Abstract
The aim of this study was to investigate the presence of heavy metals/metalloids (Pb, Cd, Hg, Cu, Fe, Zn, As) in the muscle tissue of fish from the Danube River (two locations: Zemun and Grocka). For the purpose of heavy metal determination in fish muscle, 120 samples of six different fish species, Prussian carp, barbel, bream, carp, pike perch, and catfish were collected. For determining heavy metals, we used microwave oven digestion and atomic absorption spectrometer methods. The highest average content of Pb (0.084 ± 0.004 mg kg−1), Cd (0.082 ± 0.003 mg kg−1), Hg (0.466 ± 0.006 mg kg−1), and As (0.333 ± 0.007 mg kg−1) was found in the muscle of carp (an omnivorous fish) from Grocka, while the highest average level of Fe (13.60 ± 0.03 mg kg−1) was deposited in bream (also omnivorous) from Zemun. Also, the average Cu level (1.62 ± 0.13 mg kg−1) was the highest in catfish muscle (a carnivorous fish) from Grocka, while the highest Zn content (11.16 ± 0.17 mg kg−1) was determined in muscle of Prussian carp (an omnivorous fish) from Zemun. The highest content of heavy metals (Cu, Fe, and Zn, respectively) in muscle of the six different types of fish from both locations was symmetrically arranged by species (catfish, barbel, and Prussian carp, respectively). Concentrations of Pb, Hg, and As in the Danube River fish muscle were under the maximum residual levels prescribed by the European Union (EU) and the maximum allowed concentrations (MAC) for Serbia. On the other hand, in all fish muscle from both locations (Zemun and Grocka), higher concentrations of Cd than prescribed (MAC) were found, with the exception of bream and pike perch.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The water pollution sources (atmospheric deposition, geologic weathering and the discharge of agricultural waste products, industrial and urban sewage discharges) of heavy metals are one of the greatest threats to biota because of their persistence, and possible bioaccumulation and biomagnification in food chains (Maceda-Veiga et al. 2012). Many studies have described the bioaccumulative capacity of heavy metals in a variety of aquatic organisms, and therefore, in fish, because they are located at the top of the food chain in aquatic systems (Janjić et al. 2015; Verep et al. 2012; Jovičić et al. 2015). Direct transfer of heavy metals from sediment in aquatic organisms is considered the main route or mode of transition in many aquatic species (metals accumulate in bottom feeders and also penetrate upwards in the food chain via biomagnification) (Colin et al. 2016a, 2016b; Cole et al. 2009; Vallod and Sarrazin 2010; Subotić et al. 2013), while the further consumption of fish can cause disease in humans. Because of this, the consumption of fish is considered one of the most important sources of human exposure to heavy metals (Milanov et al. 2016; Squadrone et al. 2013). The level of bioaccumulation and bioavailability of heavy metals in fish tissues is under the influence of biotic and abiotic factors, such as biological habitats of fish, different organic and inorganic chemical forms of metals in water, water temperature and pH, the concentration of oxygen in the water, as well as gender, age, body weight, and physiological condition of the fish (Kehrig et al. 2013; Has-Schön et al. 2006). Eating habits can have a big influence on the accumulation of toxic elements in various fish tissues (Milanov et al. 2016; Kehrig et al. 2013; Lenhardt et al. 2012; Zrnčić et al. 2013). Carnivorous fish accumulate higher levels of metals due to their biomagnification ability compared to species that are at a lower trophic level. (Subotić et al. 2013). On the other hand, the elements cadmium (Cd) and arsenic (As) are related to sediments, which can be indirect and long-term sources of contamination of fish that feed on benthic organisms (Noël et al. 2013).
Research suggests that high mercury content in fish may reduce the cardioprotective effect of fish intake (Guallar et al. 2002; Castro-Gonzalez and Mendez-Armenta 2008). Cadmium, lead, and arsenic (Cd, Pb, and As) are also associated with serious adverse effects on the health of children and adults (Velev et al. 2009; Janković et al. 2013). Many studies showed that bioaccumulation causes significant impacts on the ecosystem worldwide (Burger and Gochfeld 2005; Andreji et al. 2006; Falco et al. 2006). The heavy metals iron, copper, and zinc (Fe, Cu, and Zn) belong to the group of microelements, nutritive substances that are essential for important biochemical processes in all living organisms (Wei et al. 2014; Monroy et al. 2014). However, if present in high concentrations, these elements can lead to undesirable effects on human and animal health (Storelli et al. 2007; Mitic et al. 2015).
