Abstract
Recently, the biofabrication of metal nanoparticles has gained wide interest owing to its inherent features such as swift, simplicity, eco-friendliness, and cheaper costs. Different green-reducing agents led to the production of nanoparticles with varying toxicity on insects. In the current study, silver nanoparticles (AgNPs) were successfully synthesized using Habenaria plantaginea leaf extract. Ag nanoparticles were studied by UV–Vis spectroscopy (UV-Vis), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), atomic force microscopy (AFM), scanning electron microscopy (SEM) coupled with energy-dispersive spectroscopy (EDS), and transmission electron microscopy (TEM). H. plantaginea extract and AgNPs were tested for mosquito larvicidal activity on Anopheles stephensi, Aedes aegypti, Culex quinquefasciatus, An. subpictus, Ae. albopictus, and Cx. tritaeniorhynchus. LC50 values were 102.51, 111.99, 123.47, 123.96, 136.56, 149.42 μg/ml and 12.23, 13.38, 14.78, 14.37, 15.39, 16.89 μg/ml, respectively. Moreover, H. plantaginea aqueous extract and AgNPs were tested against the non-target species Anisops bouvieri, Diplonychus indicus, Poecilia reticulata, and Gambusia affinis obtaining LC50 values ranging from 831.82 to 36,212.67 μg/ml. Overall, this study showed the effectiveness of H. plantaginea-fabricated nanoparticles on a wide range of important mosquito vectors, highlighting their scarce toxicity on four natural enemies predating mosquito larvae and pupae.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mosquitoes (Diptera: Culicidae) are more than 3600 species distributed worldwide, belonging to 37 well-recognized genera. Mosquitoes have a long slender body, long legs, and long needle-shaped mouthparts. Some species mainly bite at dusk (e.g., Anopheles), during the night (e.g., Culex) or even during the whole daylight period (e.g., Aedes albopictus). Mosquitoes act as vectors for key life-threatening diseases, such as malaria, yellow fever, dengue fever, chikungunya fever, filariasis, encephalitis, and West Nile and Zika viruses, in almost all tropical and subtropical countries, as well as many other parts of the world (Govindarajan et al. 2012; Benelli, 2015a, b; WHO 2014; Dhiman et al. 2010; Benelli et al. 2016; Benelli et al. 2017a,b,c). Humans have used plant parts, related products, and metabolites in vector control since early historical times. Indeed, plants produce many chemicals, some of which have medicinal and insecticidal properties (Pavela and Benelli 2016a,b; Naqqash et al. 2016; Benelli and Mehlhorn 2016; Pavela 2015; WHO 1980).
The field of nanotechnology is one of the most active areas of research in current material science. The synthesis and characterization of noble metal nanoparticles such as silver, gold, and platinum are an emerging field of research due to their important applications in biotechnology, bioengineering, water treatment, electronics, magnetics, optoelectronics, and pest control (Rafiuddin 2013; Murugan et al. 2015; Benelli and Lukehart 2017). To deal with the abovementioned issues, in latest years, a wide array of plant-borne preparations has been proposed for the green synthesis of nanoparticles, without using high pressure, energy, temperature, or extremely toxic chemicals (Rajan et al. 2015).
Recently, emerging a number of botanicals are screened successfully for nanosynthesis of silver nanoparticles (Ag NPs), including Acalypha indica (Krishnaraj et al. 2010), Citrus limon (Prathna et al. 2011), Allium sativum (Rastogi and Arunachalam 2011), Trianthema decandra (Geethalakshmi and Sarada 2012), Terminalia chebula (Edison and Sethuraman 2012), Mimusops elengi (Prakash et al. 2013), Ficus religiosa (Antony et al. 2013), Morinda citrifolia (Suman et al. 2013), Piper pedicellatum (Tamuly et al. 2013), Artemisia nilagirica (Vijayakumar et al. 2013), Tephrosia purpurea (Ajitha et al. 2014), Tribulus terrestris (Ashokkumar et al. 2014), Anacardium occidentale (Balavigneswaran et al. 2014), Melia dubia (Kathiravan et al. 2014), Pulicaria glutinosa (Khan et al. 2014), Boerhaavia diffusa (Kumar et al. 2014), Azadirachta indica (Nazeruddin et al. 2014; Murugan et al. 2016), and Delonix elata (Sathiya and Akilandeswari 2014). Notably, it has been underlined that different green-reducing herbal preparations led to the production of metal and metal oxide nanoparticles with varying toxicity on insect pests and vectors (see Benelli 2016a,b for reviews).
