Abstract
Low methane production and high levels of heavy metal in pig slurries limit the feasibility of anaerobic digestion of pig manure. In this study, changes in the methane production and bioavailability of heavy metals in the anaerobic digestion of diluted pig manure were evaluated using single and combined action of microscale zero-valence iron (ZVI) and magnetite. After 30 days of anaerobic digestion, the methane yield ranged from 246.9 to 334.5 mL/g VS added, which increased by 20–26% in the group added with microscale ZVI and/or magnetite relative to that in the control group. Results of the first-order kinetic model revealed that addition of microscale ZVI and/or magnetite increased the biogas production potential, rather than the biogas production rate constant. These treatments also changed the distribution of chemical fractions for heavy metal. The addition of ZVI decreased the bioavailability of Cu and Zn in the solid digested residues. Moreover, a better performance was observed in the combined action of microscale ZVI and magnetite, and the ZVI anaerobic corrosion end-product, magnetite, might help enhance methane production through direct interspecies electron transfer in ZVI-anaerobic digestion process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intensive confined livestock and poultry production systems generate large quantities of manure by-products, which cause serious contamination of groundwater and/or surface water. More than 209.3 million tons of pig manure was generated in China in 2009 (Zhang et al. 2014). Effective management and unitization of pig manure have been a particular concern for government and scientific community in China. Anaerobic digestion is one of the most promising and widely utilized methods for organic matter treatment because of its advantageous properties in the pollutant removal and renewable bioenergy production. However, low hydrolysis rate and methane yield restrict the economic feasibility of anaerobic digestion because of the limiting steps of fiber hydrolysis and high ammonium inhibition (Bonmati et al. 2001; Ferreira et al. 2014). Therefore, a cost-effective approach for enhancing methane production from anaerobic digestion of pig manure must be developed.
Feed additives containing potential toxic elements, such as Cu and Zn, are extensively applied as essential nutrients and supplements to improve health and feed efficiency (Bolan et al. 2004). A major portion of the metals ingested is excreted in feces and urine; the majority of the metals reach the feces. During anaerobic digestion, the decomposition of organic substances caused an increase in the heavy metal concentration of digestate (Dąbrowska and Rosińska 2012; Jin and Chang 2011). In a previous study, digested slurry samples from 21 large-scale anaerobic digestion plants in the Jiangsu Province of China were analyzed; the total Zn and Cu concentrations in solid fraction of digestate were 399.7–671.2 and 113.6–312.9 mg/kg, respectively (Jin and Chang 2011). The digested slurries for pig manure are commonly used as organic fertilizers or soil amendments because of their ability to alter soil properties, such as plant nutrient availability, soil reaction, organic matter content, and water holding capacity (Bolan et al. 2004). Therefore, the high levels of Cu and Zn in digested pig slurries may cause potential environmental hazards.
The chemical forms of heavy metals, rather than the total heavy metal concentration, are important to determine the mobility, toxicity, and bioavailability of these metals (Hsu and Lo 2000; Liu et al. 2007). Sequential fractionation schemes are commonly used to determine metal distribution in different chemical forms, namely, soluble, exchangeable, bound to carbonates, bound to Fe–Mn oxides, bound to organic matter, and residual. The transfer and bioavailability of heavy metal during anaerobic digestion have been widely studied. Marcato et al. (2009) assessed the shift of Cu and Zn bioavailability from pig slurry before and after anaerobic digestion through chemical and biological approaches; chemical assessments revealed the lower mobility of metals, but biological assessment indicated contradictory results. Dong et al. (2013) reported that high-solid anaerobic digestion increased the bioavailability of Cu, Zn, Ni, and Cr but decreased the bioavailability of Pb. Therefore, new measures must be examined to reduce metal bioavailability during anaerobic digestion of pig manure.
