Abstract
Application of modified sintering ferric-carbon ceramics (SFC) and sintering-free ferric-carbon ceramics (SFFC) based on coal ash and scrap iron for pretreatment of tetracycline (TET) wastewater was investigated in this article. Physical property, morphological character, toxic metal leaching content, and crystal component were studied to explore the application possibility of novel ceramics in micro-electrolysis reactors. The influences of operating conditions including influent pH, hydraulic retention time (HRT), and air-water ratio (A/W) on the removal of tetracycline were studied. The results showed that SFC and SFFC were suitable for application in micro-electrolysis reactors. The optimum conditions of SFC reactor were pH of 3, HRT of 7 h, and A/W of 10. For SFFC reactor, the optimum conditions were pH of 2, HRT of 7 h, and A/W of 15. In general, the TET removal efficiency of SFC reactor was better than that of SFFC reactor. However, the harden resistance of SFFC was better than that of SFC. Furthermore, the biodegradability of TET wastewater was improved greatly after micro-electrolysis pretreatment for both SFC and SFFC reactors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tetracycline wastewater is a kind of typical pharmaceutical wastewater with the characteristics of high organic contents, high salinity, poor biodegradability, and strong biotoxicity (de Cazes et al. 2014). As a result of their complex chemical structure and strong antibacterial effect, the tetracycline wastewater cannot be treated completely by traditional biological treatment process. The residual tetracycline (TET) would be released into the environment and spread in the water, which would result in the development of antibiotic-resistant bacteria and the adverse health effects to humans (Du et al. 2014; Yang et al. 2016). Furthermore, some studies indicated that the resistant bacteria would develop and spread more quickly in the TET wastewater with direct biological treatment (Auerbach et al. 2007; Yang et al. 2015). Therefore, it is necessary to develop methods for the pretreatment of TET wastewater. At present, the main methods for TET wastewater pretreatment were ozone oxidation, Fenton, and electrolysis (Belkheiri et al. 2015). When these chemical oxidation methods were applied, large amount of chemical reagent and electric power would be consumed, which resulted in high treatment cost. However, as one of the important electrochemical treatment methods, the iron-carbon micro-electrolysis has been used for treating some refractory wastewater such as dyes wastewater, coking wastewater, and pharmaceutical wastewater (Han et al. 2016; Wu et al. 2011; Xing et al. 2016). It could utilize the waste for the effective degradation of TET and reduce the cost of TET pretreatment.
The iron-carbon micro-electrolysis was based on the iron corrosion theory (Bell et al. 2003; Guan et al. 2012). When iron and carbon were in contact with wastewater, numerous electrons were supplied from iron anode and transformed to carbon cathode. In this way, many microscopic galvanic cells that had iron anode and carbon cathode were formed. The half-cell reactions can be represented as follows (Zhu et al. 2014):
-
$$ \begin{array}{l}\mathrm{Anode}\left(\mathrm{oxidation}\right):2\mathrm{Fe}\to 2{\mathrm{Fe}}^{2+}+4{\mathrm{e}}^{-},\mathrm{E}\Big({\mathrm{Fe}}^{2+}/\mathrm{Fe}\Big)=-0.44\ \mathrm{V}\kern1em \\ {}\mathrm{Cathode}\left(\mathrm{reduction}\right):\mathrm{Acidic}:\ 2{\mathrm{H}}^{+}+2{\mathrm{e}}^{-}\to {\mathrm{H}}_2\uparrow, \mathrm{E}\Big({\mathrm{H}}^{+}/{\mathrm{H}}_2\Big)=0\ \mathrm{V}\kern1em \\ {}{\mathrm{O}}_2+4{\mathrm{H}}^{+}+4{\mathrm{e}}^{-}\to 2{\mathrm{H}}_2\mathrm{O},\mathrm{E}\left({\mathrm{O}}_2/{\mathrm{H}}_2\mathrm{O}\right)=+1.23\ \mathrm{V}\kern1em \\ {}\mathrm{Neutral}\ \mathrm{to}\ \mathrm{alkaline}:{\mathrm{O}}_2+2{\mathrm{H}}_2\mathrm{O}+4{\mathrm{e}}^{-}\to 4{\mathrm{O}\mathrm{H}}^{-},\mathrm{E}\left({\mathrm{O}}_2/{\mathrm{O}\mathrm{H}}^{-}\right)=+0.40\ \mathrm{V}\kern1em \end{array} $$