Habenaria plantaginea Lindl. is a terrestrial species belonging to the family Orchidaceae. It is an endemic plant of south India (Mabberley 2008; Medhi and Chakrabarti 2009). It is very common in the Indian forests of eastern peninsular flora from Periyakombai Hill, at 450–650 m a.s.l. An ovoid-globose tuber giving rise to an erect, glabrous, bracteates stem carrying 3 to 7, sub-basal, clustered, elliptical-oblong to oblong-lanceolate, subacute to acute, sub-sessile, basally clasping leaves that blooms in the late summer and early fill on an erect, laxly 5 to 9 flowered, glabrous, 2 to 7 cm long inflorescence with lanceolate, acuminate, largest towards the base, setaceous margined floral bracts carrying faintly fragrant flowers (Chowdhery 2009). H. plantaginea tubers are used in folk medicine to treat cough, asthma, helminthiasis, and snake bites (Maridass et al. 2008; Singh and Sanjiv 2009), as well as for the treatment of tuberculosis and paralysis (Mohammad 2011).
The use of environmentally benign materials such as green-fabricated metal and metal oxide nanoparticles offers numerous benefits of eco-friendliness and compatibility for larvicidal and oviposition-deterrent applications (Benelli and Govindarajan 2017). Here, we proposed a swift method of green synthesis of Ag nanocrystals using the aqueous leaf extract of H. plantaginea. Bio-fabricated AgNPs were characterized by UV–vis. spectroscopy, FTIR, XRD, AFM, SEM with EDX, and TEM analyses. The mosquito larvicidal potential of H. plantaginea leaf extract and H. plantaginea-fabricated AgNPs was tested on six species, An. stephensi, Ae. aegypti, Cx. quinquefasciatus, An. subpictus, Ae. albopictus, and Cx. tritaeniorhynchus. Moreover, we investigated the impact of H. plantaginea aqueous extract and H. plantaginea-fabricated AgNPs on four non-target species predating Culicidae young instars.
Materials and methods
Chemicals
Silver nitrate (AgNO3) was purchased from Merck (Germany). Normal saline, double distilled water, and demineralized water were used throughout the experiments.
Preparation of H. plantaginea leaf extract and nanosynthesis
H. plantaginea leaves were collected during February 2016 in Kodiyakkarai forest, Nagapattinam district, Tamil Nadu, India. The species identity was confirmed at the Department of Botany, Annamalai University, India. The extract was prepared using 50 g of dried leaf powder in 500 mL of boiled and cooled distilled water, following the method reported by Benelli and Govindarajan (2017).
The H. plantaginea aqueous extract was challenged with silver nitrate solution (1 mM) under controlled parameters and ambient conditions. The bioreduction was monitored by UV–Vis spectroscopy. AgNPs were characterized by UV–Vis spectroscopy, FTIR, XRD, EDX, SEM, AFM, and TEM.
Larvicidal efficacy
All mosquitoes tested here are laboratory-reared strains of Indian origin. They were originally collected 10 years ago from Vector Control Research Centre, Pondicherry Following the method by Govindarajan and Benelli (2016), the six mosquito vectors were reared under laboratory condition (25–28 °C). The larvicidal activity was studied as indicated by WHO (2005). The aqueous extract and green-synthesized AgNPs were prepared at different concentrations of 50–300 and 6–35 μg/ml, respectively. Twenty-five reared early third instar-stage larvae were tested in each replicate. Five replicates were done for each concentration. Mortality of the larvae was calculated after 24 h (Benelli et al. 2017d).
Toxicity on non-target predators
We followed the method by Sivagnaname and Kalyanasundaram (2004). The effect of aqueous extract and AgNPs was evaluated on A. bouvieri, D. indicus, P. reticulata, and G. affinis. We tested the four non-target organisms as indicated by Benelli et al. (2017d). The aqueous extract of H. plantaginea and AgNPs were evaluated at doses >50 times higher if compared to the LC50 values estimated for the six mosquito larvae. Ten replicates were done for each dose, plus four replicates of untreated controls.
Data analysis
Mosquito and natural enemy mortality rates were presented as means ± SD. All the statistical analyses were performed by SPSS version 11.5. LC50 and LC90 values were estimated using probit analysis (Finney 1971), and non-significant chi-squares were calculated (Benelli 2017). In assays focusing on toxicity against non-target organisms, the Suitability Index (SI) was calculated as described by Deo et al. (1988). A probability level of P < 0.05 was used for the significance of differences between values.