Zero-valence iron (ZVI) is non-toxic, abundant, cheap, and easy to produce; as such, ZVI has been successfully applied in the remediation/treatment of groundwater and wastewater contaminated with less biodegradable pollutants, such as heavy metals and chlorinated organic compounds (Fu et al. 2014; Xiao et al. 2013). Previous studies showed that ZVI addition to anaerobic digestion system increased the methane production efficiency by decreasing the oxidation–reduction potential; ZVI also acted as an acid buffer and an effective electron donor to improve the activity of microorganisms and the abundance of hydrogen-consuming microorganisms (Feng et al. 2014; Xiao et al. 2013; Zhen et al. 2015). For example, Wu et al. (2015) found that addition of an appropriate dosage of ZVI positively influenced chemical oxygen demand (COD) removal and methane production during the anaerobic treatment of swine wastewater. Besides, metal bioavailability may be decreased because of the interaction of ZVI with Fe, Mn, and Al oxides (Donner et al. 2013; Moore et al. 1998). The mechanism of bioavailability change in heavy metal by ZVI is highly complex and involves adsorption, reduction, surface precipitation, and co-precipitation with various iron corrosion products (Fu et al. 2014). Suanon et al. (2016) recently demonstrated that nanoscale ZVI and magnetite could regulate the bioavailability of metals during anaerobic digestion of sewage sludge. Compared with microscale ZVI and magnetite, nanoscale ZVI and magnetite have a higher specific surface area, adsorption capacity, and reactivity (Fu et al. 2014; Zhen et al. 2015), so different influence of nanoscale and microscale materials on methane production and metal bioavailability might happen in the anaerobic systems and this interested information has not been reported yet. And simultaneously, a poor fundamental understanding in the role of ZVI and its anaerobic corrosion end-product (magnetite) still greatly hampered their application in decreasing the bioavailability of metals.
Magnetite is the major end-product of ZVI anaerobic corrosion (Ruhl et al. 2014; Zhu et al. 2014). Magnetite can be inorganically or biologically generated; this compound is ubiquitous in igneous, metamorphic, and sedimentary environments (Perez-Gonzalez et al. 2010). Previous studies revealed that magnetite functions as an electron conduit to improve the rates of organic biodegradation and methane production under anaerobic conditions, because magnetite accelerates syntrophic or cooperative metabolism between electron-donating and electron-accepting microorganisms through direct interspecies electron transfer (Aulenta et al. 2014; Cruz Viggi et al. 2014; Kato et al. 2012a). In contrast to nanoscale magnetite, microscale magnetite has been scarcely investigated. So far, the combined effect of ZVI and magnetite on the methane production and bioavailability of heavy metals has not been reported yet, particularly for anaerobic digestion of pig manure.
This study aims to determine the effect of the single and combined action of microscale ZVI and magnetite on the methane production and bioavailability of heavy metals during batch anaerobic digestion of diluted pig manure. The methane production, first-order model simulation, and changes in different parameters, such as pH, volatile fatty acids (VFAs), COD, ammonia nitrogen, and total alkalinity (TA) of the digested liquid, were studied. Moreover, changes in the chemical fractions of heavy metals, such as Cu and Zn, were assessed by sequential fractionation schemes. Therefore, the role of ZVI and magnetite in change of methane production and heavy metal bioavailability during anaerobic digestion of diluted pig manure was further evaluated.
Materials and methods
Pig manure and inoculum
Pig manure was collected from a large-scale pig farm in Anhui Province, China, in August 2015. The manure was stored in the dark at 4 °C for less than a week. Microscale ZVI (>98% purity; 400 mesh) and microscale magnetite (>99% purity; 400 mesh) were purchased from Tianjin Bodi-Chemical Co. Ltd. (China) and were not any further treated before use.
An anaerobic culture was obtained from the anaerobic digestion of swine wastewater in our laboratory; the culture was used as seed and concentrated before use as inoculum. Table 1 shows the chemical characteristics of the feedstock and anaerobic culture.
Batch anaerobic digestion
Previous study reported that the optimal microscale ZVI loading was 20 g/L in anaerobic digestion of waste activated sludge (Feng et al. 2014), and simultaneously, Wu et al. (2015) further proved that the ZVI concentration of 50 g/L had a negative effect on microbial activity. Recently, Gacitua et al. (2014) found that 10 g/L magnetite nanoparticles effectively enhanced the electrocatalytic activity. So, 20 g/L ZVI and 10 g/L magnetite were selected as adding dosages in the present study. Batch anaerobic digestion was conducted in a 1-L jar. The following four experimental groups were prepared: R1, without ZVI and magnetite as control; R2, with ZVI; R3, with magnetite; and R4, with ZVI and magnetite. The experimental design of the batch anaerobic digestion is presented in Table 2. This experiment was designed to investigate changes in the process performance and bioavailability of heavy metals.
The feedstock mixture was added to the reactor, which was immediately sealed with butyl rubber stoppers after flushing with nitrogen gas for 2 min. The anaerobic digestion reactions were maintained at 35 ± 1 °C in an incubator. Each reactor was manually mixed twice daily to avoid stratification during the 30 days of anaerobic digestion. Each experimental group was conducted in triplicate. The sample from anaerobic digestion was collected and centrifuged, and the solid phase was used to analyze the chemical fractions of Cu and Zn.