The numerous micro-electrolysis reactions could break the construction of many non-biodegradable organic pollutants. When the traditional micro-electrolysis process was used for organic wastewater treatment, iron powder and carbon powder were directly added into the reactor with a certain ratio, which had many disadvantages such as low Fe utilization, easy jamming, and short serviceable cycle etc. (Han et al. 2016; Xing et al. 2016). In order to overcome these shortages, the iron-carbon granular ceramic media was prepared and applied (de Mendonça et al. 2016; Wu et al. 2011). Nevertheless, large quantities of farmland clay were consumed to produce ceramics with previous preparation technology resulting in the loss of farmland. On the other hand, coal ash and scrap iron were both common solid wastes, which contained different kinds of toxic substances (Zhang et al. 2007). With the growing consumption of coal and steel in China, the amount of coal ash and scrap iron increased greatly (Yao et al. 2014). If the large amount of coal ash and scrap iron cannot be treated properly, they would lead to the large occupation of land, the pollution of underground water, and the waste of resources. As the coal ash contained some inorganic matter and unburnt carbon, it could partly replace farmland clay and coal. The addition of scrap iron could reduce the consumption of quality iron. Thus, coal ash and scrap iron mixed with clay were used to produce ferric-carbon (Fe-C) ceramic particles to solve the treatment and resource utilization of these solids. Partial micro-electrolysis ceramics have been applied in actual wastewater treatment (Wu et al. 2011). Nevertheless, the manufacturing processes were uncontrollable in reality, especially the sintering process. As some solid waste was added in raw material, the sintering temperature and heating uniformity were changeable and hard to control, which reduced the yield and quality of Fe-C ceramics. In order to make the manufacturing process adapt to the actual situation, the sintering ferric-carbon ceramics (SFC) with modified manufacturing technology and the sintering-free ferric-carbon ceramics (SFFC) based on coal ash and scrap iron were prepared in this article.
In our study, two novel Fe-C micro-electrolysis ceramics were prepared and used for the TET wastewater pretreatment to improve the biodegradability of TET wastewater, increase the TET removal efficiency, and slow down the growth of antibiotic-resistant bacteria. At present, few researches were focused on the application of Fe-C ceramics for TET wastewater pretreatment. In this article, three aspects were investigated:
-
1.
The preparation of two novel ceramic-ferric-carbon micro-electrolysis fillers (SFC and SFFC) and their application for the pretreatment of TET wastewater
-
2.
The optimum conditions of pH, hydraulic retention time (HRT), and air-water ratio (A/W) for the TET wastewater pretreatment in SFC and SFFC reactors
-
3.
The analysis and comparison of the performance of SFC and SFFC on the removal of TET, chemical oxygen demand (COD), and total organic carbon (TOC).
Materials and methods
Raw materials
Scrap iron, coal ash, clay, and carbon powder were raw materials of SFC. Scrap iron, coal ash, clay, carbon powder, and cement were used to make SFFC. Scrap iron was obtained from Jinan steel-making plant. Coal ash and carbon powder were from Jinan fossil-fired power plant. Clay was obtained from a brickfield in Zibo, Shandong Province. All the original materials were dried at 105 °C for 4 h, crushed to pass sieve no. 100 (the diameter of mesh was 0.154 mm), and stored in polyethylene vessels to avoid humidification until being used. The binder solution was made of polyvinyl alcohol, sodium carboxymethylcellulose, and deionized water with a certain ratio. In order to simplify the degradation process of the actual TET wastewater, a certain amount of crystallization mother liquor of tetracycline hydrochloride was taken from Shandong Lukang Pharmaceutical Co., Ltd., whose main composition was tetracycline hydrochloride. According to our previous researches and plan (Yang et al. 2015), the crystallization mother liquor was dissolved in tap water to obtain the synthesized wastewater with 150 mg/L of TET. Then, the dilute wastewater was used as influent tetracycline wastewater. The specific characters of the TET wastewater are as follows: TET of 150–210 mg/L; TOC of 100–170 mg/L; COD of 150–300 mg/L; and total nitrogen of 10–18 mg/L.
Manufacturing process of SFC and SFFC
For SFC, mass ratio of scrap iron, carbon powder, clay, and coal ash were 4:1:6:2. And the mass ratio of scrap iron, carbon powder, clay, coal ash, and cement in SFFC were (30–32):8:20:25:15. According to our previous studies (Wu et al. 2011), three steps for the manufacturing operation of SFC and SFFC were as follows:
-
First: Mixing and pelleting. The raw materials of SFC and SFFC were mixed with certain mass ratio described before. Then, the mixture was stirred in a dry powder stirrer (B10-20B, made in China) for about 10.0 min. The mixture was poured into a pelletizer (QJ300, China) to produce pellets (about 7.00 wt% of binder solution was added in this process). These obtained pellets with a diameter of 5–6 mm were selected by two sieves (the diameters of meshes were 5.00 and 6.00 mm). Then, the pellets of SFC and SFFC were dried in draught cupboard at room temperature (22 °C) for about 24.0 h.
