Abstract
The study focuses on the estimation of health risk from nitrate present in the drinking water and vegetables in Nagpur and Bhandara districts in the state of Maharashtra, India. Drinking water samples from 77 locations from the rural as well as urban areas and 22 varieties of vegetable were collected and analyzed for the presence of nitrate for a period of 1 year (two seasons). The daily intake of nitrate from these water and vegetable samples was then computed and compared with standard acceptable intake levels to assess the associated health risk. The mean nitrate concentration of 59 drinking water samples exceeded the Bureau of Indian Standards limit of 45 mg/L in drinking water. The rural and urban areas were found to have mean nitrate concentration in drinking water as 45.69 ± 2.08 and 22.53 ± 1.97 mg/L, respectively. The estimated daily intake of drinking water samples from 55 study sites had nitrate concentration far below the safety margin indicating serious health risk. The sanitation survey conducted in 12 households reported contaminated source with positive E. coli count in 20 samples as the major factor of health risk. The average nitrate concentration was maximum in beetroot (1349.38 mg/kg) followed by spinach (1288.75 mg/kg) and amaranthus (1007.64 mg/kg). Among the samples, four varieties of the vegetables exceeded the acceptable daily intake (ADI) with an assumption of 0.5 kg consumption of vegetables for an average of a 60-kg individual. Therefore, irrigation of these locally grown vegetables should be monitored periodically for nitrogen accumulation by the crop above the ADI limit. The application of nitrogenous fertilizers should also be minimized in the rural areas to help protect the nitrate contamination in groundwater sources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Depletion of water sources with increasing demands for drinking, agriculture, and other purposes has led to water stress in India (Kumar et al. 2005). Groundwater as a primary drinking water source serves about 85% of the rural population of India for their daily water needs (Singh and Khan 2014). Water pollution is a widespread problem due to the presence of inorganic contaminants with nitrate being the most prevalent contaminant (Ravikanth et al. 2015). In many countries, the significantly growing nitrate contamination, deteriorating the groundwater quality, has been of concern presently.
Nitrate, a form of nitrogen, enters the environment naturally by nitrogen fixation. The excess nitrate in soil not used by plant roots (Kundu et al. 2009) due to weaker soil retention and higher solubility easily finds its way to the groundwater sources (Almasri 2007). Several anthropogenic activities—the application of nitrogenous fertilizers, municipal wastewater, septic system leakage, urban drainage reject, refuse dumps, sewage discharge, industrial discharge, contaminated land, unhygienic sanitation practices, landfills, animal waste, and soluble nitrogen compounds contained by geologic materials—aggravate the nitrate contamination (Chigor et al. 2012; CGWB 2014). The urine and excreta of farm animals such as cows and buffaloes (Wakida and Lerner 2005) and poor casings (Vough et al. 2006) have also been linked to nitrate contamination in shallow groundwater.
Maharashtra is the third largest fertilizer consumer in the country with 64.81 kg of nitrogen fertilizer consumption per hectare during 2009–2010 (Indian fertilizer scenario 2010). The rise in consumption of fertilizers from 2000 t in 1974–75 to 18,000 t in 2010–11 (Mandanna 2011) in India has subsequently increased the threat of nitrate contamination due to agricultural runoff. Due to the increasing levels of nitrate, governmental organizations have prescribed permissible limits for nitrate in drinking water as 45 mg/L NO3− (Bureau of Indian Standards 2012) and have a guideline value of 50 mg/L (WHO 2011) above which it can pose serious health hazards.
In India, the occurrence of excessive nitrate (>50 mg/L as NO3, the maximum permissible limit recommended by WHO) in groundwater has been found in one third of the total Indian states (NEERI 1991). An alarming concentration of nitrate up to 879.65 mg/L has been reported in the Nalgonda district of Andhra Pradesh. The concentration of nitrate in groundwater sources of different states reported by several researchers is listed in Table 1. This table clearly shows that nitrate contamination has affected southern and northeastern India and states in central India also.
Vegetables contributing to 80% of the individual’s daily diet are the leading source of average nitrate intake (Ziarati 2012). Depending on the method of cultivation, light intensity, rate and timing of fertilizer application, soil characteristics, and daytime temperature, variable amounts of nitrates may be found in vegetables (Thomson 2004). Several vegetables including spinach, beetroot, cabbage, celery, radish, broccoli, and lettuce have been found having nitrate concentrations greater than 1000 mg/kg. Green leafy vegetables have been reported to contain higher nitrate concentrations (Thomson et al. 2007). The consumption of fennels has also been found to be associated with fatal nitrate toxicosis in cattle (Costagliola et al. 2014).