Kinetic evaluation and calculation
Hydrolysis is a rate-limiting step in anaerobic decomposition of pig manure (Ferreira et al. 2014). The first-order kinetic model for assessing biogas production (Liang et al. 2014) may be expressed as Eq. (1), where B t (mL/g) is the cumulative biogas yield at the t day; B 0 (mL/g) is the biogas production potential and calculated by a previously reported method (Chen and Hashimoto 1978) to be equal to the intercept by linear fitting the plot of B t against 1/t; k (/day) is the biogas production rate constant; and t (days) is the digestion time.
The bioavailability of heavy metals could be determined using the ratio of φ in Eq. (1) (Dong et al. 2013; Liu et al. 2007), where C(a) is the sum of the concentrations of exchangeable, carbonate, Fe–Mn oxide, and organic matter fractions and C(t) is the sum of C(a) and residual concentrations.
Analytical methods
According to Tessier et al. (1979) and Liu et al. (2007), the five fractions of heavy metals are defined as follows: (1) exchangeable fraction (1 M MgCl2 at pH 7), (2) carbonate fraction (1 M sodium acetate at pH 5), (3) Fe–Mn oxide fraction (0.04 M NH2OH·HCl in 25% acetic acid), (4) organic matter fraction (0.02 M HNO3 and 30% H2O2 at pH 2 and 85 °C followed by 3.2 M ammonium acetate in 20% HNO3), and (5) residual fraction (HF–HNO3–HClO4 digestion). After each successive extraction, the supernatant was obtained by centrifugation at 4000 rpm for 20 min. The supernatant was filtered into 0.45-μm membrane, and the volume was adjusted to 25 mL by adding deionized water. Three extraction replicates were conducted for each sample. Cu and Zn contents were determined by inductively coupled plasma emission spectrometry (iCAP 6300 Series).
The liquid sample from anaerobic digestates was centrifuged at 4000 rpm for 5 min and the centrifugal supernatant was used for analysis of TA, COD, NH4 +–N, and VFAs. Standard methods were used to measure TS, VS, COD, and NH4 +–N (APHA 1995). Biogas production was measured by water displacement method (Liang et al. 2014) and reported at 0 °C and 1.013 × 105 Pa. Methane content was determined with an Orsat-type gas analyzer (APHA 1995). VFAs were measured by colorimetric ferric hydroxamate method with acetic acid as standard (Liang et al. 2016). TA was determined by titrating a sample with standard HCl to pH 4.3 (Björnsson et al. 2001). C, H, N, and S contents were determined with an elemental analyzer (Vario EL Cube, Germany).
Statistical analysis
One-way ANOVA was performed using SPSS 14.0 (Windows version) software to evaluate significant differences in data; the confidence interval was set at p ≤ 0.05.
Results and discussion
Change in the methane production
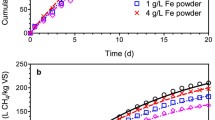
Figure 1 presents the time course of biogas production within 30 days of anaerobic digestion of pig manure. The biogas production efficiency in anaerobic digestion of pig manure is summarized in Table 3. The daily biogas production rate increased rapidly during the 6 days of anaerobic digestion and then decreased between days 7 and 18. The maximum daily biogas production rate was found on day 6 and reached 2366 mL/day for R1, 2135 mL/day for R2, 2125 mL/day for R3, and 2070 mL/day for R4. After digestion for 30 days, the specific biogas and methane yields ranged from 447.5 to 500.0 mL/g VS added and from 246.9 to 334.5 mL/g VS added, respectively (Table 3). The specific methane yield obtained is consistent with the methane yield (263.5–354.7 mL/g VS added) reported for anaerobic digestion of pig manure for 40 days of digestion (Zhang et al. 2014). The results are also close to that of thermal steam-explored pig manure (200–329 mL/g VS added) but higher than that of raw pig manure (159 mL/g VS added) (Ferreira et al. 2014). An increase of the methane content from 59.2% for control group (R1) to 65.9–66.9% for added ZVI and/or magnetite was observed. However, the mean value of methane content was not significantly different based on the statistical analysis.
