and Marks 2002). The recommended dietary allowance (RDA) of Se is 55 μg/day by WHO (1986), which are 70 and 50 μg/day for males and females, respectively, by the US Food and Nutrition Board (1980). No observed adverse effect levels (NOAELs) set by WHO (1986) are 240 μg/day (4 μg/kg bw/day), and upper tolerable level set by the Institute of Medicine (IOM 2000) is 400 μg/day. Accordingly, the Se status in humans should be evaluated within the narrow range between moderate and toxic levels. Probable daily intake (PDI) widely ranged from <10 μg/day for people living in Se-deficient areas to 4990 μg/day for habitants living in selenosis-dense areas in Enshi (Yang et al. 1983). Dhillon and Dhillon (1991) and Hira et al. (2004) examined environmental Se levels and health conditions of individuals inhabiting seleniferous areas in India and found that long-term consumption of natural Se-rich crops and forage leads to high Se intake in humans (PDI of males and females = 632 ± 31.2 and 475 ± 52.8 μg/day, respectively).
However, signs and symptoms of Se poisoning in Ziyang had been reported 30 years ago; no systematical studies have documented the Se levels in food and water, and levels of Se exposure of local populations. In our study, the total Se contents in soil, food, water, and human hair were determined in this area: (1) to investigate the Se status in soil, crops, and water from a Se-contaminated village and two other nearby villages; (2) to estimate PDI and to assess the potential risk of Se poisoning of local dwellers; and (3) to further verify Se status of inhabitants by levels in human hair. In addition, this study was conducted with an aim to provide a reference for the improvement of the health conditions of individuals living in Se-toxic areas.
Materials and methods
Sample collection
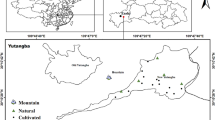
Two sampling campaigns were carried out. (1) In 2009, 26 soil samples, 16 corn samples, 7 rape seeds, 6 rice samples, and 18 water samples were collected from Naore village; 21 hair samples (male = 13; female = 8) were also collected. (2) In 2012, 138 sampling sites for soil samples were set in three villages (Naore (NR), Lin Benhe (LBH), Shuang Hekou (SHK)) in Shuang’an countryside in Ziyang; corresponding plant samples of 103 soil sampling sites and other 151 plant samples were also collected in these three villages. Stream water (n = 29), drinking water (n = 10), lotus pond water (n = 4), and hair of residents (n = 45, male = 19; female = 26) were also collected to estimate the daily dietary intake and for risk assessment; approximately 2.0 g of hair samples (about 1–3-cm length) was collected from the nape to the scalp (or occipital) region during the interview and investigation on habitants. For soil samples, five samples were obtained randomly from a 20 m × 20 m unit, and the five topsoil (0–20 cm) samples were mixed to prepare a composite sample for each site. Longitude and latitude, as well as the altitude of each sampling site, were recorded by GPS (Fig. 1). The edible parts of fruits and vegetables were collected for analysis. Water samples were obtained from rivers, irrigation channels, and residential water wells.
Sample preparation and analysis
Soil samples were air-dried, ground, sieved to <0.15 mm, and stored in plastic bags before analysis was conducted. Cereal samples were washed with tap water to remove soil and dust, and then washed with deionized water to scour the samples completely. The fresh weight of plant samples was measured; after that, the enzymes of the cereal samples were deactivated at 90 °C for 30 min and oven-dried at 60 °C to a constant weight. After the samples were ground, the cereal samples were stored in polyethylene plastic bags at 25 °C for analysis. Fruits and vegetables were washed with deionized water, air-dried, pulverized with a beater, and stored. HNO3 was used to acidify the water samples that were then preserved in a refrigerator. Hair samples were washed with detergent and ethanol and subsequently stored in polyethylene plastic bags.
Total Se levels in soil, plant, and hair samples were determined in accordance with previously described methods (Gao et al. 2011; Wang et al. 2012a), with some modifications. Different HNO3–HClO4 proportions were used to digest soil (3:2, v/v), plant samples (dried), and hair samples (4:1, v/v). Food samples and plant samples were digested the same way, while water samples used a mixture of concentrated HNO3 and 30% H2O2 under conditions similar to those of soil samples. Soil and plant samples were treated and analyzed in accordance with a previously described method (Yu et al. 2015).
The Se content was determined through hydride generation atomic fluorescence spectrometry (AFS-930) in accordance with the standard method (GB/T 5009.93-2003; Ministry of Health of the People’s Republic of China 2010); the method detection limit was 0.1 μg/L.