Nutritional, environmental, and physiological factors all contribute to the accumulation of nitrates in plants (Anjana et al. 2007). Accumulation of nitrates in high concentration by leafy vegetables such as lettuce, spinach, and some of the root crops like beetroot can be attributed to the tendency of accumulation of high concentration of nitrates in the leaves, roots, petioles, and/or stems of certain plants (Food standards Australia New Zealand 2013).
Nitrates also added as food additives in processed foods serve as preservatives and antimicrobial agents (Abdulrazak et al. 2014). In meat products and cheese, nitrate has been permitted for use as an additive (Thomson 2004).
Consumption of water and vegetables having excessive levels of nitrate can pose serious health hazards particularly in infants. Toxicity of nitrates causes methemoglobinemia, carcinogenicity, goiter, birth defects, abortions, histopathological changes in cardiac muscles, diabetes mellitus, and livestock poisoning (Gupta et al. 2008). Nitrate ingestion may lead to oxygen deprivation in infants of below 6 months of age causing methemoglobinemia or blue baby syndrome (Shrimali and Singh 2001). Approximately 20% of the nitrate secreted in saliva is converted to nitrite by the microorganisms present in the oral cavity in individuals (Cortesi et al. 2015) with high rate of conversion in contrast to normal individuals with 5% (Costagliola et al. 2014) conversion (Thomson et al. 2007). The radical conversion of nitrate to nitrite post ingestion forming carcinogenic N-nitrosamines on reaction with amines and amides can lead to potential cancer risk (Raczuk et al. 2014). Nitrates can also cause interference to the normal iodine metabolism of the thyroid gland (Ward et al. 2010).
In view of the current health concerns from the consumption of nitrates in foods, governmental agencies have stipulated a minimum acceptable level of consumption for dietary nitrates referred to as acceptable daily intake (ADI). The European Commission’s (EC) Scientific Committee for Food (SCF) established the ADI of nitrate as 3.7 mg/kg body weight (ECETOC 1988). This ADI limit was then reconfirmed by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in 2002 (EFSA Panel on Contaminants in the Food Chain 2008).
The rural population comprises approximately 32% of the total population in Nagpur and 81% in the Bhandara district (Census 2011). These rural areas depend mostly on groundwater as their potable water source. The current status of nitrate in various locations of Nagpur and Bhandara district remains unexplored with very few studies giving way to conduct further studies on the aspect. In the pretext of the above-mentioned health hazards and distinct water supply sources, this study aims at determining nitrate concentrations in the drinking water and vegetable sources in rural and urban areas of the Nagpur and Bhandara districts to identify the risk associated with the dietary nitrate intake and daily nitrate intake.
Methodology
Study area selection
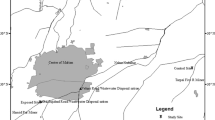
Figure 1 the study sites are located in the rural and urban settings of Nagpur (21.1458° N, 79.0882° E) and Bhandara (21.0736° N, 79.8297° E) districts. The sampling sites located in various rural and urban settings of Nagpur and Bhandara districts were selected on the basis of preliminary screening based on the nitrate concentrations found in different villages of the districts. With the Bhandara district being an agricultural center for the farmers and the Nagpur district facing issues with treatment of wastewater, their vulnerability towards nitrate contamination is increased. The Nagpur district is divided into 14 sub-divisions (CGWB 2009). The total area of the Nagpur district is 9892 km2 with an urban area of 364.66 km2 (47.9% of the district) and a rural area of 9657.93 km2 (52.1% of the district) (MSME, Government of India 2012). The total population of the district is 4,653,570 (Census 2011). The urban areas are the places with a municipality, corporation, cantonment board or notified town area committee, etc., comprising a population density of at least 5000 people per 400 km2, while all of the areas not categorized as urban comes in the periphery of rural areas comprising about 68.84% persons out of the total population. It is situated at the geographical center of India surrounding the region being an undulating plateau drained by the Kanhan and Pench rivers in the center, the Wardha River in the west, and the Wainganga River in the east. Except the monsoon season from June to September, hot summer and dryness persist in the district throughout the year. The rural area of Bhandara is inhabited by 80.52% of the population (Census 2011).