The addition of ZVI into the anaerobic systems increased the methane yields; the specific methane yield (R2) based on VS content increased by 20% compared with the control group (R1) (Table 3). This result agrees with those of previous reports (Feng et al. 2014; Wu et al. 2015; Zhen et al. 2015). For example, Wu et al. (2015) observed that the methane yield initially increased with increasing amount of ZVI added, but high ZVI concentrations (>50 g/L) weakened the ZVI-facilitated effect on the anaerobic digestion of swine wastewater. Suanon et al. (2016) also found that an increase in 45.8% of methane yield occurred at the anaerobic digestion of waste sludge under nanoscale ZVI with a dose of 0.5%, and this enhancing extent of nanoscale ZVI was higher than that of microscale ZVI in this study. This might be ascribed to the nanoscale ZVI with the higher reacting capacity. The enhancing effect of ZVI on methane production during anaerobic digestion may be attributed to the following reasons. First, the anaerobic corrosion of ZVI generated H2, which could be effectively utilized by hydrogenotrophic methanogens to produce CH4 and/or homoacetogenic bacteria to produce acetate (Daniels et al. 1987; Wu et al. 2015; Zhen et al. 2015). Second, ZVI could directly serve as an electron donor for reducing CO2 to CH4 via autotrophic methanogenesis (Feng et al. 2014; Karri et al. 2005; Zhen et al. 2015). However, the methane production was only 2.0 mL for 20 g/L ZVI, which is relatively lower than the increase in methane yield (Feng et al. 2014). Karri et al. (2005) also reported that the net methane production from this contribution accounted for only 0.7% of the electron equivalents supplied with 46.6 g/L ZVI. The final and most important reason may be that ZVI addition accelerated the hydrolysis, acidification, and methanation steps of anaerobic digestion by increasing the enzyme activity and improving the abundance of hydrogen-consuming microorganisms (Feng et al. 2014; Kong et al. 2016; Zhen et al. 2015), thereby creating beneficial conditions for anaerobic digestion.
In this study, the methane yield of R3 after the addition of 10 g/L magnetite increased by 22% (Table 3). Suanon et al. (2016) found that the methane yield from sewage sludge increased by 25.6% at the addition of 0.5% (w/w) nanoscale magnetite, whereas methane production was inhibited at the 1% level. Moreover, recent study reported that the methane yield from cattle dung slurry increased from 140.3 mL/g VS for the control group to 302.5 mL/g VS for the amended group with the addition of 20 g/L nanoscale magnetite (Abdelsalam et al. 2016). This can be explained by the fact that magnetite can compensate for the lack of the electron transfer functions of a multi-heme c-type cytochrome (Liu et al. 2015a) and accelerates syntrophic metabolism between microorganisms through direct interspecies electron transfer. By contrast, the no increase in methane yield was found in anaerobic digestion propionate or butyrate (Cruz Viggi et al. 2014; Li et al. 2015; Yamada et al. 2015) and acetate or ethanol (Kato et al. 2012b) by adding micro- or nanoscale magnetite. The different enhancements of methane yield for various digested substrates depend on the biodegradable characteristics of the digested substrate.
The methane yield of R4, with 20 g/L ZVI and 10 g/L magnetite, was significantly higher than that in the other groups to achieve the highest methane yield of 334.5 ± 7.8 mL/g VS added as well as the highest methane content (Table 3). Liu et al. (2015b) also demonstrated that the rusty iron scrap was more effective than the iron powder for improving methane production from waste activated sludge; the rusty scrap had a corrosion layer containing magnetite. This observation suggested that cooperation occurred between ZVI and magnetite.
Change in the kinetic parameters
The modeling results of biogas production with the first-order kinetic model are shown in Table 4. All the plots showed good linearity, with coefficients of determination greater than 0.9. The biogas production rate constants (k) ranged from 0.098 to 0.103/day. This value is higher than the results of the anaerobic digestion of pig manure (0.0104–0.0458/day) (Zhang et al. 2014), Spartina alterniflora (0.028–0.052/day) (Liang et al. 2014), and waste activated sludge (0.071–0.083/day) (Liu et al. 2015b). Moreover, the biogas production potential (B 0) of pig manure ranged from 460.6 to 520.7 mL/g TS, and the degradation extent (B 30 /B 0) ranged from 0.85 to 0.86. No significant differences in the degradation extent were found among the four experimental groups.
Compared with the k value of the control group, ZVI and/or magnetite addition did not increase the k value. But a higher biogas production potential (B 0) was found in amended groups with ZVI and/or magnetite addition and increased by 8.9–13% compared with control group (R1) (Table 4). This trend suggested that the addition of microscale ZVI and/or magnetite increased the biogas production potential, rather than the biogas production rate constant. A similar result was reported with ZVI addition to the anaerobic digestion of waste sludge (Liu et al. 2015b). By contrast, the biogas production rate increased, but the biogas production potential remained invariable for easily degradable and simple organic matter, such as propionate, butyrate, acetate, and ethanol (Cruz Viggi et al. 2014; Kato et al. 2012b; Li et al. 2015; Yamada et al. 2015). This trend might be attributed to differences in the biodegradable characteristics of the digested substrate.
