Abstract
Processed milk waste (MW) presents a serious problem within the dairy industries due to its high polluting load. Its chemical oxygen demand (COD) can reach values as high as 80,000 mg O2 L−1. This study proposes to reduce the organic load of those wastes using thermal coagulation and recover residual valuable components via fermentation. Thermal process results showed that the COD removal rates exceeded 40% when samples were treated at temperature above 60 °C to reach 72% at 100 °C. Clarified supernatants resulting from thermal treatment of the samples at the temperatures of 60 (MW60), 80 (MW80), and 100 °C (MW100) were fermented using lactic acid bacteria strains without pH control. Lactic strains recorded important final cell yields (5–7 g L−1). Growth mediums prepared using the thermally treated MW produced 73% of the bacterial biomass recorded with a conventional culture medium. At the end of fermentation, mediums were found exhausted from several valuable components. Industrial scale implementation of the proposed process for the recycling of industrial MWs is described and discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental and economic concerns associated with food waste have recently raised the public awareness. The dairy industry is a major part of the food industry that generate high amount of wastewater (Kosseva 2011). Noncompliant products, unusable milk due to processing errors, as well as milk that has been rinsed from the tanks, lines, etc., are handled as waste (MedTest 2012; Russ and Meyer-Pittroff 2004). In some developing countries, along the marketing chain, milk loss is mainly due to spillage and spoilage. These losses are occasioned by poor access to markets, poor milk handling practices, and irregular power supply in milk processing plants (Lore et al. 2005). For developing regions, waste of milk during postharvest handling and storage, as well as at the distribution level, is relatively high (Gustavsson et al. 2011). In Tunisia, there are about 61 dairy industries ensuring an average of 465 million liters of drinking milk per year (APII 2014). Processing losses in the factory may most likely reach 3% (MedTest 2012). Another important cause of food loss is the stock removed from retail shelves because it has reached its “sell-by” date. Such losses chiefly apply to fresh perishable items. Nearly half of these retail losses came from fluid milk and other dairy products (Kantor et al. 1997). The withdrawal of damaged products or those over their sell-by date from the market creates an important amount of dairy wastes that is unsuitable for sale. Nevertheless, limited indications are generally reported in the literature about wasted products.
According to the World Bank Group, the waste load equivalents of specific milk constituents are as follows: 1 kg of milk fat is equivalent to 3 kg of COD, 1 kg of lactose is equivalent to 1.13 kg of COD, and 1 kg protein is on average equivalent to 1.36 kg of COD (Group 1999). This would make the wastewater polluting index much higher than the specified standards when residual dairy products join the plant wastewater. Given the environmental implications of increasing processed dairy manufacture, the treatment of residual products has to be considered. Although in some cases, it could be used for animal feed (Scholten et al. 1999), their high water content is a limiting factor (Alonso et al. 2010). To meet stringent effluent discharge criteria, aerobic biological treatments relying on conventional activated sludge plants are generally employed (Tocchi et al. 2013). Membrane, chemical, and simultaneously physical-chemical methods were investigated to reduce the dairy wastewater organic load. However, these processes have an inherent drawback due to high operating costs from either the use of external acid sources or flocculating agents (Seesuriyachan et al. 2009). Thus, a growing effort from the research community and industries has been observed recently to develop efficient technologies for the treatment and/or reuse for dairy wasted products. Biological processes have been proved to be suitable cost-effective mean of pollution removal from dairy industry organic waste (Kasmi 2016). In this context, Seesuriyachan et al. (2009) have performed dairy wastewater bio coagulation using Lactobacillus casei for protein recovery. Meanwhile, Alonso et al. (2010) have fermented yoghurt whey from expired date products using Lactobacillus casei for lactic acid production. Combined physical-chemical and fermentation processes using Candida strains were proposed to treat and reuse residual fermented dairy products with effective results (Kasmi et al. 2016).
This study recommends an upstream segregation of milk wastes (hereinafter MWs) in order to be treated separately from the dairy wastewater plant. A pretreatment process is proposed using thermal coagulation and decantation of the MWs to separate concentrated caseins and fat. Resulted wheys were clarified and their use as growth medium for lactic acid bacteria was investigated. The MW reuse as growth medium was performed using Lactobacillus plantarum and Lactococcus lactis ssp. lactis strains fermentation separately. Valuable component recovery was also assessed.
Material and methods
Sampling
Processed MW samples were collected from a nearby dairy industry. Processed MWs are either the products discarded from the production lines and designed as defective products, or the returned products collected by the dairy plant from wholesalers and distributors because of packaging defect or expired shelf life. In the plant, the defected dairy products are compacted and cardboard packaging is separated. Then, the dairy effluent was collected in a storage tank to be discharged in the plant sewage at low production periods. Sampling was performed from the plant collection tank at different periods of a working day. Treatment assays were performed within a few hours.
Sample treatment
Thermal coagulation was performed in glass vessels containing 1 l of the MW sample. Vessels are emerged in a controlled temperature heating bath. Heat treatment was performed at different bearing temperatures (20, 40, 60, 80, and 100 °C). Exposure time was extended to 15 min in a way to optimize thermal coagulation (preliminary tests are not shown). Thereafter, samples were decanted in conic devices. Volume of settleable matter in the cone as mL L−1 was recorded at regular time intervals, and decantation is considered to be completed when constant values are obtained. Then, supernatants were poured out and filtrated to separate solid-liquid fractions. COD values of both supernatants and filtrates were assessed.