Sampling
Drinking water samples from 77 households of rural as well as urban settings of the Nagpur district were collected (Table 2). In total, 231 samples were collected from Savner, Bhandara, Parseoni, Kuhi, Katol, Mahadula, Hingna, Umred, and Kamptee Tehsil and urban colonies of the Nagpur and Bhandara districts (Fig. 2) for post-monsoon and pre-monsoon seasons. The water samples for nitrate analysis were collected in 1-L polyethylene bottles, and the water samples for microbiological testing were collected in 500-mL autoclaved plastic bottles. One milliliter of sodium thiosulfate was added in autoclaved bottles for the removal of any residual chlorine or other halogen from the samples as per method 9060A (APHA 2012). The collected sample bottles were stored at 0–4 °C. All samples were transported to the laboratory within 5 h and tested for the presence of coliform and nitrates within 12 h.
A total of 234 samples of fresh vegetables were also collected from the Nagpur and Bhandara districts in ziplock bags for both the pre- and post-monsoon seasons. Test samples were homogenized and used for nitrate determination.
Determination of nitrates
Chemicals
All the standard chemicals and reagents used were of AR grade. Sodium nitrate (99.0%) was purchased from Alfa Aesar. HCl (38–40%), zinc sulfate (ZnSO4·7H2O), and acetic acid were purchased from Fischer Scientific. Sodium tetraborate, zinc metal, sulfanilic acid, and N-(1-napthyl)-ethylene-diammonium dichloride were purchased from Sigma-Aldrich. Cadmium sulfate and ammonium hydroxide were purchased from Merck.
Nitrate was determined in drinking water samples by a Perkin-Elmer (EL-2373) UV/VIS spectrophotometer by the APHA Method 4500-NO3− B at the 220-nm wavelength (APHA 2012), and in vegetable samples, it was determined using the Association Official Analytical Chemists method (AOAC) official method 993.03 (AOAC 1994).
Determination of total and fecal coliform and turbidity
In order to account for the sanitation risks involved in maintaining the adequate quality of water for drinking purposes, additional parameters as testing of coliform and turbidity were also included in the study. The membrane filter (MF) technique was used for the detection and estimation of total coliforms and fecal coliforms as per APHA (2012) (9222 D/B method). All the values of fecal coliforms were reported as colony-forming units per 100 mL. Turbidity was measured using a nephelometer by Eutech by method 2130 (APHA 2012) and reported in NTU.
Sanitation and health survey
The sanitation and health survey of the population was carried out in some of the areas to explore the variability of nitrate concentration from source to household level. The structured survey was designed based on the water quality, storage, and handling practices. An observational survey was also part of the sanitation survey including identification of potential sources of contamination due to the unhealthy surrounding. In total, a survey of 12 individuals residing in 6 different areas (R16, R17, R19, U13, U20, and U29) was completed. A detailed questionnaire was prepared on seven aspects: general, source, sedimentation tank, storage tank, pipelines, and household storage. The source information was collected for the groundwater well from rural areas while information on sedimentation tank, storage tank, and pipeline leakage was collected from urban areas. A hazard level was then predicted on the basis of the survey comprehending the situation of the area.
Health risk estimation
The acceptable daily intake (ADI) for dietary nitrates is 3.7 mg/kg body weight according to JECFA which accounts to 432 mg/kg maximum nitrate level in vegetables for the daily consumption of 0.5 kg vegetables per person. The estimated daily intake was calculated as
Similarly, for drinking water, estimated daily intake was calculated as
The calculated EDI was then compared with the ADI for the assessment of health hazards associated with the consumption of nitrate-containing vegetables.
Results and discussion
Concentration of nitrate in drinking water samples
The nitrate concentration range in rural and urban areas is presented in Fig. 3. In order to identify the possible sources of nitrate in water, sampling was performed for two different seasons. Nitrate concentration ranged from 1.89 to 134.08 mg/L in rural areas during pre-monsoon and 2.05 to 223 mg/L during post-monsoon (Fig. 4). The average nitrate concentration in urban areas varied from 0.58 to 108.89 mg/L during pre-monsoon and 126.31 to 0.92 mg/L during the post-monsoon season (Fig. 5). Seasonal studies on nitrate pollution have reported nitrate contamination higher in either post- or pre-monsoon season depending on different factors such as non-point source pollution, agricultural practices, and downward flow of nitrate-rich water during heavy pumping. The nitrate pollution in the Suvarnamukhi river basin, Karnataka, increased during the post-monsoon season (October) with non-point sources of pollution being the major factor (Radhika et al. 2011). In our study also, there was a slight difference in nitrate concentration measured during the pre-monsoon season and the post-monsoon season. The average nitrate concentration was higher during the post-monsoon season in both rural and urban areas which can be possibly attributed to the seepage of fertilizers from soil applied by farmers during the rainy season.