Change in pH, COD, VFAs, NH4 +–N, and TA concentrations
Figure 2 shows the variations in pH, VFAs, COD, NH4 +–N, and TA contents of the liquid with increasing duration of anaerobic digestion. The pH rapidly decreased from 8.2 to 7.0 in the first 5 days of digestion, and ZVI and/or magnetite addition slowed down the extent of pH reduction (Fig. 2a). The anaerobic corrosion of ZVI generated hydroxyl ions, thereby increasing the buffering capacity (Zhen et al. 2015). Similar phenomena were described in literature (Kong et al. 2016; Zhen et al. 2015). Kong et al. (2016) observed that the addition of ZVI to the acidogenic reactors effectively reduced the extent of acidification during anaerobic digestion of food waste.
The rapid decrease in pH was attributed to the rapid accumulation of COD and VFAs (Fig. 2b, c). The VFA and COD content peaked at 7 days of digestion, and the maximum VFA contents reached 7890 mg/L for R1, 6990 mg/L for R2, 6250 mg/L for R3, and 6790 mg/L for R4. Siegert and Banks (2005) found that the inhibitory effect of VFAs on the biogas production and methane content was evident above 6 g/L. In the present study, a rapid reduction of biogas production rate was observed after the 7th day of digestion (Figs. 1a), which indicates that anaerobic digestion may be partly inhibited by the accumulated VFAs. The highest VFA content was obtained by the control group (R1), whereas a higher COD value was reached in the R2, R3, and R4 groups with ZVI and/or magnetite addition. This different trend of peak value occurred at COD and VFAs for control and amended groups suggested that a higher inhibition in the methane-producing process might happen at initial stage for control group, when a lower biogas production rate was considered at the 7th–13th days’ digestion for control group (Fig. 1a).
Ammonium nitrogen might inhibit microbial activity during anaerobic digestion of pig manure (Bonmati et al. 2001). The ammonium nitrogen content ranged from 200 to 500 mg/L for the four experimental groups (Fig. 2d). Rajagopal et al. (2013) found that ammonium nitrogen content ranging from 200 to 1000 mg/L had no antagonistic effects on anaerobic digestion. Hence, ammonium nitrogen inhibition did not occur during the anaerobic digestion of pig manure. TA concentrations ranged from 2300 to 3200 mg/L CaCO3 (Figs. 2e), which belonged to the normal range for healthy anaerobic digesters.
Change in the bioavailability of Cu and Zn
Suanon et al. (2016) studied the effect of nanoscale ZVI and magnetite on the bioavailability of metals during anaerobic digestion of waste activated sludge; the results showed that up to 90% of the metals remained in the solid phase. Hence, the chemical fraction of metals in the solid phase of digested pig manure was only determined in the present study.
The initial heavy metal content of mixed slurries was calculated based on the TS weight and metal content of raw pig manure and anaerobic culture (Table 1) and reached 179 mg/kg for Cu and 441 mg/kg for Zn, respectively. After 30 days of digestion, the heavy metal content of the solid phase from digested pig manure reached 202–330 mg/kg for Cu and 532–739 mg/kg for Zn, respectively. The heavy metal content of the solid phase from anaerobic digestion agreed with the results reported by Jin and Chang (2011) (113.6–312.9 mg/kg for Cu and 399.7–671.2 mg/kg for Zn). With anaerobic digestion, the heavy metal content was concentrated by 1.1–1.8 times for Cu and 1.2–1.7 times for Zn compared with the total content before anaerobic digestion. Similar phenomena were reported in anaerobic digestion (Dąbrowska and Rosińska 2012; Jin and Chang 2011) and compost processes (Liu et al. 2007) because of weight loss through organic matter decomposition. A check on the results of sequential extraction procedure was conducted by the overall recovery rates which were equal to the ratio of the sum of exchangeable, carbonate, Fe–Mn oxide, organic matter, and residual fractions, and the total concentrations of Cu and Zn from HF–HNO3–HClO4 digestion procedure. The overall recovery rates of Cu and Zn reached 87.3–106.7% in the present study, indicating that sequential extraction method was exact and reliable in detecting the speciation of Cu and Zn from anaerobic digestion of pig manure.