-
Second: Sintering treatment for SFC and steam conservation for SFFC. The dried pellets of SFC were placed into electric carbide furnace (SX2-4-13, made in China) and sintered at 600 °C for 25 min in anoxic conditions. The pellets were put in the center of the heated zone. The ceramics of SFFC were placed in high-pressure steam sterilization pot at 80 °C for 2 h.
-
Third: Cooling and natural curing treatment. After the sintering and steam conservation process, the pellets were kept in draught cupboard until they cooled down to room temperature (22 °C). Then, these pellets were maintained under natural conditions for about 7 days.
Characterization test of SFC and SFFC
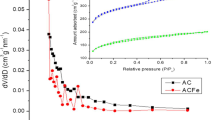
In order to compare the physical properties of SFC and SFFC, the physical properties (grain density, water absorption, bulk density, and voidage) were tested according to the national standards (GB/T17431.2-2010, China). Then, the crystal components of raw materials and finished pellets were analyzed by X-ray diffraction (XRD) (Rigaku D/MAX-YA diffractometer) (Jasiewicz et al. 2015). The micro-structure and chemical components in the surface of SFC and SFFC before and after use were determined by scanning electron microscope (SEM) and energy dispersive X-ray fluorescence spectrometer (EDX) (Hitachi S-520, Japan). The BET surface area of the ceramics was characterized under nitrogen adsorption/desorption isotherms at 77 K using automated surface area and pore size (JW-BK122W, China). Finally, trace elements in lixivium of two new ceramics were checked out to verify whether they could be applied into actual wastewater treatment without secondary pollution.
The lab-scale experimental setup
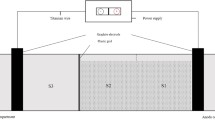
Two same lab-scale cylindrical reactors are indicated in Fig. 1. The reactors made from polymethyl methacrylate had a diameter of 80 mm with height of 70 cm. Water inlet and tray aerator were installed at the bottom of reactor. The raw wastewater was fed into the reactor with a feed pump. The supporting layer was set at 10 cm height from the bottom to hold up the fillers and distributes water and air equally. SFC (3.5 L) and SFFC (3.5 L) were filled in the two reactors with the height of 50 cm. The effective volume of the reactor was about 1 L, which was measured by pumping the water. On the top, there was a buffer zone with a height of 8 cm to stop media from being washed away. The treated effluent was collected by an effluent tank.
Running of the SFC and SFFC reactor
In order to exclude the adsorption effect of new fillers to tetracycline, the TET wastewater was continuously pumped into the two reactors for 5 days. At this time, the basic operation parameters were as follows: pH of 5, HRT of 8 h, and the fillers height of 50 cm. Then, pH, HRT, A/W, and filler height were changed, respectively, with other operating conditions invariant. The reactors were backwashed every 10 days according to the effluent water quality. The backwashing step was continued for 0.5 h. Every operating condition was continuously running for 5 days with the adaptive phase of 2 days. Three samples were taken in running phase (surplus 3 days). The optimum conditions were determined by the removal of TET, TOC, and COD.
Analytical methods
In this research, all the samples collected from both influent and effluent were stored at 4 °C for less than 24 h before measured. The concentrations of COD were measured according to standard methods (SEPAC. 2002a). In order to exclude the influence of Fe2+ ion, the pH of the effluent water samples was firstly adjusted to 10 by sodium hydroxide solution, and then COD of the samples was measured. TOC was determined by a TC/TN analyzer (Shimadzu TOC-V CPH, Japan). The high-performance liquid chromatography (HPLC, Shimadzu, Japan) was used for the measurement of tetracycline according to Cetecioglu et al. (2013). Biochemical oxygen demand (BOD5) was measured by BOD rapid monitor (BOD Track, HACH, America). Measurements of every item for each sample were repeated for three times, and the average value was selected for the determination.
Results and discussion
Comparison of properties between SFC and SFFC
The bulk density, grain density, voidage, and water absorption of SFC and SFFC are shown in Table 1. Comparing with the national standard, both SFC and SFFC had low bulk density and water absorption. Although raw materials and manufacturing processes were different, the physical properties of SFC and SFFC were quite similar. Due to the promontory on the surface of SFFC, bulk density of SFFC was a little lower than that of SFC with higher grain density. And specific surface area of SFFC was also higher than SFC. For SFFC, cement was easy to bind with other raw materials and form some small granule. Then, these small granules were glued together by more binder solution and cement, resulting in a mass of promontory on the surface of SFFC. But for SFC, with the action of binder solution, the raw material was adhered together little by little to form big smooth particles.
Figure 2 shows the crystal components analysis of SFC and SFFC before and after manufacture. The crystal structure of SFC changed a lot after sintering. For initial materials of SFC, SiO2, Fe, and C were detected with large signal. But the signal of Fe, C, and partial SiO2 disappeared and the intensity of all signals decreased a lot after sintering. This was because the atoms of Fe, C, and partial Si would move to the inside level of each other when SFC was sintered at 600 °C (Li et al. 2015). Then, they contacted with each other more closely (Tunyogi et al. 2010). But for SFFC, the characteristic XRD patterns changed a little after manufacture. When SFFC was made, Fe, C, and clay were just binded by cement and binder solution. This was a physical process. There was no great energy to make Fe, C, and clay react.