Growth medium preparation: Supernatants clarification
The recuperated MW supernatants were clarified to remove residual fat and caseins according to the protocol proposed by Moulin et al. (1976). The supernatants pH was set to 4.6 (caseins isoelectric point) with HCl (1 N); then, they were sterilized for 5 min at 102 °C. After cooling, samples were filtrated (using Whattman filter). Filtration was repeated several times until clear serum was obtained. The filtrate pH was adjusted to 7 with NaOH solution (1 N) and then sterilized at 120 °C for 20 min.
Microorganism
Lactobacillus plantarum and Lactococcus lactis ssp. lactis strains were obtained from the Laboratory of Microbiology in the Water Researches and Technologies Center (Tunisia). Strains were maintained frozen (in 40% v/v glycerol at −20 °C) until use. These two strains were cultured on MRS broth (de Man Rogosa and Sharpe, Biolife, Italy) or in solid plates (containing 1.5% w/w agar) incubated at 37 °C.
Microbial inoculum preparation and culture conditions
A loopful of Lb. plantarum and Lc. lactis grown on MRS agar plates was used to inoculate separately, 100-mL Erlenmeyer flasks containing each one 50 mL of MRS broth media. Flasks were incubated at 37 °C in a shaker at 150 rpm for 18 h. The obtained actively grown cells were used as a seed culture to inoculate 250 mL 6% (v/v) containing 100 mL of pretreated whey adjusted at pH 6. All inoculated Erlenmeyer flasks were incubated at 37 °C under mechanical agitation at 150 rpm for 24 h. All experiments were performed in triplicate.
Bacterial growth
Bacterial growth was monitored through the optical density measure at 600 nm (Thermo Spectronic UV1 equipped with VISIONlite™ software). Each culture was centrifuged for 15 min at 4000 rpm, and subsequently, the pellet was used to determine cell dry weight by gravimetric analysis after drying at 105 °C in an oven to constant weight. Cell dry mass is expressed in g L−1. The total cell count (CFU mL−1) was determined using a standard agar plate technique. The appropriately diluted samples were plated on MRS agar beforehand prepared; inoculated plates were incubated at 37 °C for 48 h to get separated colonies.
Analytical methods
pH, conductivity (mS cm−1), and TDS (g L−1) of the treated samples were determined at different points during heating using multiparameters device Consort C860. To estimate MW heat coagulation efficiency during heat treatment, the chemical oxygen demand (COD) of supernatants was measured using the potassium dichromate colorimetric method by an opened reflux system as reported by Rodier et al. (2009). MW whey characterization was performed through mineral content assessments as follows: Phosphorous content was determined using molybdenum blue spectrophotometric method (ISO 8556:1986), calcium concentration was determined using EDTA method, and magnesium content was determined using hydrotimetric method (Rodier et al. 2009). Iron was measured using an atomic absorption spectrophotometer (Rodier et al. 2009). Chloride content was determined using the Sherwood analyzer Model 926. Sodium and potassium content was determined using flame photometric method. Ammonium was determined using titrimetric dosage method (Rodier et al. 2009). Reducing sugars were quantified using Bertrand method based on cuprometric titration after sample defecation (AFNOR 1971). The protein content was obtained using the Kjeldahl nitrogen determination method, considering 6.38 as the protein conversion factor (Rombaut and Dewettinck 2007). The titratable acidity was measured by titration with 1 N NaOH in the presence of phenolphthalein, and it is expressed in the amount of lactic acid (grams) in the sample (AFNOR 1980). Ash content was determined by gravimetric difference after ashing following AOAC method 945.46 and using high-temperature muffle furnace. Total fat analysis was done according to the Gerber Method based on acido-butyrometric determination that consists on an attack of whey with sulfuric acid and separation of the released fat using Nova Safety Gerber centrifuge in the presence of isoamyl alcohol (AFNOR 2001).
Statistical analysis
MW whey characteristics were analyzed statically by the one-way ANOVA. The test of Student-Newman-Keuls was applied to determine the least significant differences (LSD) among means at P < 0.05 using IBM® SPSS version 20.0.0 software.
Results and discussion
Thermal coagulation and pH variation
Heat treatment was performed for MW samples. The parameters of pH, conductivity, and total dissolved solids (TDS) evolution under temperature effect are illustrated in Fig. 1a–c, respectively. MW samples showed an initial pH value of around 4.6, very close to the casein isoelectric point (4.6) knowing that the pH of milk varies in the range of 6.6 to 7.0.
Colloidal and serum caseins are in an equilibrium that is dependent on pH and temperature (Newstead et al. 1977). Casein micelles in milk are stable at the pH of milk, due to the stabilizing hairy brush of κ-casein (Vasbinder 2002). So, it can be concluded that MW samples had already destabilized casein structure. Through the pH profile, it was noticed that the pH of milk decreased as its temperature increased as confirmed in a previous study (Lucey and Horne 2009). Besides, two ways of acid-induced gelation were studied in the literature: T-pH and pH-T routes. The gelation route applied affected the gelation kinetics and the final gels (curds) obtained (Vasbinder et al. 2003). MW thermal coagulation in this process matches to the pH-T route where pH was initially low; then, heat treatment was performed.