The distribution of the chemical fractions is shown in Fig. 3. The highest percentage of Cu was associated with organic matter; this value increased from 44.6% for raw slurries to 74.4–85.5% for the digested solid phase. A similar result was also reported in swine manure compost (Hsu and Lo 2000). This trend might be due to the high affinity of Cu for humic acid species (Donner et al. 2012). But Suanon et al. (2016) found that the chemical fraction of Cu was dominant in Fe–Mn oxide-bound fraction under nanoscale ZVI and magnetite after anaerobic digestion, whereas carbonate-bound and organic-bound fractions apparently decreased after anaerobic digestion. From Fig. 3a, the amount of exchangeable, carbonate, and Fe–Mn oxide fractions decreased in the solid phase after anaerobic digestion compared with raw slurries. Literature reported that exchangeable fraction is susceptible to change of ionic composition in the liquid, and carbonate fraction is readily influenced by pH variations, whereas Fe–Mn oxide fraction is unstable in reductive conditions (He et al. 2009). Hence, this change indicates that the bioavailability of Cu decreased after anaerobic digestion. The residual fraction only slightly changed, i.e., from 10.4% for raw slurry before anaerobic digestion to 7.3–11.7% for the four groups after anaerobic digestion (Fig. 3a). Compared with the control group (R1), a lower carbonate and Fe–Mn oxide fractions and a higher residual fraction occurred at amended groups. Carbonate and Fe–Mn oxide fractions are unstable, whereas residual fraction is permanently fixed in crystal lattice and stable (He et al. 2009). This indicates that the bioavailability of Cu for amended groups was lower than that of control group (R1).
Zinc was principally distributed throughout the carbonate fraction (49.6–58.9%) and Fe–Mn fraction (18.7–32.3%; Fig. 3b). A similar result was reported in the swine manure compost (Hsu and Lo 2000). Suanon et al. (2016) also reported that Zn was dominant in Fe–Mn oxide-bound fraction under nanoscale ZVI and magnetite after anaerobic digestion. By contrast, Donner et al. (2012) found that Zn was mainly partitioned to iron oxides in biosolids. The residual fraction of Zn significantly increased in the groups with added microscale ZVI and/or magnetite (p < 0.05). Moreover, the exchangeable fraction of Zn significantly decreased in the groups with the added microscale ZVI (p < 0.05). A lower Fe–Mn oxide and organic fractions and a higher residual fraction for amended groups indicate that the bioavailability of Zn for amended groups was lower than that of control group (R1).
The φ value calculated by Eq. (2) showed that the control group (R1) did not decrease the bioavailability of Cu and Zn. By contrast, ZVI addition (R2 and R4) decreased the bioavailability of Cu and Zn. Moreover, magnetite addition (R3) decreased the bioavailability of Zn and slightly increased the bioavailability of Cu (Fig. 4). Dong et al. (2013) studied the change in heavy metal speciation during high-solid anaerobic digestion of sewage sludge and found a slight increase in the values of φ for Cu and Zn after anaerobic digestion. By contrast, Suanon et al. (2016) used the total percent of water-soluble, exchangeable, and carbonate-bound fractions of heavy metal as mobility factor to assess the bioavailability of heavy metal. They found that nanoscale ZVI and magnetite decreased the bioavailability of heavy metals, including Cu and Zn.
Based on the above analysis, it can be concluded that the addition of microscale ZVI during anaerobic digestion changed the distribution of the chemical fraction and decreased the bioavailability of Cu and Zn, because ZVI might change chemical speciation by adsorption, reduction, surface precipitation, and co-precipitation with various iron corrosion products (Fu et al. 2014), thereby decreasing the bioavailability of Cu and Zn. Magnetite, the major end-product of ZVI anaerobic corrosion, might enhance the biodegradation of organic matter by direct interspecies electron transfer, thereby changing the distribution of Cu and Zn. Based on R4 with a higher performance in methane production and bioavailability reduction, it was concluded that the ZVI anaerobic corrosion end-product, magnetite, might help enhance methane production through direct interspecies electron transfer in ZVI-anaerobic digestion process.
Conclusions
After 30 days of digestion, the methane yields from the anaerobic digestion of pig manure ranged from 246.9 to 334.5 mL/g VS added. Microscale ZVI and/or magnetite addition increased 20–26% of methane yields. The results of the first-order kinetic model revealed that ZVI and/or magnetite addition increased the biogas production potential, rather than the biogas production rate constant. Furthermore, the ZVI anaerobic corrosion end-product, magnetite, might help enhance methane production through direct interspecies electron transfer in ZVI-anaerobic digestion process. Moreover, microscale ZVI and/or magnetite addition changed the distribution of the chemical fractions and regulated the bioavailability of Cu and Zn. The combined action of microscale ZVI and magnetite achieved better performance.