Quality control and statistical analysis
Quality control was assessed using Chinese national certified reference materials, duplicates, and matrix spike quality control samples; an acidified Millipore water sample was also taken as laboratory control. GBW07404 (limestone soil, Se = 0.64 ± 0.18 mg/kg), with a Se content of 0.65 ± 0.06 mg/kg (n = 3), was used as the standard soil reference material, while GBW10014 (cabbage leaves Se = 0.21 ± 0.01 mg/kg) with a Se content of 0.22 ± 0.02 mg/kg (n = 3) was used as standard plant reference material. The relative percentage difference of sample duplicates was <10%.
Calculation
Bioaccumulation factor
The bioaccumulation factor (BAF) was an indicator to evaluate the transfer of Se from soil to plants. The BAF can be calculated as the following:
Estimation of daily dietary Se intake and hazard quotient
The daily dietary intake was calculated using the following equation:
where PDI is the probable daily intake of Se, \( {C}_{Se}^i \) is Se content in food, and IR is the amount of each foodstuff consumed per capita per day, collected from the Shaanxi Statistical Yearbook 2015; in this book, the consumption data for each type of foods by local habitants were shown specifically.
Hazard quotient (HQ), which evaluates the non-carcinogenic risk of Se for dwellers in the study area, was calculated by dividing the average daily Se intake (ADSel) by the reference dose (RfD). HQ values equal to or less than 1 meant that the risk of Se poisoning can be neglected; however, an HQ value higher than 1 meant that Se exposure estimates exceeded the limit bringing elevated health risks.
ADSel was calculated by assuming average body weight (bw) to be 70 kg/adult, while the reference dose was calculated as 3.4 μg/kg/day based on the NOAEL (240 μg/kg/day) (Emmanuelle et al. 2012).
Estimation of Se content in blood
According to previous research, Se levels in the blood can be predicted through two equations: Eq. (4), which is based on the relationship between human Se intake (Baoyan and Xuelin 2000) and blood Se content, and a pharmacokinetic model Eq. (5), which is calculated using daily Se intake (Chien et al. 2003; Mahapatra et al. 2001). However, the estimated blood Se content can be confirmatory only when calculated values of Eqs. (4) and (5) are similar to each other (Qin et al. 2013).
where BSe is the Se content in blood (μg/L), PDI is the daily Se intake (μg/day), τ is the turnover time of Se (17 days), C is the steady-state concentration of Se in blood (μg/L), V is the volume of blood in the body (5.5 L), and F is the uptake fraction into blood through ingestion (50%).
Statistical analysis
The normality of Se concentration in soil, water, plant, and human hair was assessed using the Kolmogorov–Smirnov test. The logarithmetic transformations of log-normally distributed data were applied for further statistical analysis. The variance of the transformed data was performed by means of a one-way analysis of variance (ANOVA) using the Tukey HSD multi-comparisons (SPSS, version 23). Variance was considered significant for P < 0.05.
Results
Se content in the environment of Shuang’an, Ziyang
Se in soil
Se content in soil ranged from 0.21 to 36.06 mg/kg, with geometric mean of 3.02 ± 5.16 mg/kg. Soil Se content from Naore village, where the selenosis symptom was reported in 1980s, was the highest (n = 64, geometric mean = 3.79 ± 8.56 mg/kg), which was significantly higher than that in SHK (n = 46, geometric mean = 2.60 ± 1.89 mg/kg) and LBH villages (n = 49, geometric mean = 1.60 ± 1.60 mg/kg, P < 0.01, Fig. 2). Naore village had the most uneven Se distribution in soil with the variation coefficient reached 120%.
Based on the abundance and deficiency indexes proposed by Tan (1989), 54.09% of the soil samples were Se toxic (>3 mg/kg), and the proportions of Se-enriched (0.40–3.0 mg/kg) and moderate soil samples (0.18–0.40 mg/kg) were 14.47 and 23.90%, respectively. The proportions of toxic soil (3 mg/kg) from NR, SHK, and LBH villages were 71.88, 56.52, and 28.57%, respectively. Approximately 97, 93, and 86% of the soil samples from the NR, SHK, and LBH villages, respectively, exceeded the adequate Se standard (0.40 mg/kg).
Se in plants
Se content in different kinds of plants ranged from 0.02 to 17 mg/kg with a geometric mean of 0.76 ± 2.51 mg/kg (Table 2). The highest Se content (0.81 ± 3.12 mg/kg) was observed in corn, but no significant difference was found among cereal plants. The highest Se content was observed in eggplant, garlic, and pepper among vegetables (Table 1). Se content of 91 samples (36%) exceeded the Se toxicity standard (>1 mg/kg) proposed by Tan (1989). Se content in plant collected from NR (n = 81; geometric mean 1.25 ± 4.04 mg/kg) was significantly higher than that from LBH (n = 77; geometric mean 0.47 ± 1.77 mg/kg) and SHK (n = 93; geometric mean 0.48 ± 2.75 mg/kg) (P < 0.05). The proportions of plant with toxic Se level from NR, LBH, and SHK villages were 55.56, 29.87, and 23.66%, respectively.