The average nitrate concentration of 18 rural study sites was above the permissible limit of 45 mg/L as NO3 with maximum concentration as 189.32 mg/L while only four of the urban locations had average nitrate concentrations above 45 mg/L as NO3 with maximum concentration as 166.48 mg/L. The average mean nitrate concentrations in rural areas were 45.69 ± 2.08 mg/L during pre-monsoon and 53.3 ± 1.07 mg/L during post-monsoon while those in urban areas were 18.51 ± 1.31 mg/L during pre-monsoon and 21.49 ± 1.74 mg/L during the post-monsoon season. The highest average nitrate concentration recorded was 223 mg/L in sample R 13 of rural areas and 126.3 mg/L in sample U 18 of urban areas during the post-monsoon season. The higher concentration of nitrate reported in the various parts of Nagpur is in accordance with the earlier studies conducted in the region. According to Marghade et al. (2011), 75% of shallow aquifer groundwater samples were found to have high concentrations of nitrate above 45 mg/L. The variability of nitrate contamination in shallow aquifers of Nagpur has also been reported by Marghade and Malpe (2014). The groundwater samples from both shallow and deep aquifers were found to contain high nitrate concentration thereby degrading the water quality. The nitrate contamination in various parts of eastern and southern Nagpur city was observed to be contaminated with nitrate affecting the groundwater quality of the region (Afaque 2013).
Comparison of nitrate concentration in drinking water from rural and urban study sites
Figure 4 represents the mean nitrate concentration in the urban and rural areas with 22.53 and 45.69 mg/L nitrate in drinking water. The average nitrate levels of drinking water of rural areas were higher in comparison to urban areas indicating higher level of contamination of drinking water sources in the rural areas. This suggests that the groundwater contributes much of the nitrate contamination as compared to the tap water supply/storage in urban areas. Application of fertilizers in the field and extensive agricultural practices, animal waste dumping, and improper storage and sanitation practices observed in rural areas could be the main causes of higher nitrate concentration. The urban areas fed by a well-maintained water distribution network in contrast to open wells and shallow tube wells in rural areas were subjected to lesser contamination risk indicated by the presence of nitrate within the prescribed limit in the urban areas.
Sanitation survey in the study sites
Studies by different authors have found numerous causes for nitrate contamination in groundwater. Shen et al. (2011) studied nitrate contamination in the Yellow River in China and have reported that high hydraulic conductivity of the thick sandy-loam soil composed of yellow river sediments and anthropogenic waste resulted in contamination of the groundwater. Due to this, the groundwater near the irrigated area was under higher risk of nitrate contamination. Similar studies in India have found that anthropogenic activities like increase in the use of synthetic N fertilizers such as urea, calcium ammonium nitrate, ammonium phosphate, and ammonium sulfate in the agricultural sector have been major contributors to nitrates in the groundwater (Ravikanth et al. 2015). On-site sanitation systems practiced frequently in India for the instant disposal of human waste increase the vulnerability of groundwater contamination with nitrates (Pujari et al. 2007). Changes in cropping pattern, agricultural runoff, intensive livestock farming, excessive use of nitrogenous fertilizers, refuse dump runoff, and contaminated human and animal wastes amount to high nitrate concentration in the groundwater (Reddy et al. 2011; Sahoo et al. 2016; Kumar et al. 2014).
In view of the above studies, possible nitrate contamination sources in the present study areas were identified by conducting the sanitation and health survey. The questionnaire explored the contamination of the source due to the interference of animals; open defecation practices; laundry washing; bathing; effluent entering from poultry, livestock, schools, or houses; condition of the pipe from source to tank; or the garbage left by animals. Further, the questionnaire dealt with identification of the contamination of sedimentation tank, storage tank, pipeline and household storages noticing their condition, use of proper lid and filter media in tank, and maintenance and cleaning of tank. The condition of the pipe, leaking joints or valves, animal fecal matter, and garbage near pipeline in urban areas were observed. The household storage practices that could lead to contamination including poor wash practices such as not washing one’s hands with soap, using unclean storage containers and ladles, and not using an electro-chlorinator were also recorded.