Results and discussion
Toxicity on mosquitoes and non-target predators
The larvicidal activity of H. plantaginea aqueous leaf extract and H. plantaginea-synthesized AgNPs was studied here. Our results showed promising larvicidal activities (Tables 1 and 2) on the early third instar larvae of the six mosquito species after 24 h of exposure; the H. plantaginea extract had LC50 values of 102.51, 111.99, 123.47, 123.96, 136.56, and 149.42 μg/ml on An. stephensi, Ae. aegypti, Cx. quinquefasciatus, An. subpictus, Ae. albopictus, and Cx. tritaeniorhynchus, respectively (Table 1). Knowledge about mosquito botanical larvicides has been recently critically discussed by Pavela (2015). While essential oils are more difficult to formulate in a polar solvent like water, aqueous plant extracts represent an easier choice (Pavela and Benelli 2016b). However, in most of the cases, the precise analysis of their chemical constituents is difficult to conduct, requiring NMR and HPTLC. Concerning the activity of plant extracts on mosquitoes, Govindarajan et al. (2008) reported that Ac. indica leaves extracted with different solvents, i.e., benzene, chloroform, ethyl acetate, and methanol, acted as larvicides on An. stephensi with LC50 of 19.25, 27.76, 23.26, and 15.03 ppm, respectively. Later, Govindarajan (2009) investigated the bio-efficacy of Cassia fistula leaf extract with different solvents, i.e., methanol, benzene, and acetone, on larvae of the chikungunya vector Ae. aegypti, showing LC50 of 10.69, 18.27, and 23.95 mg/l, respectively. Larvicidal activity of Ficus benghalensis leaves extracted with methanol, benzene, and acetone and tested on Cx. quinquefasciatus, Ae. aegypti, and An. stephensi achieved LC50 of 41.43, 58.21, and 74.32 ppm; 56.54, 70.29, and 80.85 ppm; and 60.44, 76.41, and 89.55 ppm, respectively (Govindarajan 2010). The mosquito larvicidal potential of benzene and ethyl acetate extracts of Ervatamia coronaria and Caesalpinia pulcherrima leaves on An. stephensi, Ae. aegypti, and Cx. quinquefasciatus was showed by LC50 and LC90 values of 79.08, 89.59, and 96.15 ppm and 150.47, 166.04, and 174.10 ppm, respectively (Govindarajan et al. 2011). Rajeswary and Govindarajan (2013) studied that the mosquito larvicidal properties of Ageratina adenophora against Cx. quinquefasciatus, Ae. aegypti, and An. stephensi with the leaf methanol extract LC50 of 144.86, 132.82, and 113.08, respectively. Govindarajan and Sivakumar (2014) investigated the larvicidal effect of crude hexane, benzene, chloroform, ethyl acetate, and methanol solvent extracts of Erythrina indica on An. stephensi, Ae. aegypti, and Cx. quinquefasciatus. The highest larval mortality was obtained by a treatment with leaf methanol extract, with LC50 and LC90 values of 69.43, 75.13, and 91.41 ppm and 125.49, 134.31, and 167.14 ppm, respectively.
The use of botanical extracts to fabricate nanomaterials improves their toxicity against mosquito vectors (Benelli 2016a,b). In our assays, the larvicidal activity of H. plantaginea-synthesized AgNPs showed LC50 values of 12.23, 13.38, 14.78, 14.37, 15.39, and 16.89 μg/ml, respectively (Table 2). These values are extremely low if compared to the activity of the most tested raw plant extracts. However, in some cases, lower values have been estimated for other green-fabricated nanoparticles, showing that different botanicals used as reducing and capping agents plant also a role in determining the bioactivity of the nanoparticles. For example, Pongamia pinnata-mediated synthesized AgNPs acted as an effective mosquito larvicidal on Ae. albopictus with the LC50 of 0.25 ppm (Naik et al. 2014). The larvicidal potential of Leucas aspera-synthesized AgNPs on Ae. aegypti has been also studied, reporting an LC50 of 8.5632 mg/l (Suganya et al. 2014). Green synthesis of AgNPs using the leaf extract of Pithecellobium dulce on the filariasis vector Cx. quinquefasciatus showed an LC50 of 21.56 mg/L (Raman et al. 2012). About fungi, Agaricus bisporus-mediated fabrication of AgNPs on Culex vectors showed dose-dependent mortality rates when tested at 5 mg/L (100% mortality), 2.5 mg/L (81%), 1.25 mg/L (62%), 0.625 mg/L (28%), and 0.312 mg/L (11%) (Dhanasekaran and Thangaraj 2013). Biofabrication of AgNPs using Nerium oleander leaf extract against first to fourth instar larvae of An. stephensi led to LC50values of 20.60, 24.90, 28.22, and 33.99 ppm, respectively (Roni et al. 2013). Also, green-synthesized Ag nanocrystals fabricated with Murraya koenigii leaf extract were toxic to An. stephensi larvae (I-IV) and pupae, with LC50 of 10.82, 14.67, 19.13, 24.35, and 32.09 ppm (Suganya et al. 2013). Concerning the potential mechanisms of action of nanoparticles, very little is known and further research is still needed (Benelli 2016b).