As opposed to pH tendency, conductivity as well as total dissolved solids showed an increasing profile with temperature. Both conductivity and TDS followed a linear development shape. This might be explained by calcium concentration increase in the treated MW. In fact, the main salt ions present in milk are potassium, sodium, calcium, magnesium, chloride, and phosphate, of which calcium and phosphate are highly relevant for maintaining the integrity of the casein micelles. Caseins in the micelles are linked to calcium phosphate nanoclusters. Temperature and pH affect the equilibrium of calcium and casein present in the micelle and in the serum (Vasbinder 2002). Due to heating, calcium phosphate precipitates. However, this process is reversible and the original calcium concentration could be restored. Therefore, heat treatment causes a temporary decrease in the serum calcium concentration (Schreiber 2001). However, decreasing pH causes a release of casein and calcium into the serum (Vasbinder 2002). Thus, TDS and conductivity increase associated with pH decrease in the treated milk were probably attributed to calcium release into the serum.
Curd separation and supernatants (whey) recuperation
Following heat treatment and cooling to room temperature, samples were allowed to settle, until the steady curd (settled matter) formation. As such, supernatants could be simply poured or removed by centrifugation.
Samples treated at 20 and 40 °C presented a thin layer of supernatant even after 24 h of decantation. Supernatants were cloudy and hard to separate. This will cause clogging problems during clarification. Hence, it will be unprofitable to consider those samples. According to the treated MW samples at temperatures above 60 °C, the resulting supernatants were clear and they could be easily separated. Within 30 min of settling, the curd has occupied 70% of the sample volume. At 60 min, it represented 40% of the total volume, but after 24 h, the minimum level reached 25 and 10% for samples treated at 80 and 100 °C, respectively. It has been reported that pH-T route resulted in stronger curds with less serum separation (Vasbinder et al. 2003). A higher temperature increased clearly the dry mass content of the curd. Those results are in agreement with previous findings where maximum whey clarity corresponding to a minimum OD (optic density) was obtained following a heat treatment at 80 °C. Obtained curds could be destined to be incorporated in animal feed.
COD removal efficiency
Only samples treated at temperatures above 60 °C are considered for further investigations. To examine thermal coagulation efficiency on organic load removal of MW samples, supernatants, filtrates, and clarified filtrates were collected for COD assessment (Fig. 2). The raw MW COD was around 80,000 mg O2 L−1. Beyond 60 °C, recorded supernatant removal rates were nearby 20 and 22% following 60 and 80 °C thermal treatment, respectively. However, with the MW sample treated at 100 °C, COD removal rate was relatively important (53.3%). Thermal treatment was pushed further to investigate greater thermal treatment temperature effect on COD removal. At 110 °C, supernatant COD value was very close to that recorded with MW100.
This finding was discussed in previous works, and it was interpreted by the changes in the environment of one or more of the aromatic residues (Garrett et al. 1988) which might cause a drop in the samples organic compounds content. Nevertheless, Fauquant et al. (1985) concluded from their study that the temperature of 80 °C appeared to be a threshold value and beyond it, a sharp drop of the total nitrogen content is recorded in the clarified whey. This fact is explained by a significant distortion of some soluble proteins. Indeed, upon heat treatment of milk above 60 °C, several processes could take place, of which denaturation of whey proteins is the most obvious (Oldfield et al. 2000). Research on heat treatment of milk showed that the final composition of the mixture of whey proteins and casein micelles depends on the pH and the temperature of heat treatment (Law and Leaver 2000). After heating at around 90 °C, more than 95% of all the β-immunoglobulin and almost 80% of all the α-lactalbumin present in the milk are denatured. Almost 25% of both proteins remains in the supernatant, while 65% of all β-immunoglobulin and 50% of all α-lactalbumin are found in the curd and thus was associated with the casein micelle (Vasbinder 2002). The filtration process has allowed COD removal rate improvement reaching 28, 36, and 69.7% for MW60, MW80, and MW100 filtrates, respectively. This result was mainly attributed to the important proportion of proteins and fat content removal resulting from thermal coagulation and filtration processes. It is interesting to mention that the most important removal rate was recorded with MW100. The induced heat effect on soluble protein for samples treated at 100 °C might explain the COD values shift for MW100. Nevertheless, filtrates clarification, mainly performed to remove residual fat and caseins, revealed a moderate contribution to COD values lowering. In fact, clarification has allowed COD value decrease of around 17% for MW60 filtrate, 13% for MW80 filtrate, and only 8% for MW100 filtrate. The final recorded COD removal rates were 40, 44, and 72.1% for resulting whey previously treated at 60, 80, and 100 °C, respectively. It appears that thermal coagulation process has the most important impact on the raw MW COD removal. The thermal treatment process contribution to the total removal rate was about 50% for MW60 and MW80. However, it was about 74% for MW100 with a firm resulting curd and clear supernatant. In the meanwhile, it appears that clarification process contribution to the COD removal is as important as the thermal treatment temperature is low.