Se in water
Se contents from streams, wells, ponds, and rice fields of three villages ranged from 1.86 to 36.82 μg/L with a geometric mean of 12.35 ± 9.45 μg/L. Se content was higher in stream water (n = 35, geometric mean 15.54 ± 10.46 μg/L) than in pond water (n = 4, geometric mean 15.03 ± 3.5 μg/L), rice field water (n = 5, geometric mean 9.88 ± 0.39 μg/L), and potable water (n = 17, 7.85 ± 6.04 μg/L). Se content in potable water of NR (n = 9, geometric mean = 8.99 ± 5.19 μg/L) is higher than that in the other two villages (n = 8, geometric mean = 6.96 ± 4.28 μg/L); no significant difference was found. The highest Se content was observed in five stream water samples (36.82, 35.39, 33.43, 33.19, 30.6 μg/L) with Se content exceeding 30 μg/L. Thus, Se content in all surface and potable waters were lower than the corresponding standards set by WHO (1986), 40 μg/L) and EPA (2002), 50 μg/L).
Probable daily Se intake
Based on the intake rate of each kind of food from the Statistical Yearbook of Shaanxi Province (Shaanxi Bureau of Statistics 2015) and food Se content, Se PDI of Shuang’an habitants was 1067 μg/day (Table 2). PDI of NR habitants (1801 μg/day) was much higher than that in LBH (666 μg/day) and SHK (686 μg/day). For local dwellers, major pathways for daily Se intake include cereals, vegetables, and meats. Fruit (4 μg/day) and drinking water (24 μg/day) only slightly contributed to Se PDI.
Se content in hair and blood
The age of randomly recruited participants (n = 66) ranged from 16 to 75 years old with an average of 45 years old. Se levels in hair of local habitants varied significantly from 0.59 to 63.05 mg/kg, with a geometric mean of 12.72 ± 13.81 mg/kg. Moreover, hair Se levels of males (n = 32, geometric mean = 4.73 ± 3.77 mg/kg) were significantly lower than females (n = 34, geometric mean = 14.83 ± 16.76 mg/kg). According to Tan’s (1989) classification, 21.21% of the hair samples (n = 14) were potentially toxic (0.5–3.0 mg/kg), while 78.79% of the hair samples (n = 52) belonged to Se-toxic (>3 mg/kg). Based on the average PDI (1067 μg/day) of local habitants, calculated values of Se content in blood were 1891 and 1649 μg/L through Eqs. (4) and (5), respectively. For habitants living in NR, SHK, and LBH, the Se content in blood was 2783–2824, 1029–1317, and 1060–1347 μg/L, respectively.
Discussion
Se in soil, plant, and water
Soil Se content of Shuang’an was 3.02 ± 5.16 mg/kg, which was much higher than average Se levels of China (0.173 mg/kg, Tan et al. 2002) and worldwide (0.40 mg/kg, Fordyce 2013), but lower than that from Enshi of China (9.36 ± 18.6 mg/kg) and selenosis-affected areas in India (Jainpur) (6.5 ± 0.3 mg/kg; Jaiswal et al. 2012). Soil Se content of NR in this study was far lower than that reported by Luo et al. (2003, 26 mg/kg) and Fang and Wu (2004, 16.9 mg/kg) in the same place, but it is still significantly higher than that of LBH and SHK. Se in soil mostly comes from weathering of parent material (Qin et al. 2013; Wang and Gao 2001; Yu et al. 2014). In the study area, Se was controlled by the bedrock, a series of pyritic carbonaceous slates, and volcanic substances that created a wide and flat area where the Naore village is located. Because of the thin soil layer, rocks crop out from the floor of the valley and weather into Se-rich soils (Luo et al. 2003).