The concentrations of 10 metal elements in lixivium of two new ceramics are listed in Table 2. All metal concentrations were much lower than the national threshold determined by the national standard (GB 5085.3-2007, China). In addition, the concentrations of Fe and Ca were larger than other metal elements, which were benefitted for subsequent biological advanced treatment (Lissalde et al. 2016). These results proved that the two novel ceramics could be used as water treatment materials without secondary pollution. Due to the addition of cement, concentrations of all elements in lixivium of SFFC were less than that in SFC, suggesting that SFFC had stronger acid resistance than SFC.
The surface and fracture surface micro-structures of SFC and SFFC are shown in Fig. 3. A large amount of apophysis and mesoporous could be found on both SFC and SFFC surface. The rough surfaces increased the contact area between fillers and wastewater. In addition, many mesoporous existed on fracture surface, which indicated that many mesoporous could run through the ceramics. Some TET wastewater could be stored in the mesoporous, increasing the contacting time of TET with ceramics, which was beneficial for electrodegradation of TET. Due to the above analysis, SFC and SFFC were suitably applied in the electrobaths for TET wastewater treatment.
Influence of influent pH on the performance of SFC and SFFC
In this period, the experiment was operated at HRT of 10 h without aeration. Then, influent pH was successively changed from 1 to 10. The removal rates of TET, TOC, and COD are listed in Fig. 4. For SFC and SFFC, the removal rates decreased with increasing pH. This was because the corrosion rate of Fe would increase with the gradually enhanced acid influent (Wang et al. 2013), which was useful for micro-electrolysis reaction and the degradation of TET. When pH increased from 1 to 3, all the removal rates decreased slowly and kept high. But all the removal rates began to decrease quickly when pH was above 4. For SFFC, the inflection point was under the pH of 2. When influent pH of SFFC reactor was more than 2, the removal rates of TET, TOC, and COD quickly decreased from 75, 73, and 70% to 22, 13, and 12%, respectively. Then, effluent pH gradually became over 7.5 and unstable for anaerobic biological treatment (Yang et al. 2015). But when influent pH was too low, the corrosion rate of ceramics was accelerated while the entire removal rates increased slightly. Then, the effluent pH of SFC and SFFC was about 5–6 and unsuitable for subsequent biological treatment. Due to the removal efficiency and the sustainability of ceramics, the optimum influent pH of SFC and SFFC was selected as 3 and 2, respectively. The optimum influent pH of SFFC was lower than that of SFC for the reason that the addition of cement impeded the contact of Fe, C, and hydrogen ion, which was adverse for the electron transport. Therefore, more hydrogen ion in the influent should be provided to improve the contact possibility. Of course, the entire highest removal efficiency of SFC reactor higher than that of SFFC reactor was also caused by the addition of cement. When influent pH was same, the effluent pH of SFFC reactor was higher than that of SFC reactor for the reason that the alkaline cement in SFFC neutralized part of H+. It could be seen that the removal efficiencies of TET were higher than that of TOC and COD for both SFC and SFFC at the same influent pH values. It was because that some functional groups with small bond energy in TET were broken by micro-electrolysis reaction firstly and decomposed to low-molecular weight organics. Then, the small molecular substance was gradually removed by some reactions such as micro-electrolysis reaction, oxidation reaction, reduction reaction, and hydrolysis reaction (Wu et al. 2012). So, the removal rate of TET was higher than that of TOC and COD.