In our non-target assays, we tested H. plantaginea-synthesized AgNPs and the related aqueous plant extract on insects (A. bouvieri and D. indicus) and fishes (P. reticulata and G. affinis). We observed moderate toxicity on A. bouvieri, D. indicus, P. reticulata, and G. affinis with LC50 values ranging from 831 to 36,212 μg/ml (Tables 3 and 4). The estimated SI indicated that H. plantaginea-fabricated AgNPs were less toxic to the four non-target organisms if compared to the targeted mosquito larval populations (Table 5). Recently, a growing number of research evidences highlighted the eco-friendly nature of plant extracts, essential oils as well as AgNPs fabricated using botanicals on non-target species predating mosquitoes (Benelli 2016a,b). For example, the Atlantia monophylla extract was safe to aquatic mosquito predators G. affinis, P. reticulata, and D. indicus, with LC50 values of 23.4, 21.3, and 5.7 mg/l (Sivagnaname and Kalyanasundaram 2004). Acacia caesia-synthesized AgNPs were safer to three non-target A. bouvieri, D. indicus, and G. affinis, with LC50 ranging from 684 to 2245 μg/ml (Benelli et al. 2017d). Essential oil from Amomum subulatum was safer to D. indicus, G. affinis, and P. reticulata with LC50 range of 3123–9104 μg/ml (Govindarajan et al. 2017). Also, green-synthesized AgNPs obtained using the leaf extract of Rubus ellipticus as reducing and capping agent were scarcely toxic to A. bouvieri, D. indicus, and G. affinis, with LC50 from 896 to 2261 μg/ml (AlQahtani et al. 2017). Further researches aimed to understand the fate and long-term toxicity of residual nano-Ag concentrations in water bodies are ongoing (Banumathi et al. 2017).
Bionanosynthesis and nanocharacterization
UV–Vis spectroscopy
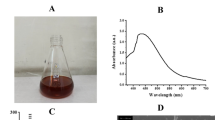
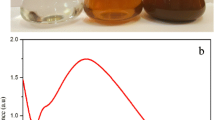
The reduction of silver nitrate to AgNP by the leaf extract of H. plantaginea was confirmed by measuring the UV–Vis spectrum of the nano- suspension. The silver nitrate solution was added to the yellow aqueous leaf extract. The color change to brown confirmed the formation of AgNP (Fig. 1a). This color change was due to the reduction of Ag+ to Ag0 by various biomolecules present in the leaf extract. The absorption spectrum of AgNP was recorded and is depicted in Fig. 1b. The AgNP showed a characteristic absorption peak at a wavelength of 466 nm due to surface plasmon resonance (SPR). The spherical shape of the AgNP was confirmed by the kmax of 466 nm. According to the literature (Prathna et al. 2011), absorption bands in the range 400–480 nm in the UV–Vis spectrum correspond to spherical-shaped metallic nanoparticles. The presence of a single peak in the figure corroborated the spherical shape of H. plantaginea-synthesized AgNPs according to Mie theory (Prathna et al. 2011).
XRD and EDX analyses
The X-ray diffraction (XRD) profile of H. plantaginea-synthesized AgNPs is depicted in Fig. 2a. The four distinct peaks at 2θ = 38.52, 44.28, 63.97, and 78.34 were interpreted as (1 1 1), (2 0 0), (2 2 0), and (3 1 1) lattice planes respectively, showing the face-centered cubic (fcc) structure of metallic Ag (Maity et al. 2011, 2012).
Energy-dispersive spectrum revealed the presence of elemental silver in the sample (Fig. 2b), as underlined by the sharp peak at 3 keV. The occurrence of other peaks (O and C) was presumably related with capping action of metabolites from H. plantaginea extract (Madhumitha et al. 2015).
FTIR spectroscopy
As shown by Fig. 3, the FTIR spectrum exhibited a number of major peaks at 3461.11, 2918.18, 2849.30, 2427.91, 1639.84, 1382.55, 1115.14, 1046.26, 841.64, and 788.97 cm−1. The peak at 3461.11 could be due to –OH stretching from alcohols and phenols in the leaf extract. The small band at 2918.18 can be due to the C–H stretching of alkanes. The possible presence of carboxylic acid (O–H stretch) was observed at 2849.30 and 2427.91. The medium band observed at 1639.84 implied the stretching vibrations of C=C functional groups of aromatics. A strong peak at 1382.55 denoted the bending vibrations of sulfate (S=O stretching) and suggested the possible binding of S-NPs with the proteins present in the extract. The small peaks at 1115.14 can be due to the P=O stretching of phosphine oxide present in the extract. The band observed at 1046.26, 841.64, and 788.97 can indicate P=OR and S=OR stretching modes of esters from the H. plantaginea extract (Mishra and Sardar 2012; Tamboli and Lee 2013; Perni et al. 2014).