According to Mei’s (1985) study, high Se content in soil accompanies with high Se levels in plants. In this study, 40% of vegetable and 32% of cereal significantly exceeded the Se toxicity standard (1 mg/kg; Tan 1989). In addition, Se content of plant from NR was significantly higher than that from LBH and SHK. Se content in corn (0.81 ± 3.12 mg/kg) was much higher than that from Hubei Enshi (0.43 mg/kg, Huang et al. 2013), Shaanxi Yao County (0.08 mg/kg, Fang et al. 2003), and Heilongjiang Tailai (0.03 mg/kg, Tan and Huang 1991), but lower than that from Hubei Enshi (3.76 mg/kg, Qin et al. 2013) and Venezuela (14 mg/kg, Fordyce 2013). Rice Se levels from this study area were lower than that from Venezuela (18 mg/kg, Fordyce 2013) and Hubei Enshi (2.11 mg/kg, Qin et al. 2013), but higher than that from the other areas in China (Huang et al. 2010; Gao et al. 2011) and USA (0.136 mg/kg, Fordyce 2013). Thus, Se levels in plants were high within the scope of China and the world (Table 3).
In addition to the soil Se content, differences in the capacity of Se uptake for plant are another decisive factor which can be reflected by the accumulation coefficient (Table 1). Eggplant and pepper are two kinds of vegetables with accumulation coefficient greater than 1. Corn accumulates more Se than rice; this is due to the different Se uptake ability of plants and the Se speciation of soil. Under the flooded conditions of rice field, selenate is more likely to be reduced to selenite which is less available than selenate (Li et al. 2007). In addition, another environmental influence such as Se level in irrigation water is also the crucial factor that determined Se content in plants.
Se in water is also highly determined by soil Se content. Stream water had an average Se level of 12.35 ± 9.45 μg/L (1.86–36.82 μg/L, Fig. 3), which was comparable to that from the Šobov Slovakia seleniferous area (0.12–32.2 μg/L, Bujdoš et al. 2005) and Punjab, India (0.12–69.5 μg/L, Dhillon and Dhillon 2003), but lower than that in Enshi, China (0.46–275 μg/L, Fordyce 2013). The five water samples with Se content bigger than 30 μg/L were all collected from confluence reaches of streams, where rock erosion is more significant than in the other parts, and more Se erodes into water. However, Se content in potable water was relatively low, and no significant difference between NR village and other two villages was found. The geometric mean of Se content in potable water was 7.85 ± 6.04 μg/L, which is lower than the maximum permissible level (MPL) set by US EPA, WHO, European Union, and Chinese Minister of Health (Table 4).
Daily Se intake
The daily Se intake of local residents in NR, LBH, and SHK was 1801, 666, and 686 μg/day, respectively. The average Se intake of studied area was 1067 μg/day, which exceeded tolerable upper Se intake limit (400 μg/day) suggested by WHO (1986) and Chinese Nutrition Society (2001). The average Se intake of this study was higher than those in other Se-rich areas such as Venezuela (200–350 μg/day, Combs and Combs 1986), USA (106 μg/day, Food and Nutrition Board and Institute of Medicine 2000), Greenland (235 μg/day, Hansen et al. 2004), Japan (127.5 μg/day, Miyazaki et al. 2002), India (475–632 μg/day, Hira et al. 2004), Canada (98–224 μg/day, Gissel-Nielsen 1998), and New Zealand (19–80 μg/day, Combs 2001). In contrast, Se intake for residents living in Enshi of Hubei (4990 mg/kg, Yang et al. 1983) was 5 times and 2.8 times higher than the average PDI of this study area and NR village, respectively.
The Se intake is determined not only by the Se content in food but also the diet structure. The staple food of residents living in Ziyang Shuang’an is cereal, and the diet structure is quite unitary. Although corn had lower Se levels than vegetable and meat, 46.65% of inhabitants’ daily Se intake comes from cereal consumption. Because of high Se content in meat, it also makes a considerable contribution (20.99%) to daily Se intake. The higher Se in meat may attribute to animals that require more Se than plants (Klapec et al. 2004). The contribution of eggs and potable water to daily Se intake is less than 15% (Fig. 4). These findings were consistent with those from a previous research where cereals were the major contributors of Se intake, followed by fish and meat, with vegetables and fruits contributing the least (Choi et al. 2009; Gao et al. 2011). However, research on Se PDI in Saudi Arabia showed that daily Se intake was mainly from meat and that eggs and cereals only account for approximately 16% of daily Se intake, and these differences may be due to variable dietary habits and distinct Se levels in consumed food (Fordyce 2013; Yu et al. 2015).