Influence of HRT on the performance of SFC and SFFC
The optimum operational pH of SFC and SFFC reactor has been selected as 3 and 2 in the “Influence of influent pH on the performance of SFC and SFFC” section. Then, the effects of 10 HRTs were selected and analyzed in this section to determine the optimum TET loads of SFC and SFFC. The aeration intensity measured by A/W was 15:1 for the two reactors. The changed removal efficiencies of TET, TOC, and COD with HRT are listed in Fig. 4. It can be seen that all removal efficiencies increased with longer HRT for SFC and SFFC reactors. This was because that the contact time of wastewater and ceramics increased with longer HRT and the time of electrodegradation of TET also increased (Zhou et al. 2013). When HRT increased from 1 to 6 h, the entire removal rates increased quickly. But all the removal rates increased slowly when HRT was more than 7 h for both SFC and SFFC reactors. As influents of SFC and SFFC reactors were acidic, Fe would react with hydrogen ion (H+) at the beginning. Then, the concentration of H+ decreased quickly with time going, and the wastewater would become alkaline from acidic. At the same time, a fraction of Fe reacted with oxygen (O2) and water (H2O) to produce hydroxide ion (OH−), which enhanced the change of wastewater from acidic to alkaline. In addition, Fe3+ may precipitate as Fe(OH)3, which resulted in the flocculation precipitation process in the wastewater (Zagklis et al. 2012). Especially when pH was more than 4, most Fe3+ would precipitated as Fe(OH)3 and the flocculation precipitation process would greatly enhanced the removal of TET (Millero 1998). Then, most Fe3+ would be discharged in the form of Fe(OH)3 sedimentation. Therefore, with the increasing HRT from 1 to 6 h, the micro-electrolysis reaction time constantly increased, resulting in the quick increase of the entire removal rates and the increasing effluent pH. However, the wastewater has already become alkaline from acidic, and the micro-electrolysis reaction would be greatly restrained when HRT was too long. Hence, the reaction time was not restriction factors anymore, and the removal rates increased slightly when HRT was more than 7 h. Due to all the removal efficiencies, HRT of 7 h was the best operating choice for both SFC and SFFC reactors. It could be seen that the treatment effect in SFC reactor was also better than that in SFFC reactor with similar HRT. This was because of the same reason in the “Influence of influent pH on the performance of SFC and SFFC” section.
Influence of A/W on the performance of SFC and SFFC
Different reactions would happen in cathode region under different concentrations of DO (Sirés and Brillas 2012). So, aeration intensity was an important influencing factor for the removal of TET. In this period, the operating conditions were as follows: pH of SFC and SFFC were 3 and 2, HRT was 7 h, and then A/W of 0, 5, 10, 15, 20, and 25 were selected to obtain the influence of aeration intensity on the TET removal. The results are shown in Fig. 5. For both SFC and SFFC reactors, the removal of TET, TOC, and COD increased with A/W, and then stayed stable, since the electrode voltages in aerated reactor were higher than that in anaerobic reactor, which was discussed above. Furthermore, when oxygen participated in the cathodic reaction, more energy was provided for micro-electrolysis process and some secondary reaction such as Fenton reaction enhanced (Koltsakidou et al. 2016). However, all the removal rates increased a little at A/W of 15 for the fact that DO in wastewater had reached the gas-saturated solution and its influence on removal of TET became weak. Furthermore, effluent pH increased with the enhancing aeration intensity. Especially when A/W was higher than 15, effluent pH increased greatly for both SFC and SFFC reactors. The effluent pH of SFC reactor was over 7.2 at A/W of 15, which was considered unsuitable for biological treatment (Winkler et al. 2011). Due to the removal efficiencies of TET and the change of effluent pH, A/W of 10–15 was considered as the best aeration intensity.
Influence of media heights on removal of TET
According to the previous description, the best operation of SFC reactor was pH of 3, HRT of 7 h, and A/W of 10–15. For SFFC reactor, the best operation was pH of 2, HRT of 7 h, and A/W of 10–15. In order to determine the change of TET with media height, seven media heights were selected to determine the best operations. The results are displayed in Fig. 5c, d. For SFC reactor, the removal efficiencies of TET, TOC, and COD increased quickly when media height increased from 0 to 5 cm, and then increased slowly. The TET removal rate kept stable when media height was about 15 cm. However, the TOC and COD removal rates stayed stable when media height was about 25 and 35 cm, respectively, which were behind TET. The delay of COD and TOC for keeping stable indicated that TET was decomposed firstly, which has been discussed in the “Influence of influent pH on the performance of SFC and SFFC” section. The change of effluent pH with media height in SFC reactor was similar to that of TOC removal rate, which also proved this process. The changes of TET, TOC, and COD in SFFC reactor were different from those in SFC reactor. The TET and TOC removal rates increased quickly when media heights increased from 0 to 5 cm, and then stayed stable when media heights were about 15 and 45 cm. The media height when TOC removal rate stayed stable in SFFC reactor was higher than that in SFC reactor for the reason that SFC had higher reaction activity than SFFC. Because the intensity of micro-electrolysis reaction in SFFC reactor was not enough for the degradation of COD, the COD removal rate increased slowly when height was 0–25 cm. After most of TET was degraded by Fe-C micro-electrolysis, partial energy that produced by micro-electrolysis was used for the removal of COD, so COD removal rate increased quickly when media height was 25–35 cm. In the end, the COD removal rate gradually became stable. The change of effluent pH with media height in SFFC reactor was similar to that of TOC removal rate, which reflected that H+ was gradually consumed by Fe-C micro-electrolysis. In this part, it could be concluded that the media height of 45 cm could fully meet the requirement for the removal process of TET wastewater for both SFC and SFFC reactors.
Under the best operating condition, the TET removal rates of SFC and SFFC could reach to about 95 and 90%, which was better than the conventional micro-electrolysis systems (Sirés and Brillas 2012).