Fermentation
Characterization of the clarified whey
In the aim to determine heat coagulation temperature effect on the quality of the collected liquid fraction, the clarified wheys (serums) resulting from MW samples previously treated at 60, 80, and 100 °C (hereinafter CMW60, CMW80, and CMW100) as described previously in the paragraph 2.3. were characterized. Table 1 presents different serums compositions. The described heat treatment and clarification processes of MW yielded yellowish serums with high contents of sugars (33.7–38 g L−1). No substantial difference was recorded between serums in terms of sugar content (P < 0.05). The yellowish color of whey is caused by the presence of riboflavin known as vitamin B2 (De Wit 2001). The majority of milk lactose remains in the liquid fraction, constituting the main fraction of the organic load (Ghaly and Kamal 2004). Fat and protein contents are also partially responsible of organic contamination (Prazeres et al. 2012). However, after fat removal, the whey protein content is in the range of 3.2–9.2 g L−1. Significant difference was recorded with CMW100 protein content (3.2 g L−1) compared to CMW60 and CMW80 (9.2 and 8.9 g L−1). This result is probably attributed to the importance of protein denaturation at 100 °C, since at such temperature, destabilized proteins aggregate, and they are no longer soluble in the whey. Besides, the inorganic contamination of whey is attributable to the presence of mineral salts as reported in the literature (Venetsaneas et al. 2009), principally sodium (4.1–6.2 g L−1), potassium (about 2 g L−1), chlorides (about 1.2 g L−1), and calcium (1.6–7.0 g L−1). The high mineral content in acid whey is due to the dissolution of calcium and phosphorous from casein micelles during acidification down to pH 4.6 (De Wit 2001). The whey calcium content was slightly changed due to the heat treatment from 60 to 100 °C as reported by Fauquant et al. (1985) where thermocalcic clarification of whey was studied. Thermal treatment at different temperatures affected significantly protein, potassium, calcium, sodium, magnesium, ammonium, and ash content (P < 0.05) in the resulting MW serums. However, no significant effect was noticed for sugar, iron chlorides, and phosphorous concentrations.
From the obtained results, it appears that clarified thermally treated MW cannot be directly discharged in the environment without further treatment and/or valorization, since it is a nutrient-rich effluent (Prazeres et al. 2012). One of the promising ways to use the lactose in whey is to use it as a low-cost carbon source for the production of organic acids by fermentation using lactic acid bacteria (Fu and Mathews 1999). It was well-known that for maintaining a good bacterial growth, culture media are generally provided with adequate amounts of minerals using yeast extract. The yeast extract contains specially (in μg g−1 dry weight of yeast extract), Fe (150), Cu (71), Mg (1270), and Zn (74) (van Niel and Hahn-Hägerdal 1999). The initial chemically defined media realized by Aller et al. (2014) contained eight trace minerals from which magnesium proved to be essential. It has been demonstrated by MacLeod and Snell (1947) that potassium ion is also required in large amounts compared to Mg and Mn minerals by all lactic acid bacteria. MW serums composition showed clearly that mediums contain valuable nutrients (sugar, ammonium, magnesium, potassium, etc.) that could be recovered through lactic acid bacteria fermentation.
Clarified MW fermentation using Lc. lactis and Lb. plantarum
Bacterial growth
The main carbon energy source spectrum accessible for lactic acid bacteria (LAB) has been widened significantly. Reports of new possible substrates are frequently published, and the utilization of industrial side streams is a growing trend (Taskila and Ojamo 2013). Among lactic acid bacteria, Lc. lactis ssp. lactis and Lb. platarum have been the most studied in terms of physiology, genetics, and applications (Samaržija et al. 2001). This is likely to be the result of their major importance as starter cultures in the production of fermented food. The major functions of these two species in dairy fermentation are the production of lactic acid from lactose, hydrolysis of casein, and citric acid fermentation (Samaržija et al. 2001). Lc. lactis ssp. lactis is used extensively in the dairy industry as a starter culture for a variety of products. To investigate the effect of MW, different treatment temperatures on the lactic acid bacteria fermentation, CMW60, CMW80, and CMW100, were inoculated separately with Lc. lactis ssp. lactis and Lb. platarum strains. At the end of the fermentation, bacterial growth, cell viability, and titratable acidity were evaluated. Results summarized in Table 2 show that Lc. Lactis ssp. lactis exhibited a better cell growth compared to Lb. plantarum with the different fermented serums. Important bacterial growth associated with important lactic acid production was recorded with the fermented mediums previously treated at 60 and 80 °C. The best bacterial growth rates were recorded with CMW60 where Lc. lactis ssp. lactis and Lb. plantarum biomass concentrations were of 7.2 and 5.3 g L−1, respectively. Both strains grown present a slight decrease for CMW80 (6.8 and 5.1 g L−1, respectively). However, biomass concentrations with CMW100 were limited to 4.3 g L−1 for Lc. lactis ssp. lactis and 4.0 g L−1 for Lb. plantarum. Furthermore, it is worthy to mention that the titratable acidity (TA) production increase is attributed to the lactic acid bacteria population increase. Lactic acid production recorded using Lc. lactis ssp. lactis and Lb. plantarum was respectively 10.2 and 7.8 g L−1 with CMW60 and 8.6 g L−1 equally with CMW80. An average acidity production decrease of 24 and 34%, respectively, was observed with CMW100. It has been demonstrated that Lc. lactis ssp. lactis is capable of sustainable growth in extreme levels of stress, compared to other