AFM, SEM, and TEM
H. plantaginea-synthesized AgNPs studied using AFM showed polydispersed and spherical structures, with size from 0.1 to 29 nm (Fig. 4). SEM (Fig. 5a) highlighted well-defined spherical H. plantaginea-synthesized AgNPs. The average particle size within the selected area of SEM was 50 nm. The result is comparable with the Annona squamosa leaf extract-mediated silver nanoparticles reported by Kumar et al. (2012). Lastly, TEM (Fig. 5b) revealed that H. plantaginea-synthesized AgNPs are spherical in shape and are uniformly distributed without significant agglomeration. The crystalline nature of AgNPs is in good agreement with XRD results. H. plantaginea-synthesized AgNP size ranges from 20 to 50 nm, with average size of 37.8 nm (see also Roopan et al. 2013).
Conclusions
Overall, the H. plantaginea-mediated nanosynthesis led to the production of nanoparticle homogeneous in size, with crystalline structure, as showed by TEM, AFM, and XRD data, respectively. Furthermore, our experiments showed the high efficacy of H. plantaginea-fabricated nanocrystals against a wide range of important mosquito vectors, highlighting their scarce toxicity on four natural enemies predating mosquito larvae and pupae.
References
Ajitha B, Reddy YAK, Reddy PS (2014) Biogenic nano-scale silver particles by Tephrosia purpurea leaf extract and their inborn antimicrobial activity. Spectrochim Acta Part A Mol Biomol Spectrosc 121:164–172
AlQahtani FS, AlShebly MM, Govindarajan M, Senthilmurugan S, Vijayan P, Benelli G (2017) Green and facile biosynthesis of silver nanocomposites using the aqueous extract of Rubus ellipticus leaves: toxicity and oviposition deterrent activity against Zika virus, malaria and filariasis mosquito vectors. J Asia Pac Entomol 20:157–164
Antony JJ, Sithika MAA, Joseph TA, Suriyakalaa U, Sankarganesh A, Siva D, Kalaiselvi S, Achiraman S (2013) In vivo antitumor activity of biosynthesised silver nanoparticles using Ficus religiosa as a nano factory in DAL induced mice model. Colloids Surf B Biointerfaces 108:185–190
Ashokkumar S, Ravi S, Kathiravan V, Velmurugan S (2014) Synthesis, characterization and catalytic activity of silver nanoparticles using Tribulus terrestris leaf extract. Spectrochim Acta Part A Mol Biomol Spectrosc 121:88–93
Balavigneswaran CK, Sujin Jeba Kumar T, Moses Packiaraj R, Prakash S (2014) Rapid detection of Cr (VI) by AgNPs probe produced by Anacardium occidentale fresh leaf extracts. Appl Nanosci 4:367–378
Banumathi B, Vaseeharan B, Suganya P, Citarasu T, Govindarajan M, Alharbi NS et al. (2017) Toxicity of camellia sinensis-fabricated silver nanoparticles on invertebrate and vertebrate organisms: morphological abnormalities and DNA damages. J Clust Sci. doi:10.1007/s10876-017-1201-5
Benelli G (2015a) Research in mosquito control: current challenges for a brighter future. Parasitol Res 114:2801–2805
Benelli G (2015b) Plant-borne ovicides in the fight against mosquito vectors of medical and veterinary importance: a systematic review. Parasitol Res 114:3201–3212
Benelli G (2016a) Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: a review. Parasitol Res 115:23–34
Benelli G (2016b) Green synthesized nanoparticles in the fight against mosquito-borne diseases and cancer—a brief review. Enzyme Microbial Technol 95:58–68
Benelli G (2017) Commentary: data analysis in bionanoscience—issues to watch for. J Clust Sci 28:11–14
Benelli G, Govindarajan M (2017) Green-synthesized mosquito oviposition attractants and ovicides: towards a nanoparticle-based “lure and kill” approach?. J Clust Sci 28:287–308
Benelli G, Kadaikunnan S, Alharbi NS, Govindarajan M (2017d) Biophysical characterization of Acacia caesia-fabricated silver nanoparticles: effectiveness on mosquito vectors of public health relevance and impact on non-target aquatic biocontrol agents. Environ Sci Pollut Res DOI 10.1007/s11356-017-8482-y
Benelli G, Lo Iacono A, Canale A, Mehlhorn H (2016) Mosquito vectors and the spread of cancer: an overlooked connection? Parasitol Res 115:2131–2137
Benelli G, Lukehart CM (2017) Special issue: applications of green-synthesized nanoparticles in pharmacology, parasitology and entomology. J Clust Sci 28:1–2
Benelli G, Mehlhorn H (2016) Declining malaria, rising dengue and Zika virus: insights for mosquito vector control. Parasitol Res 115:1747–1754