The improper sanitation practices, open wells without fencing to safeguard from animals, open defecation practices, and improper storage practices were frequently noticed in the rural areas of study. Leaked pipelines, sewage discharge, wastewater discharge, and inappropriate waste dumping sites were identified as the possible reasons for contamination of nitrate in the urban study areas.
According to a sanitary survey completed in the following areas, sanitation practices were improper. Handling stored water with dirty ladle or unwashed hands was the major cause. The area nearby groundwater wells was unhealthy in 70% of the studied sites. The storage tank cleaning was not performed at regular intervals. The household practices of storing water were improper, and the vessel used for storing water was not cleaned every alternative day. The household storage practices contributed to 37% of the nitrate contamination followed by source, storage tank, pipeline, and sedimentation tank (Fig. 6).
The improper sanitation practices, open wells without fencing to safeguard from animals, open defecation practices, and improper storage practices were frequently noticed in the rural areas of study. Leaked pipelines, sewage discharge, wastewater discharge, and inappropriate waste dumping sites were identified as the possible reasons for contamination of nitrate in the urban study areas.
Tests of coliform and turbidity were also examined in the above samples to distinguish the gap between sanitation practices followed by the people. The presence of total coliform in the drinking water sample indicates the non-portability as a 100-mL sample should have 0 total coliform count according to the Indian standards.
The presence of coliform in 20 samples (Table 3) indicated the bacterial contamination and potential health risk in the drinking water of the area according to the regulations (Bureau of Indian Standards 2012).
Nitrate concentration in sampled vegetables
Average nitrate concentrations of different vegetables are summarized in Table 4. Nitrate was found in at least one sample of each vegetable. These results were found to be in accordance with the ability of the vegetables to accumulate nitrate. The smallest amount of nitrate was accumulated by carrot and capsicum. Beetroot, spinach, and amaranthus were found to have a maximum average concentration of nitrates (1349.38, 1288.75, and 1007.64 mg/kg).
Calculation of EDI and its comparison with ADI
According to Raczuk et al. (2014), if a daily consumption of 0.5 kg fresh vegetables per person is assumed, maximum nitrate levels in vegetables are 432 mg/kg. Using this as benchmark, beetroot, spinach, amaranthus, and fenugreek all exceeded this threshold ADI limit (Table 5) indicating risk from nitrates. In a study by Qiu et al. (2014) on the relationship of water and nitrate accumulation of vegetables, it has been concluded that there is a positive linear correlation between nitrate and water content in the plants. Higher content of water in tissue, petiole, and shoot sap was associated with higher nitrate accumulation indicating that differences in water content could influence genotypic variation of nitrates in vegetables. The presence of a nitrate reductase enzyme also influences nitrate as, in lettuce (high in nitrate), it has lower activity compared to higher activity in peas (low nitrate level) (Cortesi et al. 2015). These contributing factors may enhance the nitrate content of vegetables thereby exceeding the standard ADI limits. Therefore, much attention is required to minimize the accumulation of nitrates by these vegetables and their consumption at a higher rate.
The calculation of EDI for drinking water samples also confirmed the threat to human health due to excessive nitrate contamination. Nitrate intake with low safety margin was depicted by 22 study sites. The daily water consumption from the rest of the areas were far below the safety margin (ADI: EDI <1) depicting the highest hazard level due to excessive nitrate contamination (Fig. 7). The nitrate contamination level was higher in rural areas as compared to urban settings as the hazard from ingested drinking water in rural areas; according to above results, these areas were more prone to nitrate contamination. This leads to a significant conclusion that groundwater contaminated by nitrate in rural areas has to be monitored and regulated to help mitigate the nitrate contamination in the subsequent areas. Also, none of the water sources which had nitrate concentration from the studied sites were reported to lie within the range of the medium (10–20) or high (20–30) and very high (>30) safety margin, which is noteworthy.
There could be many factors that are responsible for the increasing concentration of nitrates in vegetables. As reported by Abdulrazak et al. (2014), soil type and even agricultural practices could affect the variability in nitrate concentrations in crops. Differences in species, strain, environmental pollution, and light conditions and differing degrees of nitrate absorption result in differing amounts of nitrate concentration in various vegetables (Kharsyntiew et al. 2014).
Thus, our study is in agreement with the studies carried out so far indicating green leafy and highly rooted vegetables to contain a greater concentration of nitrates.