lactic acid strains (Kim et al. 1999). In fact, the microorganism has an enzymatic system that hydrolyzes the disaccharide and then metabolizes the glucose using the glycolytic pathway (Cock and de Stouvenel 2006). However, Lc. lactis ssp. lactis requires certain metabolites in the growth medium although it has a genetic potential to synthesize some of them. Synthetic medium for Lc. lactis ssp. lactis should contain at least six amino acids (isoleucine, valine, leucine, histidine, methionine, and glutamic acid) and seven vitamins (biotin, pyridoxal, folic acid, riboflavin, nicotinamide, thiamine, and pantothenic acid) (Reiter and Oram 1962). All the amino acid requirements are naturally present in milk at sufficient rates (Payne-Botha and Bigwood 1959). Both essential and nonessential amino acids were similarly affected by autoclaving, 30% lower than in controls (Yeung et al. 2005). Strain growth and cell viability seem to be not affected by this deficit, since the number of viable cells was as important as the final cell concentration obtained with the minimal growth medium containing glucose, acetate, vitamins, and eight amino acids yielding around 1.6 × 109 cells mL−1 (Jensen and Hammer 1993). However, this amino acids and vitamin deficit has slightly affected Lactobacillus plantarum growth in CMW60 and CMW100. Lb. plantarum cultivated on CMW80 cell density was in the order of 1010 CFU mL−1. Similar results have been achieved with nutrient requirements improved medium by Saguir and de Nadra (2007). Although Lb. plantarum is more resistant towards lactic acid than many other microorganisms, its growth is still strongly inhibited by the high concentrations of this organic acid reached during fermentation (Russell and Diez-Gonzalez 1997). Besides, as the presence of lactic acid influences the growth rate of the microorganism, changes in gene expression are expected that are not caused by the primary effect of the sodium lactate but by the secondary effect of an altered absolute or relative growth rate (Pieterse et al. 2005). This behavior could explain bacterial growth limitation on CMW60 with Lb. plantarum fermentation compared to Lc. lactis ssp. lactis. Since these experiments were under uncontrolled conditions, pH values were dropped with lactic acid accumulation. Such factors were probably responsible for a great reduction of the stationary phase length that decreased in the decline phase due to product inhibition (Fu and Mathews 1999).
Valuable components recovery
The recorded consumption rates using both LAB strains are illustrated in Fig. 3. Important amounts were obtained for iron (44–79%) and calcium (44–67%). Less important consumption rates were recorded for magnesium (2–34%), sodium (1–36%), and potassium (2–10%). Lc. lactis ssp. lactis strain minerals consumption rates were always more important than Lb. plantarum consumption for the same culture medium, except for potassium. This comes perfectly in proportion with Lc. lactis ssp. lactis growth and organic acid production compared to Lb. plantarum recorded data. Both strains presented slight difference in iron, calcium, and magnesium consumption rates for CMW60 and CMW80, since those media presented initially very similar composition, compared to CMW100 medium composition.
In the literature, information about mineral assimilation behavior and metabolism of those strains is barely available. Thus, Lb. plantarum has been found to increase absorption of iron (Fe) from normally low iron bioavailability meals (Bering et al. 2007). This fact may be in relation with its aptitude to iron assimilation that exceeded 65% with CMW80. Nevertheless, Lc. lactis iron consumption rate in the same culture medium reached 75%. Such minerals assimilation aptitude might need further investigations of the tested strains abilities, especially since it was reported that Lb. plantarum does not require iron (Archibald 1983).
Industrial scale of the milk waste recycling process
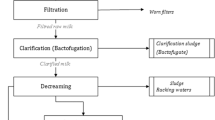
The proposed process for the treatment and reuse of MW could be implemented in an industrial scale. The approach of the industrial treatment process scale up supposes that any milk processing plant is considered as a unit to which a fermentation plant could be connected to produce valuable products using the resulting whey from thermally pretreated milk residuals. Other dairy product factories could be considered because of their residual product whey such as yoghurt or fermented milks (Alonso et al. 2010; Kasmi et al. 2016). Several studies showed significant economic, environmental, and social benefits resulting from the reuse of dairy residuals. Therefore, the reuse unit in stand-alone plant located close to dairy industries areas could be considered. If applied in small-scale plant, this approach has the advantage of modest investment cost and low environmental impact. Nevertheless, the highly loaded dairy wastes could be collected in a common collect center to be processed by the proposed bioconversion plant for lactic acid and/or LAB production. Figure 4 illustrates the industrial scale MW recycling process flowchart. Collected milk residuals have to be homogenized (mixed-up) to maintain an input product quality as stable as possible for thermal coagulation. Thermal pretreatment could be performed in a temperature-controlled tank at 60 °C for 15 min. For energy-saving reasons, the desired temperature could be reached using the steam generated by the industrial boiler if the recycling unit is connected to the milk processing factory. Whey separation could be performed using centrifuge decanter. The recuperated curd containing mainly proteins and fat will be destined for animal feed (Scholten et al. 1999). Separated whey has to be clarified to stabilize the fermentation medium. Clarification could be performed as described previously via successive pH adjustment, filtration, and sterilization according the protocol proposed by Moulin et al. (1976).