Risk assessment of Se poisoning
The average HQ of this area was as high as 4.34, which was calculated using the average daily selenium intake and recommend daily allowance. HQ for NR, LBH, and SHK was 7.50, 2.78, and 2.86, respectively; selenosis area of NR was nearly three times of the other two villages. All these HQ values were much higher than 1 and the results in Indian Se-rich areas (0.98, Dhillon and Dhillon 2015). The high HQ value indicated that residents living in this area were at a huge risk of Se toxicity, especially for habitants living in NR village. Se in hair, which acts as the terminal of the rock–soil–plant–human body ecosystem, can verify the Se status of people (Gao et al. 2011; Chen et al. 2013). The geometric mean of hair Se level in local inhabitants was 7.05 ± 13.81 mg/kg. The result was lower than that in Enshi Se-rich areas (21.5 ± 20 mg/kg, Xia et al. 2000) and India seleniferous areas (28.21 mg/kg, Dhillon and Dhillon 1991). However, Se content in human hair was two times of toxic standard suggesting by Tan (1989), which further indicates Se poisoning risk in study area. In addition, Se content of female hair was threefold of that in male hair, which is in agreement with previous studies (Hira et al. 2004), indicating that high Se exposure levels in women would be a matter of concern in future Se risk assessments.
In addition to hair Se, blood Se is also a favored biomarker when comparing the Se status between different living conditions (Pestitschek et al. 2013). According to WHO (1986), Se toxicity was observed in hair and nails at whole blood Se levels ranging from 1020 to 1854 μg/L. EPA sets the NOAEL of Se content in blood at 1000 μg/L and the lowest observable adverse effect level (LOAEL) at 1350 μg/L for blood Se (EPA 1992). Calculation of blood Se content through daily Se intake and hair Se content showed that the blood Se status of inhabitants from NR (2783–2824 μg/L) was twice as high as that in LBH (1029–1317 μg/L) and SHK (1060–1347 μg/L), indicating that habitants living in NR were facing a greater risk of Se poisoning. The calculated blood Se status of local residents was much higher than that of NOAEL and results in other Se-rich areas such as Amazon (284.3 μg/L, Lemire et al. 2006; 228.4 μg/L, Lemire et al. 2012) and Greenland (488 μg/L, Hansen et al. 2004), which further confirmed the huge Se poisoning risk for local inhabitants.
Despite the non-negligible risk reflected by daily Se intake and Se content in hair and blood, there have been no cases of Se poisoning in the last 30 years, which was in agreement with the result in Greenland and Brazilian Amazon populations despite their high Se intake (Hansen et al. 2004; Lemire et al. 2012). Previous research found that human exposed to high environmental Se levels may tolerate higher Se contents through homeostatic adjustment (ATSDR 2003; WHO 1986) and may even develop mechanisms to take advantage of such the high Se intake (Mattson 2008). Zhu et al. (2009) also suggested that because SeMet is the major Se species in plant Se, which is less toxic than SeCys and inorganic Se, Se poisoning may have disappeared. The Institute of Medicine (IOM 2000) emphasized that SeMet may not entirely undergo homeostatic regulatory processes as a consequence of non-specific accumulation. As such, it may act as a storage pool for Se and play an important role in adjusting Se status balance. Although the risk of Se toxicity may have been reduced, further research should be performed to examine Se species, their conversion in staple food, and Se intake of humans.
Conclusion
High Se levels were observed in the soil samples from Ziyang Shuang’an and distributed unevenly; approximately 72% of the soil was classified as Se toxic. The Se content in plants varied and exhibited an ability to accumulate, with a geometric mean of 0.76 ± 2.51 mg/kg. Se content of soil and plant in NR was significant higher than that in adjacent villages LBH and SHK. Stream water yielded the highest Se level, with a geometric mean of 12.35 ± 10.46 μg/L. The Se content in potable water (7.85 ± 6.04 μg/L) was lower than the standard of WHO and EPA. The high Se content in hair and the calculated daily Se intake indicated that the inhabitants of Shuang’an in Ziyang were at a high risk of Se poisoning, especially for habitants of NR.
Selenosis was not evident in our study population, whose Se intake is obtained from traditional diet. Nevertheless, local government should consider agronomic measures to regulate the availability of Se. Increased awareness and enhanced knowledge are keys to modifying the single-diet structure, reducing dependence on cereals, and increasing the amount of imported food.