Discussion
It could be concluded from the “Influence of media heights on removal of TET” section that the major removal of TET, COD, and TOC happened at 0–10 cm height and ended at about 25–35 cm. After the study of determining the best operation, some SFC and SFFC examples were taken out at the media height of 5–10 and 25–35 cm to detect the inner reason for the good performance of SFC and SFFC on treating TET wastewater. After treating TET wastewater for about 120 days, SFC was brown while SFFC was black when they were taken out. Furthermore, SFC was seriously hardened and difficult to be separated. While SFFC did not become hardened, they were easily separated. Because the composition of SFC and SFFC was different, their structure was different too. The reaction rate of SFC was extremely rapid; Fe quickly fell off from SFC. Only part of Fe reacted with H+ and dissolved in water, so a large amount of Fe stayed on the surface of SFC, and then bonded with each other (Morgan et al. 2010). Thus, SFC was brown and became hardened. For SFFC, their reaction rate was slow, Fe could react with H+ and dissolve in water slowly, so little Fe would stay on surface of SFFC. However, C gradually appeared on the surface with the consumption of Fe. So, SFFC were black and did not harden. This phenomenon indicated that the hardening resistance of SFFC was better than that of SFC. In order to describe this process in detail, the micro-structure of SFC and SFFC observed by SEM is listed in Fig. 6. For SFC, comparing with the primary ceramics, the signs of corrosion were shown on the surface of all the samples. The corrosion of ceramics from 5 cm was serious than that from 25 cm for the reason that the major degradation of TET happened at 5 cm height. Furthermore, the fracture surface of SFC at 25 cm did not show the signs of corrosion. However, serious corrosion signs were extended from the surface to the center of SFC that was taken from 5 cm height. This phenomenon also indicated that the major degradation happened at 5 cm height. After treating TET wastewater, serious corrosion made the surface of SFC become rougher and the mesoporous became bigger than before, which was more benefited for the electrodegradation of TET as the contact area of TET and ceramics was increased further. The change of SFFC was similar to SFC; the surface and fracture surface of SFC at 5 cm showed more serious corrosion than those of SFFC at 25 cm. The corrosion also made SFFC increase the contact area with TET. It could be seen that SFC had more serious corrosion than SFFC by comparing the used SFC and SFFC at the same media height. The serious corrosion made the surface of SFC alveolate. The honeycomb structural body made SFC hardened more easily than SFFC.
Micro-structure of SFC and SFFC after they were used. a Surface of SFC at 5 cm fillers height. b Fracture surface of SFC at 5 cm fillers height. c Surface of SFC at 25 cm fillers height. d Fracture surface of SFC at 25 cm fillers height. e Surface of SFFC at 5 cm fillers height. f Fracture surface of SFFC at 5 cm fillers height. g Surface of SFFC at 25 cm fillers height. h Fracture surface of SFFC at 25 cm fillers height
In order to reflect the micro-electrolysis process intuitively, the change of different material composition on surface of SFC and SFFC was measured by EDX. The EDX spectrums of two ceramics are listed in Fig. SM-1. The results are shown in Table 3. Comparing the original and used ceramic fillers, it could be seen that the iron content decreased greatly for the reason that iron reacted with H+ and formed Fe2+. Fe2+ could be dissolved in the water. The corrosion of iron was the foundation of micro-electrolysis. Due to the loss of Fe, the mass percent of C and O increased. Furthermore, carbon was not dissolved in the water. The contents of other minor elements like Ca, K, Ti, and S increased for the reason that the raw material contained some minerals, such as illite, kaolinite, and vermiculite, which were made up of these elements and oxygen (Zhu et al. 2003). These minerals had acid and alkali resistance and could not be dissolved in the water easily. Mn mainly existed in cement, so Mn was not detected in SFC and its content in SFFC increased. However, the contents of Si and Al decreased. This could be inferred that montmorillonite in the clay could absorb water to swelling and fell off from the ceramics. Furthermore, aluminum oxide in the coal cinder could be dissolved in acid or alkaline solution, which resulted in the loss of Al. After using for the same time, more Fe was consumed by SFC than that by SFFC, which indicated that the serviceable cycle of SFFC was longer than that of SFC. When the reaction activity of SFC and SFFC was too low, in order to make SFC and SFFC recycled, they would be cleaned by hydrochloric acid whose pH was 1. From the above, it could be concluded that the reaction rate of Fe in SFC was more than SFFC under the best operation condition. As the initial content of Fe in SFC and SFFC was the same, the consumption rate and amount of Fe in SFC was more than that in SFFC in the same time; hence, the working life and cycle criterion of SFC would be less than SFFC. This suggested that the recyclability of SFFC was better than SFC.