Benelli G, Pavela R, Iannarelli R, Petrelli R, Cappellacci L, Cianfaglione K, Afshar FH, Nicoletti M, Canale A, Maggi F (2017b) Synergized mixtures of Apiaceae essential oils and related plant-borne compounds: larvicidal effectiveness on the filariasis vector Culex quinquefasciatus say. Ind Crop Prod 96:186–195
Benelli G, Pavela R, Maggi F, Petrelli R, Nicoletti M (2017a) Commentary: making green pesticides greener? The potential of plant products for nanosynthesis and pest control. J Clust Sci 28:3–10
Benelli G, Rajeswary M, Govindarajan M (2017c) Towards green oviposition deterrents? Effectiveness of Syzygium lanceolatum (Myrtaceae) essential oil against six mosquito vectors and impact on four aquatic biological control agents. Environ Sci Poll Res doi: 10.1007/s11356-016-8146-3
Chowdhery HJ (2009) Orchid diversity in north–eastern states of India. J Orchid Soc India 23(1–2):19–42
Deo PG, Hasan SB, Majumdar SK (1988) Toxicity and suitability of some insecticides for household use. Int Pest Control 30:118–129
Dhanasekaran D, Thangaraj R (2013) Evaluation of larvicidal activity of biogenic nanoparticles against filariasis causing Culex mosquito vector. Asian Pac J Trop Dis 31:74–179
Dhiman RC, Pahwa S, Dhillon GPS, Dash AP (2010) Climate change and threat of vector-borne diseases in India: are we prepared? Parasitol Res 106(4):763–773
Edison TJI, Sethuraman MG (2012) Instant green synthesis of silver nanoparticles using Terminalia chebula fruit extract and evaluation of their catalytic activity on reduction of methylene blue. Process Biochem 47:1351
Finney DJ (1971) Probit analysis. Cambridge University Press, London, pp 68–72
Geethalakshmi R, Sarada DVL (2012) Gold and silver nanoparticles from Trianthema decandra: synthesis, characterization, and antimicrobial properties. Int J Nanomedicine 7:5375–5384
Govindarajan M (2009) Bioefficacy of Cassia fistula Linn. (Leguminosae) leaf extract against chikungunya vector, Aedes aegypti (Diptera: Culicidae). Eur Rev Med Pharmacol Sci l13:99–103
Govindarajan M (2010) Larvicidal efficacy of Ficus benghalensis L. plant leaf extract against Culex quinquefasciatus say. Aedes aegypti L. and Anopheles stephensi L. (Diptera: Culicidae). Eur Rev Med Pharmacol Sci 14:107–111
Govindarajan M, Benelli G (2016) Artemisia absinthium-borne compounds as novel larvicides: effectiveness against six mosquito vectors and acute toxicity on non-target aquatic organisms. Parasitol Res 115:4649–4661
Govindarajan M, Jebanesan A, Pushpanathan T, Samidurai K (2008) Studies on effect of Acalypha indica L. (Euphorbiaceae) leaf extracts on the malarial vector, Anopheles stephensi Liston (Diptera: Culicidae). Parasitol Res 103:691–695
Govindarajan M, Mathivanan T, Elumalai K, Krishnappa K, Anandan A (2011) Mosquito larvicidal, ovicidal and repellent properties of botanical extracts against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res 109:353–367
Govindarajan M, Sivakumar R, Amsath A, Niraimathi S (2012) Larvicidal efficacy of botanical extracts against two important vector mosquitoes. Eur Rev Med Pharmacol Sci 16(3):386–392
Govindarajan M, Sivakumar R (2014) Larvicidal, ovicidal, and adulticidal efficacy of Erythrina indica (lam.) (family: Fabaceae) against Anopheles stephensi, Aedes aegypti, and Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res 113:777–791
Govindarajan M, Rajeswary M, Senthilmurugan S, Vijayan P, Alharbi NS, Kadaikunnan S, Khaled J M, Benelli G (2017) Larvicidal activity of the essential oil from Amomum subulatum Roxb. (Zingiberaceae) against Anopheles subpictus, Aedes albopictus and Culex tritaeniorhynchus (Diptera: Culicidae), and non-target impact on four mosquito natural enemies. Physiol Mol Plant Path doi:10.1016/j.pmpp.2017.01.003
Kathiravan V, Ravi S, Ashokkumar S (2014) Synthesis of silver nanoparticles from Melia dubia leaf extract and their in vitro anticancer activity. Spectrochim Acta Part A Mol Biomol Spectrosc 130:116–121
Khan M, Khan ST, Khan M, Adil SF, Musarrat J, AlKhedhairy AA, Alkhathlan HZ (2014) Antibacterial properties of silver nanoparticles synthesized using Pulicaria glutinosa plant extract as a green bioreductant. Int J Nanomedicine 9:3551–3565
Krishnaraj C, Jagan EG, Rajasekar S, Selvakumar P, Kalaichelvan PT, Mohan N (2010) Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf B Biointerfaces 76:50–56