References
Agency for Toxic Substances and Disease Registry (ATSDR) (2003) Toxicological profile for selenium. U.S. Department of Health and Human Services, Public Health Services, Atlanta, GA, http://www.atsdr. cdc.gov/toxprofiles/tp92.html
Aro A, Alfthan G, Varo P (1995) Effects of supplementation of fertilizers on human selenium status in Finland. Analyst 120(3):841–843
Bañuelos GS, Tang J, Hou Y, Yin X, Yuan L (2015) Environmental pathways and dietary intake of selenium in a selenium rich rural community in China: a natural biofortification case study. Global Advances in Selenium Research from Theory to Application: Proceedings of the 4th International Conference on Selenium in the Environment and Human Health 2015. CRC Press, pp. 147
Baoyan M, Xuelin Z (2000) Regional ecological risk assessment of selenium in Jilin Province, China. Sci Total Environ 262(1):103–110
Bujdoš M, Muľová A, Kubova J, Medveď J (2005) Selenium fractionation and speciation in rocks, soils, waters and plants in polluted surface mine environment. Environ Geol 47(3):353–360
Burk RF (2002) Selenium, an antioxidant nutrient. Nutrition in Clinical Care An Official Publication of Tufts University 5(2):75–79
Caussat L, Lelièvre M, Ministère de la Santé, des Solidarités (2007) Les échangesd’expériences en matière de retraites au sein de l’ue: acquis et développementsfuturs. RetraiteEtSociété 1:127–155
Chen YJ, Liu YJ, Tian LX et al (2013) Effect of dietary vitamin E and selenium supplementation on growth, body composition, and antioxidant defense mechanism in juvenile largemouth bass (Micropterus salmoides) fed oxidized fish oil [J]. Fish Physiol Biochem 39(3):593–604
Chien LC, Yeh CY, Huang SY, Shieh MJ, Han BC (2003) Pharmacokinetic model of daily selenium intake from contaminated seafood in Taiwan. Sci Total Environ 311(1–3):57–64
Chilimba ADC, Young SD, Black CR, Rogerson KB, Ander EL, Watts MJ, Lammel J, Broadley MR (2011) Maize grain and soil surveys reveal suboptimal dietary selenium intake is widespread in Malawi. Scientific Reports 1(8):72
Chinese Nutrition Society (2001) Dietary reference intake of Chinese residents [M]. China Light Industry Press, Beijing, pp 56–102 (In Chinese)
Combs GF (2001) Selenium in global food systems. Br J Nutr 85(05):517–547
Choi YJ, Kim JY, Lee HS, Kim C, Hwang IK, Park HK, Oh CH, (2009) Selenium content in representative Korean foods. J Food Compos Anal 22 (2):117–122
Combs GF, Combs SB (1986) The biological availability of selenium in foods and feeds. The Role of Selenium in Nutrition:p127–p177
Dhillon KS, Dhillon SK (1991) Selenium toxicity in soils, plants and animals in some parts of Punjab, India. Int J Environ Stud 37(1–2):15–24
Dhillon K, Dhillon S (2003) Quality of underground water and its contribution towards selenium enrichment of the soil–plant system for a seleniferous region of northwest India. J Hydrol 272(1):120–130
Dhillon KS, Dhillon SK (2015) Selenium in groundwater and its contribution towards daily dietary Se intake under different hydrogeological zones of Punjab, India. J Hydrol 533:615–626
Emmanuelle B, Virginie M, Fabienne S, Isabelle I, Martine PG, Bernard L et al (2012) Selenium exposure in subjects living in areas with high selenium concentrated drinking water: results of a french integrated exposure assessment survey. Environ Int 40(1):155–161
Fang W, Wu P (2004) Elevated selenium and other mineral element concentrations in soil and plant tissue in bone coal sites in Haoping area, Ziyang County, China. Plant & Soil 261(261):135–146
Fang W, Wu P, Hu R, Huang Z (2003) Environmental Se–Mo–B deficiency and its possible effects on crops and Keshan–Beck disease (KBD) in the Chousang area, Yao County, Shaanxi Province, China. Environ Geochem Health 25(2):267–280
Finglas PM (2000) Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B 6, folate, vitamin B 12, pantothenic acid, biotin and choline. Trends Food Sci Technol 11(8):296–297
Food and Nutrition Board & Institute of Medicine (2000) Dietary reference intakes: vitamin C, vitamin E, selenium, and carotenoids. National Academy Press, Washington, DC
Fordyce FM (2007) Selenium geochemistry and health. Ambio 36(1):94–97
Fordyce FM (2013) Selenium deficiency and toxicity in the environment [M]. Essentials of Medical Geology:375–416
Gao J, Liu Y, Huang Y, Lin ZQ, Bañuelos GS, Lam MHW, Yin X (2011) Daily selenium intake in a moderate selenium deficiency area of Suzhou, China. Food Chem 126(3):1088–1093
Gbadebo AM, Babalola OO, Ajigbotesho FL (2010) Selenium concentration in food and blood of residents of Abeokuta Metropolis, Southwestern Nigeria. J Geochem Explor 107(2):175–179
Gissel-Nielsen G (1998) Effects of selenium supplementation of field crops. Environmental Chemistry of Selenium:99–112
Hansen JC, Deutch B, Pedersen HS (2004) Selenium status in Greenland Inuit. Sci Total Environ 331(1):207–214
Hira CK, Partal K, Dhillon KS (2004) Dietary selenium intake by men and women in high and low selenium areas of Punjab. Public Health Nutr 7(1):39–43