The present study thus affirmed that the drinking water consumed by inhabitants from piped water supply was much safer in comparison to the one from shallow wells and the estimated daily intake of nitrate from drinking water and vegetables in maximum cases was below the safety margin. Therefore, the major contamination sources that may include extensive application of fertilizers in agricultural areas (Almasri and Kaluarachchi 2004) and leachate from waste disposal sites (Vasanthi et al. 2008) should be monitored to minimize the risk of nitrates.
Conclusion
Nitrate pollution of drinking water is widespread, globally affecting the ecosystems and inherent human health. The high amount of nitrates in some of the leafy vegetables also has the potential to increase the health disease burden of humans. The unhygienic sanitation and health practices followed in rural study sites could also amount to greater risk of nitrate contamination which was confirmed by the sanitation survey indicating the higher percentage of area near groundwater being polluted along with unhealthy household water storage practices as the major contributing factors. This article provides an aid to understand the severity of nitrate pollution and the consequent risks associated with planning and control measures to deal with them.
The current study focused on the identification of the extent of nitrate pollution in rural and urban areas of Nagpur and Bhandara regions with risk assessment. The comparison of nitrate profiles in the groundwater and surface water of rural and urban areas depicted significant groundwater contamination of nitrate in rural areas. Among the vegetables, four varieties exceeded the acceptable daily intake level for nitrate. Further, the risk associated with nitrate contamination assessed by a sanitation survey and the EDI-ADI comparison was found to be higher indicating the dire need for preventive measures. Thus, control measures to restrict the entry of nitrates at a larger extent to the humans are necessitated. These preventive measures may include minimizing the application of nitrogenous fertilizers in the agricultural areas, pre-treatment of nitrate-rich water originating from industries and waste dumping sites, practicing healthy water handling and storage and hygienic conditions, promoting growth of crop cover on soil to reduce leaching during the monsoon season, and evolving stringent laws to help protect the nitrate contamination in such areas. The government along with public participation should focus on nitrate-prone areas depicted in the article to help mitigate the nitrate pollution thereby maintaining better sanitation practices.
References
Abdulrazak S, Otie D, Oniwapele YA (2014) Concentration of nitrate and nitrite in some selected cereals sourced within Kaduna state, Nigeria. Online Journal of Animal and Feed Research 4:37–41
Afaque M (2013) Groundwater information, Nagpur district, Maharashtra. Central Groundwater Board, Ministry of water resources, Government of India http://cgwb.gov.in/District_Profile/Maharashtra/Nagpur.pdf. Accessed 11 Feb 2016
Almasri MN (2007) Nitrate contamination of groundwater: a conceptual management framework. Environ Impact Assess Rev 27:220–242
Almasri MN, Kaluarachchi JJ (2004) Assessment and management of long-term nitrate pollution of groundwater in agriculture-dominated watersheds. J Hydrol 295:1–4
Anjana, Umar S, Iqbal M (2007) Nitrate accumulation in plants, factors affecting the process, and human health implications. A review. Journal of Agronomy for sustainable development 27:45–57
AOAC Official Method 993.03 (1994) Nitrate in baby foods, spectrophotometric method. Journal of AOAC Interntional 77:425
APHA (2012) Standard methods for the examination of water and wastewater, 22nd edn. American public health association, San Francisco
Brindha K, Rajesh R, Murugan R, Elango L (2010) Natural and anthropogenic influence on the fluoride and nitrate concentration of groundwater in parts of Nalgonda district, Andhra Pradesh, India. J Appl Geochem 12:231–241
Bureau of Indian Standards (2012) Indian standard: drinking water – Specification (second revision), IS 10500. New Delhi, India
Census, Census of India (2011) http://www.census2011.co.in/ Accessed 22 Dec 2015