The pretreatment of TET wastewater was aimed to make the subsequent biological treatment of TET easy. The biodegradability of TET wastewater was one of these important indexes, which could show the feasibility of biological treatment. The biodegradability was usually represented by biochemical oxygen demand (BOD)/COD (Verenich and Kallas 2002). The results (Table 4) were measured under the best operation condition. It could be seen that the biodegradability of TET wastewater was improved greatly after micro-electrolysis pretreatment for both SFC and SFFC reactors. The TET wastewater became biodegradable from almost non-biodegradable. Particularly necessary to point out that the original TET wastewater just contained tetracycline and did not contained other organic substance. In all, the micro-electrolysis pretreatment completed the aims of our study well.
Conclusion
-
1.
According to the physical and chemical properties, SFC and SFFC were suitable for the micro-electrolysis pretreatment of TET wastewater.
-
2.
The optimum conditions of SFC reactor for TET wastewater treatment were pH of 3, HRT of 7 h, and A/W of 10. For SFFC reactor, the optimum conditions were pH of 2, HRT of 7 h, and A/W of 15.
-
3.
In general, the TET removal efficiency of SFC reactor was better than that of SFFC reactor. However, the harden resistance of SFFC was better than that of SFC. Furthermore, the biodegradability of TET wastewater was improved greatly after micro-electrolysis pretreatment by both SFC and SFFC reactors.
References
Auerbach EA, Seyfried EE, McMahon KD (2007) Tetracycline resistance genes in activated sludge wastewater treatment plants. Water Res 41:1143–1151
Belkheiri D, Fourcade F, Geneste F, Floner D, Aït-Amar H, Amrane A (2015) Combined process for removal of tetracycline antibiotic—coupling pre-treatment with a nickel-modified graphite felt electrode and a biological treatment. Int Biodeterior Biodegradation 103:147–153
Bell LS, Devlin JF, Gillham RW, Binning PJ (2003) A sequential zero valent iron and aerobic biodegradation treatment system for nitrobenzene. J Contam Hydrol 66:201–217
Cetecioglu Z, Ince B, Gros M, Rodriguez-Mozaz S, Barceló D, Orhon D, Ince O (2013) Chronic impact of tetracycline on the biodegradation of an organic substrate mixture under anaerobic conditions. Water Res 47:2959–2969
de Cazes M, Belleville MP, Petit E, Llorca M, Rodríguez-Mozaz S, de Gunzburg J, Barceló D, Sanchez-Marcano J (2014) Design and optimization of an enzymatic membrane reactor for tetracycline degradation. Catal Today 236(Part A):146–152
de Mendonça FG, Rosmaninho MG, da Fonseca PX, Soares RR, Ardisson JD, Tristão JC, Lago RM (2016) Use of iron and bio-oil wastes to produce highly dispersed Fe/C composites for the photo-Fenton reaction. Environ Sci Pollut Res 1–6
Du J, Ren H, Geng J, Zhang Y, Xu K, Ding L (2014) Occurrence and abundance of tetracycline, sulfonamide resistance genes, and class 1 integron in five wastewater treatment plants. Environ Sci Pollut Res 21:7276–7284
Guan X, Xu X, Lu M, Li H (2012) Pretreatment of oil shale retort wastewater by acidification and ferric-carbon micro-electrolysis. Energy Procedia 17(Part B):1655–1661
Jasiewicz K, Cieslak J, Kaprzyk S, Tobola J (2015) Relative crystal stability of AlxFeNiCrCo high entropy alloys from XRD analysis and formation energy calculation. J Alloys Compd 648:307–312
Koltsakidou Α, Antonopoulou M, Sykiotou M, Εvgenidou Ε, Konstantinou I, Lambropoulou DA (2016) Photo-Fenton and Fenton-like processes for the treatment of the antineoplastic drug 5-fluorouracil under simulated solar radiation. Environ Sci Pollut Res 1–10
Han Y, Li H, Liu M, Sang Y, Liang C, Chen J (2016) Purification treatment of dyes wastewater with a novel micro-electrolysis reactor. Sep Purif Technol 170:241–247
Li G, Wang L, Li W, Xu Y (2015) Mesoporous Fe/C and core–shell Fe–Fe3C@C composites as efficient microwave absorbents. Microporous Mesoporous Mater 211:97–104
Lissalde S, Charriau A, Poulier G, Mazzella N, Buzier R, Guibaud G (2016) Overview of the Chemcatcher® for the passive sampling of various pollutants in aquatic environments part B: field handling and environmental applications for the monitoring of pollutants and their biological effects. Talanta 148:572–582
Millero FJ (1998) Solubility of Fe(III) in seawater. Earth Planet Sci Lett 154:323–329