For the clarified whey fermentation, LAB inoculum should be previously prepared in laboratory scale. Several stages of inoculum growth can be employed starting with a shake flask culture, then to a small laboratory fermenter (10 L of medium), followed by a first-stage fermenter (100–1000 L of medium), then to a second-stage fermenter (10,000 L of medium), and finally to 100,000 L of medium or greater volume in a production scale fermenter (Litchfield 2009).
Temperatures during fermentation can be controlled by cooling water coils immersed in fermenter vessels. Centrifugal separation or membrane processes can be used for harvesting cells from the medium. Centrifugation is mostly used on an industrial scale because of the low viscosity of the medium, the properties of the cells, and the large cell size. The temperature is usually kept between 5 and 15 °C during harvesting depending on the strain (Porubcan and Sellars 1979). Membrane separation technics are widely used to produce concentrated LAB biomass. An approximate 12-fold concentration can be reached by ultrafiltration without any cell damage caused by heat developing during the process (Mäyra-Mäkinen and Bigret 2004).
It is worthy to mention that the fermentation process could be optimized in a way to promote lactic acid production with cell recycling. Reusing the lactic acid bacterial cells from a batch culture has been investigated for inoculating a subsequent culture as an alternative to conventional inoculum development (Litchfield 2009). Besides, a such raw material characteristics like low cost and availability for whole year are highly required for the commercial production of lactic acid (Randhawa et al. 2012). Then, biotechnical production of lactic acid may be based on several alternative microorganisms. In commercial scale of lactic acid bacterial processes, the cells are kept in suspension in the production medium by mixing with mechanical agitators or circulating the medium from the bottom to the top of the fermenter by pumping. It was pointed out previously that Lactobacillus spp. require low oxygen concentrations for growth and lactic acid production (Litchfield 2009). The recovery of lactic acid must be improved in order to reduce lactic acid losses and to increase purity (González et al. 2008). Purification or product recovery is an important step in production of lactic acid that is associated with separation and purification of lactic acid from fermentation broth. Lactic acid can be separated and substantially purified from fermentation broths by several membrane-based unit operations (Ghaffar et al. 2014).
Consequently, the proposed MW recycling process either oriented for LAB production or for lactic acid production generates not only valuable products with considerable economic interest but also exhausted fermented medium with reduced organic load.
Conclusion
Processed MW contains important concentrations of sugar, proteins, and minerals that contribute to its polluting load increase (80,000 mg O2 L−1). Thermal coagulation treatment at 60, 80, and 100 °C, filtration, and clarification processes have resulted in important COD removal rates, reaching 72.1%. The major contribution to the COD removal was recorded using thermal treatment at different temperatures. Nevertheless, temperatures above 90 °C strongly affected whey protein availability. Recuperated serums contain lactose (33.7–38 g L−1), proteins (3.2–9.2 g L−1), and minerals that could be recovered through bacterial biomass production. CMW60, CMW80, and CMW100 were fermented separately Lc. lactis ssp. lactis and Lactobacillus plantarum, without pH control. Therefore, with CMW60 and CMW80, Lc. lactis ssp. lactis and Lb. plantarum recorded better final cell yields (of around 7 and 5 g L−1, respectively) compared to the yields obtained with CMW100. These results were in agreement with viable cells (around 1010 CFU mL−1). Valuable components of the MW have been recovered through cell biomass production. Mineral consumption rates in the medium were around 79% for iron, 67% for calcium, and 34% for magnesium by Lc. lactis ssp. lactis and around 66% for iron, 54% for calcium, and 27% for magnesium by Lb. plantarum. Thermal coagulation pretreatment and resulting whey LAB fermentation approach constitute a promising alternative for MW reuse at an industrial scale.