Central Groundwater Board (CGWB) (2009) Groundwater information. http://cgwb.gov.in/District_Profile/Maharashtra /Nagpur.pdf Accessed 2 Mar 2016
Central Groundwater Board (CGWB) (2014) Concept note on geogenic contamination of groundwater in India. http://re.indiaenvironmentportal.org.in/files/file/Geogenic%20Final.pdf Accessed 8 Dec 2015
Chakrabarty S, Sarma HP (2011) Fluoride, iron and nitrate contaminated drinking water in Kamrup district, Assam, India. Scholars Research Library Archives of Applied Science Research 3:186–192
Chigor VN, Umoh VJ, Okuofu CA, Ameh JB, Igbinosa EO, Okoh AI (2012) Water quality assessment: surface water sources used for drinking water and irrigation in Zaria, Nigeria are a public health hazard. Environmental monitoring and assessment journal 184:3389–3400
Cortesi ML, Vollano L, Peruzy MF, Marrone R, Mercogliano R (2015) Determination of nitrate and nitrite levels in infant foods marketed in Southern Italy. Journal of food 13:629–634
Costagliola A, Roperto F, Benedetto D, Anastasio A, Morrone A, Perillo A, Russo V, Papparella S, Paciello O (2014) Outbreak of fatal nitrate toxicosis associated with consumption of fennels (Foeniculum vulgare) in cattle farmed in Campania region (southern Italy). Environ Sci Pollut Res 21:6252–6257
Dar IA, Dar MA, Sankar K (2009) Nitrate contamination in groundwater of Sopore town and its environs, Kashmir, India. Arab Journal of Geosciences 3:267–272
ECETOC (1988) Technical report no. 27 on nitrate and drinking water, Brussels, European center for ecotoxicology and toxicology of chemicals. http://www.ecetoc.org/wpcontent/uploads/2014/08/ECETOC-TR-027.pdf
EFSA Panel on Contaminants in the Food Chain (2008) Nitrate in vegetables—Scientific Opinion of the Panel on Contaminants in the Food chain. EFSA J 689(April):1–79
Food Standards Australia NewZealand (2013) Survey of nitrates and nitrites in food and beverages in Australia. http://www.foodstandards.gov.au/consumer/additives/nitrate/documents/Survey%20of%20nitrates%20and%20nitrites%20Attach%201.pdf Accessed 16 Mar 2016
Ganesh KM, Suryanarayana G, Janardhana C (2015) GIS aided assessment of physico-chemical properties of the ground water from the villages of Puttaparthimandal, Anantapur district, Andhra Pradesh (India). Int J Adv Res 3:956–960
Gupta SK, Gupta RC, Chhabra SK, Eskiocak S, Gupta AB, Gupta R (2008) Health issues related to N pollution in water and air. Journal of Current Sciences 94:1469–1477
Indian fertilizer scenario (2010) Department of Fertilizers, Ministry of Chemicals and Fertilizers, Government of India
Jhariya DC, Shandilya AK , Dewangan R (2012). Nitrate pollution in the groundwater around Sagar Town, Madhya Pradesh, India. Paper presented in International Conference on Chemical, Ecology and Environmental Sciences (ICEES'2012) march 17–18, Bangkok pp 151–154
Kharsyntiew LS, Bharose R, Kumar S, Thomas T (2014) An analytical study of nitrate-nitrogen on vegetables from different markets of Allahabad. International Journal of Agricultural Sciences 10:474–476
Kumar R, Singh RD, Sharma KD (2005) Water resources in India. Special section: Water, Current Science 89:794–811
Kumar PJS, Babu PT, Delson PD (2013) Level and distribution of nitrate in groundwater in parts of Vellore district, Tamil Nadu, India. Elixir pollution Journal 55:12782–12784
Kumar PJS, Jegathambal P, James EJ (2014) Chemometric evaluation of nitrate contamination in the groundwater of a hard rock area in Dharapuram, South India. Appl Water Sci 4:397–405
Kundu MC, Mandal B, Hazra GC (2009) Nitrate and fluoride contamination in groundwater of an intensively managed agroecosystem: a functional relationship. Journal of Science of Total Environment 407:2771–2782
Mandanna PK (2011) Consumption of chemical fertilisers in India (1975–2012). Fertiliser Association of India, Ministry of Chemicals and Fertilisers, Government of India, India
Marghade D, Malpe DB (2014) Assessment of extent and variability of nitrate contamination in shallow and deep aquifers of an urban area, India. J Appl Geochem 16:45–63
Marghade D, Malpe DB, Zade AB (2011) Geochemical characterization of groundwater from northeastern part of Nagpur urban, Central India. Journal of Environmental Earth Sciences 62:1419–1430
MSME development institute, Gov. of India, Nagpur 2012. http://dcmsme.gov.in/dips/nagpur%20dips%2012-13.pdf Accessed 7 Jan 2016