Hou JW, Liu BF, Dong QA et al (1993) The investigation report of the selenium level of animal products in Ziyang County [J]. Shaanxi Agricultural Sciences 1993(2):41–42 (in Chinese)
Huang JF, Hong CL, Chen YP, Shi YP (2010) A preliminary study on the soil and crops in Jiaxing plain selenium. J Zhejiang Agric Sci 1(06):1356–1357 (in Chinese)
Huang Y, Wang Q, Gao J, Lin Z, Bañuelos GS, Yuan L, Yin X (2013) Daily dietary selenium intake in a high selenium area of Enshi, China. Nutrients 5(3):700–710
Jaiswal SK, Prakash R, Acharya R, Reddy AV, Prakash NT (2012) Selenium content in seed, oil and oil cake of Se hyperaccumulated Brassica juncea (Indian mustard) cultivated in a seleniferous region of India. Food Chem 134(1):401–404
Klapec T, Mandić ML, Grgić J et al (2004) Selenium in selected foods grown or purchased in eastern Croatia [J]. Food Chem 85(3):445–452
Lemire M, Mergler D, Fillion M, et al (2006) Elevated blood selenium levels in the Brazilian Amazon. [J]. Sci Total Environ 366(1):101–11
Lemire M, Philibert A, Fillion M, Passos CJS, Guimarães JRD, Barbosa F, Mergler D (2012) No evidence of selenosis from a selenium-rich diet in the Brazilian Amazon. Environ Int 40(2):128–136
Levander OA (1982) Clinical consequences of low selenium intake and its relationship to vitamin E. Ann N Y Acad Sci 393:70–82
Levander OA (1987) A global view of human selenium nutrition. Annu rev Nutr 7(1):227–250
Li N, Gao ZD, Luo DG, Tang X, Chen DF, Hu YH (2007) Environmental selenium content and analysis of serum selenium level in Zhoukoudian area of Beijing. Public Health in the Capital 1(1):13–17 (in Chinese)
Luo KL, Xu L, Tan JA, Wang D, Xiang L (2003) Selenium source in the selenosis area of the Daba region, South Qinling Mountain, China. Environ Geol 45(3):426–432
Mahapatra S, Tripathi R, Raghunath R, Sadasivan S (2001) Daily intake of Se by adult population of Mumbai, India. Sci Total Environ 277(1):217–223
Mattson MP (2008) Awareness of hormesis will enhance future research in basic and applied neuroscience. Crit rev Toxicol 38(7):633–639
Mcnaughton SA, Marks GC (2002) Selenium content of Australian foods: a review of literature values. Journal of Food Composition & Analysis 15(2):169–182
Mei ZQ (1985) Summary on two Se-rich areas of China. Chin J Endem 4:379–385 (In Chinese)
Ministry of Health of the People’s Republic of China (GB 5009.93-2010) (2010) National food safety standard Determination of selenium in foods. China Standards, Beijing (in Chinese)
Miyazaki Y, Koyama H, Nojiri M, Suzuki S (2002) Relationship of dietary intake of fish and non-fish selenium to serum lipids in Japanese rural coastal community. J Trace Elem med Biol 16(16):83–90
Navarro-Alarcon M, Cabrera-Vique C (2008) Selenium in food and the human body: a review. Sci Total Environ 400(1–3):115–141
Nuttall KL (2006) Evaluating selenium poisoning. Annals of Clinical & Laboratory Science 36(4):409–420
Pestitschek M, Sonneck-Koenne C, Zakavi SR, Li S, Knoll P, Mirzaei S (2013) Selenium intake and selenium blood levels: a novel food frequency questionnaire. Wiener KlinischeWochenschrift 125(5–6):160–164
Qin HB, Zhu JM, Liang L, Wang MS, Su H (2013) The bioavailability of selenium and risk assessment for human selenium poisoning in high-Se areas, China. Environ Int 52:66–74
Shaanxi Bureau of Statistics (2015) Shaanxi Statistical Yearbook. China Statistics, Beijing (in Chinese)
Sharma N, Prakash R, Srivastava A, Sadana U, Acharya R, Prakash NT, Reddy AR (2009) Profile of selenium in soil and crops in seleniferous area of Punjab, India by neutron activation analysis. J Radioanal Nucl Chem 281(1):59–62
Sirichakwal PP, Puwastien P, Polngam J, Kongkachuichai R (2005) Selenium content of thai foods. Journal of Food Composition & Analysis 18(1):47–59
Tan J. A. (1989) The atlas of endemic diseases and their environments in the People’s Republic of China. Science, Beijing (in Chinese)
Tan JA, Huang Y (1991) Selenium in geo-ecosystem and its relation to endemic diseases in China. Water air Soil Pollut 57(1):59–68
Tan JA, Zhu W, Wang W, Li R, Hou S, Wang D, Yang L (2002) Selenium in soil and endemic diseases in China. Sci Total Environ 284(1):227–235
Temmerman LD, Waegeneers N, Thiry C, Laing GD, Tack F, Ruttens A (2014) Selenium content of Belgian cultivated soils and its uptake by field crops and vegetables. Sci Total Environ 468-469:77–82
Thiry C, Ruttens A, Temmerman LD, Schneider YJ, Pussemier L (2012) Current knowledge in species-related bioavailability of selenium in food. Food Chem 130(4):767–784
U.S. Environmental Protection Agency (EPA) (1992) The integrated risk information system (IRIS). Online. Cincinnati, OH: Office of Health and Environmental Assessment, Environmental Criteria and Assessment Office
U.S. Environmental Protection Agency (EPA) (2002) Integrated risk information systems (IRIS) for selenium. National Centre for Environmental Assessment, Office of Research and Development, Washington, D.C.