Kumar PPNV, Pammi SVN, Kollu P, Satyanarayana KVV, Shameem U (2014) Green synthesis and characterization of silver nanoparticles using Boerhaavia diffusa plant extract and their antibacterial activity. Ind Crop Prod 52:562–566
Kumar R, Roopan SM, Prabhakarn A, Khanna VG, Chakroborty S (2012) Agricultural waste Annona squamosa peel extract: biosynthesis of silver nanoparticles. Spectrochim Acta A 90:173–176
Mabberley DJ (2008) Mabberley’s plant book: a portable dictionary of plants, their classification and uses, vol 3. Cambridge University Press, Cambridge, p 606
Madhumitha G, Elango G, Roopan SM (2015) Bio-functionalized doped silver nanoparticles and its antimicrobial studies. J Sol-Gel Sci Technol 73:476–483
Maity D, Bain MK, Bhowmick B, Sarkar J, Saha S, Acharya K, Chakraborty M, Chattopadhyay D (2011) In situ synthesis, characterization, and antimicrobial activity of silver nanoparticles using water soluble polymer. J Appl Polym Sci 122:2189–2196
Maity D, Mollick MMR, Mondal D, Bhowmick B, Bain MK, Bankura K, Sarkar J, Acharya K, Chattopadhyay D (2012) Synthesis of methylcellulose–silver nanocomposite and investigation of mechanical and antimicrobial properties. Carbohydr Polym 90:1818–1825
Maridass M, Zahir Hussain MI, Raju G (2008) Phytochemical survey of orchids in the Tirunelveli Hills of South India. Ethnobotanical Leaflets 12:705–712
Medhi RP, Chakrabarti S (2009) Traditional knowledge of NE people on conservation of wild orchids. Indian J Tradn Knowl 8:11–16
Mishra A, Sardar M (2012) Alpha-amylase mediated synthesis of silver nanoparticles. Sci Adv Mater 4:143–146
Mohammad HM (2011) Therapeutic orchids: traditional uses and recent advances—an overview. Fitoterapia 82:102–140
Murugan K, Benelli G, Ayyappan S, Dinesh D, Panneerselvam C, Nicoletti M, Hwang J-S, Kumar PM, Subramaniam J, Suresh U (2015) Toxicity of seaweed-synthesized silver nanoparticles against the filariasis vector Culex quinquefasciatus and its impact on predation efficiency of the cyclopoid crustacean Mesocyclops longisetus. Parasitol Res 114(6):2243–2253
Murugan K, Panneerselvam C, Samidoss CM, Madhiyazhagan P, Roni M, Subramaniam J, Dinesh D, Rajaganesh R, Paulpandi M, Wei H, Aziz AT, Alsalhi MS, Devanesan S, Nicoletti M, Pavela R, Canale A, Benelli G (2016) In vivo and in vitro effectiveness of Azadirachta indica-synthesized silver nanocrystals against Plasmodium berghei and Plasmodium falciparum, and their potential against malaria mosquitoes. Res Vet Sci 106:14–22
Naik BR, Gowreeswari GS, Singh Y, Satyavathi R, Daravath RR, Reddy PR (2014) Bio-synthesis of silver nanoparticles from leaf extract of Pongamia pinnata as an effective larvicide on dengue vector Aedes albopictus (Skuse) (Diptera: Culicidae). Adv Entomol:293–101
Naqqash MN, Gökçe A, Bakhsh A, Salim M (2016) Insecticide resistance and its molecular basis in urban insect pests. Parasitol Res 115:1363–1373
Nazeruddin GM, Prasad NR, Waghmare SR, Garadkar KM, Mulla IS (2014) Extracellular biosynthesis of silver nanoparticle using Azadirachta indica leaf extract and its anti-microbial activity. J Alloys Compd 583:272–277
Pavela R (2015) Essential oils for the development of eco-friendly mosquito larvicides: a review. Ind Crop Prod 76:174–187
Pavela R, Benelli G (2016a) Ethnobotanical knowledge on botanical repellents employed in the African region against mosquito vectors—a review. Exp Parasitol 167:103–108
Pavela R, Benelli G (2016b) Essential oils as eco-friendly biopesticides? Challenges and constraints. Tr Plant Sci 21(12):1000–1007
Perni S, Hakala V, Prokopovich P (2014) Biogenic synthesis of antimicrobial silver nanoparticles capped with L-cysteine. Colloids Surf A Physicochem Eng Asp 460:219–224
Prakash P, Gnanaprakasama P, Emmanuel R, Arokiyaraj S, Saravanan M (2013) Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf B Biointerfaces 108:255–259
Prathna TC, Chandrasekaran N, Raichur AM, Mukherjee A (2011) Biomimetic synthesis of silver nanoparticles by Citrus limon (lemon) aqueous extract and theoretical prediction of particle size. Colloids Surf B Biointerfaces 82:152–159
Rafiuddin ZZ (2013) Bio-conjugated silver nanoparticles from Ocimum sanctum and role of cetyltrimethyl ammonium bromide. Colloids Surf B Biointerfaces 108:90–94
Rajan R, Chandran K, Harper SL, Yun SI, Kalaichelvan PT (2015) Plant extract synthesized nanoparticles: an ongoing source of novel biocompatible materials. Ind Crop Prod 70:356–373