References
Abdelsalam E, Samer M, Attia Y, Abdel-Hadi M, Hassan H, Badr Y (2016) Comparison of nanoparticles effects on biogas and methane production from anaerobic digestion of cattle dung slurry. Renew Energy 87:592–598
APHA A WEF (1995) Standard methods for the examination of water and wastewater 19th ed Washington, DC: American Public Health Association
Aulenta F, Fazi S, Majone M, Rossetti S (2014) Electrically conductive magnetite particles enhance the kinetics and steer the composition of anaerobic TCE-dechlorinating cultures. Process Biochem 49:2235–2240
Björnsson L, Murto M, Jantsch TG, Mattiasson B (2001) Evaluation of new methods for the monitoring of alkalinity, dissolved hydrogen and the microbial community in anaerobic digestion. Water Res 35:2833–2840
Bolan N, Adriano D, Mahimairaja S (2004) Distribution and bioavailability of trace elements in livestock and poultry manure by-products. Crit Rev Environ Sci Technol 34:291–338
Bonmati A, Flotats X, Mateu L, Campos E (2001) Study of thermal hydrolysis as a pretreatment to mesophilic anaerobic digestion of pig slurry. Water Sci Technol 44:109–116
Chen Y, Hashimoto A Kinetics of methane fermentation. In: Biotechnol. bioeng. symp.;(United States), 1978. Dept. of Agriculture, Clay Center, NE
Cruz Viggi C, Rossetti S, Fazi S, Paiano P, Majone M, Aulenta F (2014) Magnetite particles triggering a faster and more robust syntrophic pathway of methanogenic propionate degradation. Environ Sci Technol 48:7536–7543
Dąbrowska L, Rosińska A (2012) Change of PCBs and forms of heavy metals in sewage sludge during thermophilic anaerobic digestion. Chemosphere 88:168–173
Daniels L, Belay N, Rajagopal BS, Weimer PJ (1987) Bacterial methanogenesis and growth from CO2 with elemental iron as the sole source of electrons. Science 237:509–511
Dong B, Liu X, Dai L, Dai X (2013) Changes of heavy metal speciation during high-solid anaerobic digestion of sewage sludge. Bioresour Technol 131:152–158
Donner E et al (2012) A multi-technique investigation of copper and zinc distribution, speciation and potential bioavailability in biosolids. Environ Pollut 166:57–64
Donner E, Brunetti G, Zarcinas B, Harris P, Tavakkoli E, Naidu R, Lombi E (2013) Effects of chemical amendments on the lability and speciation of metals in anaerobically digested biosolids. Environ Sci Technol 47:11157–11165
Feng Y, Zhang Y, Quan X, Chen S (2014) Enhanced anaerobic digestion of waste activated sludge digestion by the addition of zero valent iron. Water Res 52:242–250
Ferreira L, Souza T, Fdz-Polanco F, Pérez-Elvira S (2014) Thermal steam explosion pretreatment to enhance anaerobic biodegradability of the solid fraction of pig manure. Bioresour Technol 152:393–398
Fu F, Dionysiou DD, Liu H (2014) The use of zero-valent iron for groundwater remediation and wastewater treatment: a review. J Hazard Mater 267:194–205
Gacitua MA, González B, Majone M, Aulenta F (2014) Boosting the electrocatalytic activity of Desulfovibrio paquesii biocathodes with magnetite nanoparticles. International Journal of Hydrogen Energy 39:14540–14545
He MM, Tian GM, Liang XQ (2009) Phytotoxicity and speciation of copper, zinc and lead during the aerobic composting of sewage sludge. J Hazard Mater 163:671–677
Hsu J-H, Lo S-L (2000) Characterization and extractability of copper, manganese, and zinc in swine manure composts. J Environ Qual 29:447–453
Jin H, Chang Z (2011) Distribution of heavy metal contents and chemical fractions in anaerobically digested manure slurry. Appl Biochem Biotechnol 164:268–282
Karri S, Sierra-Alvarez R, Field JA (2005) Zero valent iron as an electron-donor for methanogenesis and sulfate reduction in anaerobic sludge. Biotechnol Bioeng 92:810–819
Kato S, Hashimoto K, Watanabe K (2012a) Methanogenesis facilitated by electric syntrophy via (semi) conductive iron-oxide minerals. Environ Microbiol 14:1646–1654
Kato S, Hashimoto K, Watanabe K (2012b) Microbial interspecies electron transfer via electric currents through conductive minerals. Proc Natl Acad Sci 109:10042–10046
Kong X, Wei Y, Xu S, Liu J, Li H, Liu Y, Yu S (2016) Inhibiting excessive acidification using zero-valent iron in anaerobic digestion of food waste at high organic load rates. Bioresour Technol 211:65–71