U.S. Environmental Protection Agency (EPA) (2012) Basic information about selenium in drinking waterfact sheet. United States Environmental Protection Agency, Washington, D.C.
U.S. Institute of Medicine (IOM) (2000) Dietary reference intakes for vitamin C, vitamin E, selenium and carotenoids. National Academy Press, Washington D.C
US Food and Nutrition Board (1980) Recommended dietary allowances, 9th, revised edn. National Academy of Sciences, Washington, DC
Wang Z, Gao Y (2001) Biogeochemical cycling of selenium in Chinese environments. Appl Geochem 16(11):1345–1351
Wang SS, Liang DL, Wang D, Wei W, Fu D, Lin ZQ (2012) Selenium fractionation and speciation in agriculture soils and accumulation in corn (Zea mays L.) under field conditions in Shaanxi Province, China. Sci Total Environ 427:159–164
World Health Organization (WHO) (1986) Environmental health criteria 58: selenium. International Program on Chemical Safety, Geneva, 190 pp
World Health Organization (WHO) (2011) Guidelines for Drinking-water Quality 4th Ed. [J]
Xia Y, Ha P, Hill K et al (2000) Distribution of selenium between fractions in erythrocytes, plasma, hair, and fingernails of Chinese women living in selenium-deficient, -adequate, and -excessive areas of China [J]. J Trace Elem Exp med 13(13):333–342
Yang GQ, Wang SZ, Zhou RH, Sun SZ (1983) Endemic selenium intoxication of humans in China. Am J Clin Nutr 37(5):872–881
Yang G, Yin S, Zhou R, Gu L, Yan B, Liu Y, Liu Y (1989) Studies of safe maximal daily dietary Se-intake in a seleniferous area in China. Part II: relation between Se-intake and the manifestation of clinical signs and certain biochemical alterations in blood and urine. Journal of Trace Elements & Electrolytes in Health & Disease 3(3):1717–1719
Yang H, Wang Q, Jin G, Lin Z, Bañuelos GS, Yuan L, Yin X (2013) Daily dietary selenium intake in a high selenium area of Enshi, China. Nutrients 5(3):700–710
Yu T, Yang Z, Lv Y, Hou Q, Xia X, Feng H, Zhang M, Jin L, Kan Z (2014) The origin and geochemical cycle of soil selenium in a Se-rich area of China. J Geochem Explor 139:97–108
Yu DS, Liang DL, Lei L, Zhang R, Sun X, Lin ZQ (2015) Selenium geochemical distribution in the environment and predicted human daily dietary intake in northeastern Qinghai, China. Environ Sci Pollut res 22(15):11224–11235
Zhao C, Ren J, Xue C (1993) Selenium in soils of selenium-rich areas in Ziyang County [J]. Acta Pedol sin 30(3):253–259 (In Chinese)
Zhu YG, Pilon-Smits EA, Zhao FJ, Williams PN, Meharg AA (2009) Selenium in higher plants: understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci 14(8):436–442
Acknowledgements
The authors thank the financial support provided by the National Natural Science Foundation of China (No. 41571454 and 41171379, to D. L. Liang).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Cui, Z., Huang, J., Peng, Q. et al. Risk assessment for human health in a seleniferous area, Shuang’an, China. Environ Sci Pollut Res 24, 17701–17710 (2017). https://doi.org/10.1007/s11356-017-9368-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9368-8