Rajeswary M, Govindarajan M (2013) Mosquito larvicidal and phytochemical properties of Ageratina adenophora (Asteraceae) against three important mosquitoes. J Vector Borne Dis:50141–50143
Raman N, Sudharsan S, Veerakumar V, Pravin N, Vithiya K (2012) Pithecellobium dulce mediated extra-cellular green synthesis of larvicidal silver nanoparticles. Spectrochim Acta Mol Biomol Spectrosc 96:1031–1037
Rastogi L, Arunachalam J (2011) Sunlight based irradiation strategy for rapid green synthesis of highly stable silver nanoparticles using aqueous garlic (Allium sativum) extract and their antibacterial potential. Mater Chem Phys 129:558–563
Roni M, Murugan K, Panneerselvam C, Subramaniam J, Hwang JS (2013) Evaluation of leaf aqueous extract and synthesized silver nanoparticles using Nerium oleander against Anopheles stephensi (Diptera: Culicidae). Parasitol Res 112:981–990
Roopan RSM, Madhumitha G, Rahuman AA, Kamaraj C, Bharathi A, Surendra TV (2013) Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. Ind Crop Prod 43:631–635
Sathiya CK, Akilandeswari S (2014) Fabrication and characterization of silver nanoparticles using Delonix elata leaf broth. Spectrochim Acta Mol Biomol Spectrosc 128:337–341
Singh A, Sanjiv D (2009) Medicinal orchids—an overview. Ethnobotanical Leaflets 13:399–412
Sivagnaname N, Kalyanasundaram M (2004) Laboratory evaluation of methanolic extract of Atlantia monophylla (family: Rutaceae) against immature stages of mosquitoes and non-target organisms. Mem Inst Oswaldo Cruz 99:115–118
Suganya A, Murugan K, Kovendan K, Mahesh Kumar P, Hwang JS (2013) Green synthesis of silver nanoparticles using Murraya koenigii leaf extract against Anopheles stephensi and Aedes aegypti. Parasitol Res 112:1385–1397
Suganya G, Karthi S, Shivakumar MS (2014) Larvicidal potential of silver nanoparticles synthesized from Lucas aspera leaf extracts against dengue vector Aedes aegypti. Parasitol Res 113:1673–1679
Suman TY, Rajasree SRR, Kanchana A, Elizabeth SB (2013) Biosynthesis, characterization and cytotoxic effect of plant mediated silver nanoparticles using Morinda citrifolia root extract. Colloids Surf B Biointerfaces 106:74–78
Tamboli DP, Lee DS (2013) Mechanistic antimicrobial approach of extracellularly synthesised silver nanoparticles against gram positive and gram negative bacteria. J Hazard Mater 260:878–884
Tamuly C, Hazarikaa M, Borah SCH, Das MR, Boruah MP (2013) In situ biosynthesis of Ag, Au and bimetallic nanoparticles using Piper pedicellatum C.DC: green chemistry approach. Colloids Surf B Biointerfaces 102:627–634
Vijayakumar M, Priya K, Nancy FT, Noorlidah A, Ahmed ABA (2013) Biosynthesis, characterisation and anti-bacterial effect of plant-mediated silver nanoparticles using Artemisia nilagirica. Ind Crop Prod 41:235–240
WHO (1980) Expert committee on diabetes mellitus. Second report. Technical report series 646. World Health Organization, Geneva, pp 12–15
World Health Organization (2005) Guidelines for laboratory and field testing of mosquito larvicides. Communicable disease control, prevention and eradication, WHO pesticide evaluation scheme. WHO, Geneva, WHO/CDS/WHOPES/GCDPP/1.3
World Health Organization (2014) A global brief on vector-borne diseases. WHO/DCO/WHD/2014.1
Acknowledgments
The authors extend their sincere appreciations to the Deanship of Scientific Research at King Saud University for funding the work through the research group project no. (RGP-073). The authors would like to thank the Principal and Head of the Department of Zoology, Thiru. Vi. Ka Government Arts College and the Professor and Head, Department of Zoology, Annamalai University for the laboratory facilities provided.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Santiago V. Luis
Invited contribution for the special issue Plant-borne compounds and nanoparticles: challenges for medicine, parasitology and entomology (GREEN-NANO-PEST&DRUGS).
Rights and permissions
About this article
Cite this article
Aarthi, C., Govindarajan, M., Rajaraman, P. et al. Eco-friendly and cost-effective Ag nanocrystals fabricated using the leaf extract of Habenaria plantaginea: toxicity on six mosquito vectors and four non-target species. Environ Sci Pollut Res 25, 10317–10327 (2018). https://doi.org/10.1007/s11356-017-9203-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9203-2