Morgan JLL, Wasylenki LE, Nuester J, Anbar AD (2010) Fe isotope fractionation during equilibration of Fe−organic complexes. Environ Sci Technol 44:6095–6101
Sirés I, Brillas E (2012) Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: a review. Environ Int 40:212–229
State Environmental Protection Administration of China (2002a) Monitoring and analysis methods of water and wastewater, fourth edn. China Environmental Science Press, Beijing
Tunyogi A, Tanczikó F, Bogdán C, Horváth ZE, Szilágyi E (2010) Characterisation of annealed Fe/Ag bilayers by RBS and XRD. Instrum Methods Phys Res Sect B 268:1972–1975
Verenich S, Kallas J (2002) Wet oxidation lumped kinetic model for wastewater organic burden biodegradability prediction. Environ Sci Technol 36:3335–3339
Wang X, Liu F, Tan W, Feng X, Koopal LK (2013) Transformation of hydroxycarbonate green rust into crystalline iron (hydr)oxides: influences of reaction conditions and underlying mechanisms. Chem Geol 351:57–65
Winkler MKH, Kleerebezem R, Kuenen JG, Yang J, van Loosdrecht MCM (2011) Segregation of biomass in cyclic anaerobic/aerobic granular sludge allows the enrichment of anaerobic ammonium oxidizing bacteria at low temperatures. Environ Sci Technol 45:7330–7337
Wu J, Zhang H, Oturan N, Wang Y, Chen L, Oturan MA (2012) Application of response surface methodology to the removal of the antibiotic tetracycline by electrochemical process using carbon-felt cathode and DSA (Ti/RuO2–IrO2) anode. Chemosphere 87:614–620
Wu S, Qi Y, Gao Y, Xu Y, Gao F, Yu H, Lu Y, Yue Q, Li J (2011) Preparation of ceramic-corrosion-cell fillers and application for cyclohexanone industry wastewater treatment in electrobath reactor. J Hazard Mater 196:139–144
Xing W, Li D, Li J, Hu Q, Deng S (2016) Nitrate removal and microbial analysis by combined micro-electrolysis and autotrophic denitrification. Bioresour Technol 211:240–247
Yang K, Yue Q, Han W, Kong J, Gao B, Zhao P, Duan L (2015) Effect of novel sludge and coal cinder ceramic media in combined anaerobic–aerobic bio-filter for tetracycline wastewater treatment at low temperature. Chem Eng J 277:130–139
Yang K, Yue Q, Kong J, Zhao P, Gao Y, Fu K, Gao B (2016) Microbial diversity in combined UAF–UBAF system with novel sludge and coal cinder ceramic fillers for tetracycline wastewater treatment. Chem Eng J 285:319–330
Yao ZT, Xia MS, Sarker PK, Chen T (2014) A review of the alumina recovery from coal fly ash, with a focus in China. Fuel 120:74–85
Zagklis DP, Koutsoukos PG, Paraskeva CA (2012) A combined coagulation/flocculation and membrane filtration process for the treatment of paint industry wastewaters. Ind Eng Chem Res 51:15456–15462
Zhang B-h, Wu D-y, Wang C, He S-b, Zhang Z-j, Kong H-n (2007) Simultaneous removal of ammonium and phosphate by zeolite synthesized from coal fly ash as influenced by acid treatment. J Environ Sci 19:540–545
Zhou H, Lv P, Shen Y, Wang J, Fan J (2013) Identification of degradation products of ionic liquids in an ultrasound assisted zero-valent iron activated carbon micro-electrolysis system and their degradation mechanism. Water Res 47:3514–3522
Zhu L, Yang K, Lou B, Yuan B (2003) A multi-component statistic analysis for the influence of sediment/soil composition on the sorption of a nonionic surfactant (Triton X-100) onto natural sediments/soils. Water Res 37:4792–4800
Zhu Q, Guo S, Guo C, Dai D, Jiao X, Ma T, Chen J (2014) Stability of Fe–C micro-electrolysis and biological process in treating ultra-high concentration organic wastewater. Chem Eng J 255:535–540
Acknowledgements
This research was supported by the 12th Five-Year Plan, Ministry of Science and Technology of the People’s Republic of China, Project No. 2013BAJ10B0402, and Technology Foresight Program of Shandong Province (No. 2012GGE27011) and by the grants from Tai Shan Scholar Foundation (No. ts201511003).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Bingcai Pan
Electronic supplementary material
Fig. S1.
The EDX spectrum of two ceramics after used: (a) the EDX spectrum of SFC after used; (b) the EDX spectrum of SFFC after used (DOC 55 kb)
Rights and permissions
About this article
Cite this article
Yang, K., Jin, Y., Yue, Q. et al. Comparison of two modified coal ash ferric-carbon micro-electrolysis ceramic media for pretreatment of tetracycline wastewater. Environ Sci Pollut Res 24, 12462–12473 (2017). https://doi.org/10.1007/s11356-017-8841-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8841-8