Li H, Chang J, Liu P, Fu L, Ding D, Lu Y (2015) Direct interspecies electron transfer accelerates syntrophic oxidation of butyrate in paddy soil enrichments. Environ Microbiol 17:1533–1547
Liang YG, Yin SS, Si YB, Zheng Z, Yuan SJ, Nie E, Luo XZ (2014) Effect of pretreatment and total solid content on thermophilic dry anaerobic digestion of Spartina alterniflora. Chem Eng J 237:209–216
Liang YG, Cheng B, Si YB, Cao DJ, Li DL, Chen JF (2016) Effect of solid-state NaOH pretreatment on methane production from thermophilic semi-dry anaerobic digestion of rose stalk. Water Sci Technol 73:2913–2920
Liu Y, Ma L, Li Y, Zheng L (2007) Evolution of heavy metal speciation during the aerobic composting process of sewage sludge. Chemosphere 67:1025–1032
Liu F, Rotaru AE, Shrestha PM, Malvankar NS, Nevin KP, Lovley DR (2015a) Magnetite compensates for the lack of a pilin-associated c-type cytochrome in extracellular electron exchange. Environ Microbiol 17:648–655
Liu Y, Wang Q, Zhang Y, Ni B-J (2015b) Zero valent iron significantly enhances methane production from waste activated sludge by improving biochemical methane potential rather than hydrolysis rate. Scientific Reports 5:8263
Marcato C-E, Pinelli E, Cecchi M, Winterton P, Guiresse M (2009) Bioavailability of Cu and Zn in raw and anaerobically digested pig slurry. Ecotoxicol Environ Saf 72:1538–1544
Moore P, Daniel T, Gilmour J, Shreve B, Edwards D, Wood B (1998) Decreasing metal runoff from poultry litter with aluminum sulfate. J Environ Qual 27:92–99
Perez-Gonzalez T, Jimenez-Lopez C, Neal AL, Rull-Perez F, Rodriguez-Navarro A, Fernandez-Vivas A, Iañez-Pareja E (2010) Magnetite biomineralization induced by Shewanella oneidensis. Geochim Cosmochim Acta 74:967–979
Rajagopal R, Massé DI, Singh G (2013) A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour Technol 143:632–641
Ruhl AS, Franz G, Gernert U, Jekel M (2014) Corrosion product and precipitate distribution in two-component Fe(0) permeable reactive barriers. Chem Eng J 239:26–32
Siegert I, Banks C (2005) The effect of volatile fatty acid additions on the anaerobic digestion of cellulose and glucose in batch reactors. Process Biochem 40:3412–3418
Suanon F, Sun Q, Mama D, Li J, Dimon B, Yu C-P (2016) Effect of nanoscale zero-valent iron and magnetite (Fe3O4) on the fate of metals during anaerobic digestion of sludge. Water Res 88:897–903
Tessier A, Campbell PG, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
Wu D, Zheng S, Ding A, Sun G, Yang M (2015) Performance of a zero valent iron-based anaerobic system in swine wastewater treatment. J Hazard Mater 286:1–6
Xiao X, Sheng GP, Mu Y, Yu HQ (2013) A modeling approach to describe ZVI-based anaerobic system. Water Res 47:6007–6013
Yamada C, Kato S, Ueno Y, Ishii M, Igarashi Y (2015) Conductive iron oxides accelerate thermophilic methanogenesis from acetate and propionate. J Biosci Bioeng 119:678–682
Zhang W, Lang Q, Wu S, Li W, Bah H, Dong R (2014) Anaerobic digestion characteristics of pig manures depending on various growth stages and initial substrate concentrations in a scaled pig farm in southern China. Bioresour Technol 156:63–69
Zhen G, Lu X, Li Y-Y, Liu Y, Zhao Y (2015) Influence of zero valent scrap iron (ZVSI) supply on methane production from waste activated sludge. Chem Eng J 263:461–470
Zhu L, Gao K, Jin J, Lin H, Xu X (2014) Analysis of ZVI corrosion products and their functions in the combined ZVI and anaerobic sludge system. Environ Sci Pollut Res 21:12747–12756
Acknowledgments
This work was partly supported by National Key Technology Support Program (2012BAD14B13), Anhui Provincial Natural Science Foundation (1508085SMB210), and the open project of Key Laboratory of energy saving and waste disposal of protected agriculture of Ministry of Agriculture (ECWM-2017KT-01).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Liang, Yg., Li, Xj., Zhang, J. et al. Effect of microscale ZVI/magnetite on methane production and bioavailability of heavy metals during anaerobic digestion of diluted pig manure. Environ Sci Pollut Res 24, 12328–12337 (2017). https://doi.org/10.1007/s11356-017-8832-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8832-9