References
AFNOR (1971) Lait. Détermination de la teneur en lactose NF V 04–213
AFNOR (1980) Lait. Détermination de la matière sèche. NF VO4 207. Recueil de normes françaises. Laits et produits laitiers. Méthodes d’analyse, AFNOR. Normalisation française, Paris, pp 33–34
AFNOR (2001) Lait - Détermination de la teneur en matière grasse - Méthode gravimétrique (méthode de référence). NF EN ISO 1211, pp 21
Aller K, Adamberg K, Timarova V, Seiman A, Feštšenko D, Vilu R (2014) Nutritional requirements and media development for Lactococcus lactis IL1403. Appl Microbiol Biotechnol 98(13):5871–5881
Alonso S, Herrero M, Rendueles M, Diaz M (2010) Residual yoghurt whey for lactic acid production. Biomass Bioenergy 34(7):931–938
APII (2014) Les Industries Agroalimentaires en Tunisie. Agency for the Promotion of Industry and Innovation, Report
Archibald F (1983) Lactobacillus plantarum, an organism not requiring iron. FEMS Microbiol Lett 19(1):29–32
Bering S, Sjoltov L, Wrisberg SS, Berggren A, Alenfall J, Jensen M, Hojgaard L, Tetens I, Bukhave K (2007) Viable, lyophilized lactobacilli do not increase iron absorption from a lactic acid-fermented meal in healthy young women, and no iron absorption occurs in the distal intestine. Br J Nutr 98(5):991–997
Cock LS, de Stouvenel AR (2006) Lactic acid production by a strain of Lactococcus lactis subs lactis isolated from sugar cane plants. Electron J Biotechnol 9(1):40–45
De Wit JN (2001) Lecturer’s handbook on whey products, 1st edn. EuropeanWhey Products Association, Brussels, Belgium
Fauquant J, Vieco E, Brule G, Maubois JL (1985) Clarification des lactosérums doux par agrégation thermocalcique de la matière grasse résiduelle. Lait 65(647–648):1–20
Fu W, Mathews AP (1999) Lactic acid production from lactose by Lactobacillus plantarum: kinetic model and effects of pH, substrate, and oxygen. Biochem Eng J 3(3):163–170
Garrett JM, Stairs RA, Annett RG (1988) Thermal denaturation and coagulation of whey proteins: effect of sugars. J Dairy Sci 71(1):10–16
Ghaffar T, Irshad M, Anwar Z, Aqil T, Zulifqar Z, Tariq A, Kamran M, Ehsan N, Mehmood S (2014) Recent trends in lactic acid biotechnology: a brief review on production to purification. J Rad Res Appl Sci 7(2):222–229
Ghaly AE, Kamal MA (2004) Submerged yeast fermentation of acid cheese whey for protein production and pollution potential reduction. Water Res 38(3):631–644
González MI, Alvarez S, Riera FA, Álvarez R (2008) Lactic acid recovery from whey ultrafiltrate fermentation broths and artificial solutions by nanofiltration. Desalination 228(1):84–96
Group WB (1999) Dairy industry. Pollution prevention and abatement handbook, Report. World Bank Publications, Washington, pp 295–297
Gustavsson J, Cederberg C, Sonesson U, van Otterdijk R, Meybeck A (2011) Global Food Losses And Food Waste. FAO
Jensen PR, Hammer K (1993) Minimal requirements for exponential growth of Lactococcus lactis. Appl Environ Microbiol 59(12):4363–4366
Kantor LS, Lipton K, Manchester A, Oliveira V (1997) Estimating and Addressing America’s Food Losses. Food Review
Kasmi M (2016) Biological processes as promoting way for both treatment and valorization of dairy industry effluents: a review. Waste Biomass Valor. doi:10.1007/s12649-016-9795-9797
Kasmi M, Hamdi M, Trabelsi I (2016) Eco-friendly process combining physical-chemical and biological technics for the fermented dairy products waste pretreatment and reuse. Water Sci Technol:1–9. doi:10.2166/wst.2016.477
Kim WS, Ren J, Dunn NW (1999) Differentiation of Lactococcus lactis subspecies lactis and subspecies cremoris strains by their adaptive response to stresses. FEMS Microbiol Lett 171(1):57–65
Kosseva MR (2011) Management and processing of food wastes. In: Moo-Young M (ed) Comprehensive Biotechnology, Environmental Biotechnology and Safety, Elsevier
Law AJR, Leaver J (2000) Effect of pH on the thermal denaturation of whey proteins in milk. J Agr Food Chem 48(3):672–679
Litchfield JH (2009) Lactic acid, microbially produced. Applied Microbiology: Industrial, Elsivier Inc., pp 362–372. Available from https://imtech.wikispaces.com/file/view/Lactic+Acid,+Microbially+Produced,.pdf
Lore T, Omore A, Staal S (2005) Types, levels and causes of post-harvest milk and dairy losses in sub-Saharan Africa and the Near East: Phase one synthesis report. International Livestock Research Institute, Nairobi, Kenya
Lucey LA, Horne DS (2009) Milk salts: technological significance. In: McSweeney PLH, Fox PF (eds) Advanced dairy chemistry: lactose, water, salts and minor constituents, vol 3. Springer ScienceþBusiness Media, Cork, pp 350–389
MacLeod RA, Snell EE (1947) Some mineral requirements of the lactic acid bacteria. J Biol Chem 170(1):351–365
Mäyra-Mäkinen A, Bigret M (2004) Industrial use and production of lactic acid bacteria. In: Salminen S, von Wright A, Ouwehand A. (eds) Lactic acid bacteria: microbiological and functional aspects, 3rd ed. CRC Press, Boca Raton London New York, pp 175–198