NEERI (1991) Water mission report on participation of NEERI. NEERI (National Environmental Engineering Research Institute), Nagpur
Pujari PR, Nanoti M, Nitnaware VC, Khare LA, Thacker NP, Kelkar PS (2007) Effect of on-site sanitation on groundwater contamination in basaltic environment—a case study from India. Environ Monit Assess 134:271–278
Qiu W, Wang Z, Huang C, Chen B, Yang R (2014) Nitrate accumulation in leafy vegetables and its relationship with water. J Soil Sci Plant Nutr 14:761–768
Raczuk J, Wadas W, Głozak K (2014) Nitrates and nitrites in selected vegetables purchased at supermarkets in Siedlce , Poland. Rocz Panstw Zakl Hig Journal 65:15–20
Radhika KN, Rao KLN, Krishnamurthy J, Neelakantarama JM (2011) Nitrate pollution in groundwater: its causes and effects in central part of Suvarnamukhi river basin, Karnataka. J Appl Geochem 13:1–16
Ravikanth P, Sundaraiah R, Sateesh P (2015) Fluoride and nitrate contamination in the groundwater of Kalwakurthy area, Mahabubnagar district, Telangana state, India. Indian Journal of applied research 5:1
Reddy DV, Nagabhushanam P, Peters E (2011) Village environs as source of nitrate contamination in groundwater: a case study in basaltic geo-environment in Central India. Environ Monit Assess 174:481–492
Sahoo PK, Kim K, Powell MA (2016) Managing groundwater nitrate contamination from livestock farms: implication for nitrate management guidelines. Current Pollution Reports 2:178–187
Shen Y, Lei H, Yang D, Kanae S (2011) Effects of agricultural activities on nitrate contamination of groundwater in a Yellow River irrigated region. Water quality: current trends and expected climate change impacts (Proceedings of symposium H04 held during IUGG2011 in Melbourne, Australia, IAHS Pub. 348
Shrimali M, Singh KP (2001) New methods of nitrate removal from water. Environ Pollut 112:351–359
Singh K, Khan YM (2014) Basic necessity for the survival of humans: drinking water quality in rural India. Global journal of finance and management 6:739–744
Suthar S, Bishnoib P, Singh S, Mutiyar PK, Nemaa AK, Patil NS (2009) Nitrate contamination in groundwater of some rural areas of Rajasthan, India. J Hazard Mater 171:189–199
Thomson B (2004) Nitrates and nitrites dietary exposure and risk assessment. Client report FW0392. Institute of environmental science and research limited, Christchurch Science Center, New Zealand, p 5
Thomson BM, Nokes CJ, Cressey PJ (2007) Intake and risk assessment of nitrate and nitrite from New Zealand foods and drinking water. Food Additives and Contamination Journal 24:113–121
Tiwari RN (2011) Assessment of groundwater quality and pollution potential of Jawa block, Rewa district, Madhya Pradesh, India. Proceedings of the International Academy of Ecology and Environmental Sciences 1:202–212
Vasanthi P, Kaliappan S, Srinivasaraghavan R (2008) Impact of poor solid waste management on groundwater. Environmental Monitoring Assessment 143:227–238
Vough L, Cassel E, Barao S (2006) Nitrate poisoning of livestock, North Dakota State University 839, 2–5. Available from: www.ag.ndsu.edu Accessed 4 Apr 2016
Wakida FT, Lerner DN (2005) Non-agricultural sources of groundwater nitrate: a review and case study. Journal of Water Research 39:3–16
Ward MH, Kilfoy BA, Weyer PJ, Anderson KE, Folsom AR, Cerhan JR (2010) Nitrate intake and the risk of thyroid cancer and thyroid disease. J Epidemiol 21:389–395
WHO (World health organization) (2011) Guidelines for drinking water quality, Fourth edn. World health organization, Geneva
Ziarati P (2012) Assessing the health risk of nitrate content in vegetables to the general population in Tehran-Iran. Open Access Scientific Reports 1:4
Acknowledgements
The authors would like to acknowledge the efforts by the knowledge resource center of CSIR-NEERI for authenticating the manuscript by checking plagiarism through iThenticate software and providing the manuscript no. CSIR-NEERI/KRC/2017/JAN/WTMD/2.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOCX 38 kb)
Rights and permissions
About this article
Cite this article
Taneja, P., Labhasetwar, P. & Nagarnaik, P. Nitrate in drinking water and vegetables: intake and risk assessment in rural and urban areas of Nagpur and Bhandara districts of India. Environ Sci Pollut Res 26, 2026–2037 (2019). https://doi.org/10.1007/s11356-017-9195-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9195-y