MedTest. 2012. Industrie du lait et des produits laitiers (CLC) :Secteur Alimentaire en Tunisie. UNIDO. Report
Moulin G, Ratomahenina R, Galzy P, Boze M (1976) Sélection de levure en vue de la culture sur lactosérum. Lait 56(553–554):135–142
Newstead DF, Sanderson WB, Conaghan EF (1977) Effects of whey protein concentrations and heat treatment on the heat stability of concentrated and unconcentrated milk. New Zeal J Dairy Sci 12:29–36
Oldfield DJ, Singh H, Taylor MW, Pearce KN (2000) Heat-induced interactions of β-lactoglobulin and α-lactalbumin with the casein micelle in pH-adjusted skim milk. Int Dairy J 10(8):509–518
Payne-Botha S, Bigwood EJ (1959) Amino-acid content of raw and heat-sterilized cow’s milk. Br J Nutr 13:385–389
Pieterse B, Leer RJ, Schuren FH, van der Werf MJ (2005) Unravelling the multiple effects of lactic acid stress on Lactobacillus plantarum by transcription profiling. Microbiol 151(12):3881–3894
Porubcan RS, Sellars RL (1979) Lactic starter culture concentrates. In: Peppler HT (ed) Microbiology. Van Nostrand Reinhold, Princeton, NJ, p 59
Prazeres AR, Carvalho F, Rivas J (2012) Cheese whey management: a review. J Environ Manag 110:48–68
Randhawa M, Ahmed A, Akram K (2012) Optimization of lactic acid production from cheap raw material: sugarcane molasses. Pak J Bot 44(1):333–338
Reiter B, Oram JD (1962) Nutritional studies on cheese starters. J Dairy Res 29:63–77
Rodier J, Legube B, Merlet N, Brunet R (2009) Eaux Résiduaires. In: Rodier J (ed) L'analyse de l'eau: Eaux naturelles, eaux résiduaires, eau de mer, 9th edn. Dunod, Paris
Rombaut R, Dewettinck K (2007) Thermocalcic aggregation of milk fat globule membrane fragments from acid buttermilk cheese whey. J Dairy Sci 90(6):2665–2674
Russ W, Meyer-Pittroff R (2004) Utilizing waste products from the food production and processing industries. Crit Rev Food Sci Nutr 44(1):57–62
Russell JB, Diez-Gonzalez F (1997) The Effects of Fermentation Acids on Bacterial Growth. In: Poole, RK (ed) Advances in Microbial Physiology, Vol 39, Academic Press, San Diego London Boston New York Sydney Tokyo Toronto, pp 205–234
Saguir FM, de Nadra MC (2007) Improvement of a chemically defined medium for the sustained growth of Lactobacillus plantarum: nutritional requirements. Curr Microbiol 54(6):414–418
Samaržija D, Antunac N, Havranek JL (2001) Taxonomy, physiology and growth of Lactococcus lactis: a review. Mljekarstvo 51(1):35–48
Scholten RHJ, van der Peet-Schwering CMC, Verstegen MWA, den Hartog LA, Schrama JW, Vesseur PC (1999) Fermented co-products and fermented compound diets for pigs: a review. Anim Feed Sci Technol 82(1–2):1–19
Schreiber R (2001) Heat-induced modifications in casein dispersions affecting their rennetability. Int Dairy J 11(4–7):553–558
Seesuriyachan P, Kuntiya A, Sasaki K, Techapun C (2009) Biocoagulation of dairy wastewater by Lactobacillus casei TISTR 1500 for protein recovery using micro-aerobic sequencing batch reactor (micro-aerobic SBR). Process Biochem 44(4):406–411
Taskila S, Ojamo H (2013) The Current Status and Future Expectations in Industrial Production of Lactic Acid by Lactic Acid Bacteria. In: Kongo, M. (ed), Lactic Acid Bacteria – R&D for Food, Health and Livestock Purposes, InTech, Rijeka, pp 615–632
Tocchi C, Federici E, Scargetta S, D’Annibale A, Petruccioli M (2013) Dairy wastewater polluting load and treatment performances of an industrial three-cascade-reactor plant. Process Biochem 48(5–6):941–944
van Niel EWJ, Hahn-Hägerdal B (1999) Nutrient requirements of lactococci in defined growth media. Appl Microbiol Biotechnol 52(5):617–627
Vasbinder A (2002) Casein—whey protein interactions in heated milk, Ph.D Thesis, Utrecht University. Netherlands, pp 141
Vasbinder A, Alting A, de Kruif K (2003) Quantification of heat-induced casein–whey protein interactions in milk and its relation to gelation kinetics. Colloids Surfaces B Biointerfaces 31(1–4):115–123
Venetsaneas N, Antonopoulou G, Stamatelatou K, Kornaros M, Lyberatos G (2009) Using cheese whey for hydrogen and methane generation in a two-stage continuous process with alternative pH controlling approaches. Bioresour Technol 100(15):3713–3717
Yeung CY, Lee HC, Lin SP, Yang YC, Huang FY, Chuang CK (2005) Negative effect of heat sterilization on the free amino acid concentrations in infant formula. Eur J Clin Nutr 60(1):136–141
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Kasmi, M., Hamdi, M. & Trabelsi, I. Processed milk waste recycling via thermal pretreatment and lactic acid bacteria fermentation. Environ Sci Pollut Res 24, 13604–13613 (2017). https://doi.org/10.1007/s11356-017-8932-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8932-6





 ), filtrates (
), filtrates ( ) and clarified filtrates (
) and clarified filtrates ( ) COD evolution expressed in (mg d′O2 L−1) following thermal coagulation at 60, 80, and 100 °C compared to the raw MW (
) COD evolution expressed in (mg d′O2 L−1) following thermal coagulation at 60, 80, and 100 °C compared to the raw MW ( )
)