Rivers are dynamic ecosystems and it may be difficult to detect metals in the environment, which often carries concentrations under detection limits when compared to the levels stored in biota. This further highlights the importance of fish in studies monitoring metal pollution, along with the fact that fish seem to be one of the most sensitive taxa to the long-term effects of pollution (Colin et al. 2016a). The Danube is an international river and the second largest in Europe, and therefore, is important for commercial fisheries. Grocka is downstream from Belgrade and Pančevo (a small town with developed chemical industries: fertilizer factory, refinery, and petrochemistry). It is known that the majority of industrial waste water is discharged in Serbia without any previously conducted purification treatment (Milanović et al. 2010).
Fish absorb heavy metals/metalloids from the surrounding environment depending on a variety of factors such as the characteristics of the species under consideration, the exposure period, the concentration of the element, temperature, salinity, pH, etc. Heavy metals released by anthropogenic activities will be accumulated in aquatic organisms through the food chain; as a result, human health can be at risk because of consumption of fish contaminated by toxic chemicals (Ginsberg and Toal 2009). For this reason, fish muscle is commonly analyzed to determine contaminant concentrations. The aim of this study was to assess the bioaccumulation of heavy metals/metalloid (Pb, Cd, Hg, Cu, Fe, Zn, As) in the most abundant fish species of commercial interest: omnivorous fish (Prussian carp—Carassius auratius gibelio, barbel—Barbus barbus, bream—Abramis brama, carp—Cyprinus carpio) and carnivorous fish (pike perch—Stizostedion lucioperca and catfish—Silurus glanis) from the Danube River.
Materials and methods
Study area
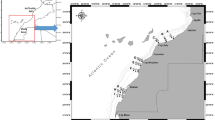
For this study, the fish sampled were landed from the River Danube from two different locations. The first site is located upstream from Belgrade, in the territory of Zemun (44° 50 ‘35 “N; 20 ° 23′ 40″ E) and the other is downstream from Belgrade in Grocka (44° 40’ 19 “N, 20 ° 43′ 11″ E) (Fig. 1).
Reagents
In order to determine the content of heavy metals, all chemicals used were analytical grade purity. Deionized water (resistivity of 18.2 MΩcm−1 at 25 °C) obtained from a deionized water system (Heming manufacturer, model PO2a LD3M) was used for all dilutions. The reagents used for microwave digestion, nitric acid, HNO3 (65%), and hydrogen peroxide, H2O2 (30%) were high pure quality (J.T. Baker). The element standard solutions (J.T. Baker) used for the calibration, were prepared by diluting stock solutions of 1000 mg L−1.
Apparatus
Digestion of fish muscle was performed using a microwave closed system MW 3000 (Anton Paar GmbH, Graz, Austria), with the cuvette model MF 100. Digestion was carried out with programs suitable for preparing samples of fish meat, with a range of 250 to 630 W.
After digestion, the content of heavy metals was determined by the atomic absorption spectrometer Perkin Elmer Analyst 700 with the MHS system (Shelton, the USA). Concentrations of Fe, Cu, and Zn were detected by flame atomic absorption spectrometry (FAAS), while analyses of Cd and Pb were performed by graphite furnace (GFAAS). Arsenic was analyzed by hydride generation (HGAAS), and content of mercury was determined by cold vapor atomic absorption spectrometry (CVAAS) with a discontinuous system (MHS 15). Operating parameters for the determination of heavy metals are shown for each element in Tables 1, 2, and 3. The content of each element was measured in peak area mode against a calibration curve.
Sampling
Fishes were caught at two locations (Zemun and Grocka) with similar levels of pollution in the Belgrade section of the Danube River. These locations were chosen because of their proximity to the confluence of the Sava River (which carries additional amounts of waste water) with the Danube River, as well as their proximity to the urban area of Belgrade on one side, and to agricultural areas on the other side of the river (Grocka is an agricultural area). The samples of each fish species were collected from professional fishermen during October 2013. Each fish from each catch was identified to species level, and a random subsample of ten individuals per species was used for metal analysis. Determination of heavy metals and arsenic was carried out on different types of fish, omnivorous (Prussian carp—Carassius auratius gibelio, barbel—Barbus barbus, bream—Abramis brama, carp—Cyprinus carpio) (Balık et al. 2003) and carnivorous (pike perch—Stizostedion lucioperca and catfish—Silurus glanis). As the juveniles of these species (<90 mm total length, TL) could not be reliably identified, they were excluded from the analysis. The fish species (Prussian carp, barbel, bream, carp, pike perch, and catfish) were caught with average lengths of 24.80 ± 3.2 cm, 54.90 ± 5.8 cm, 61.03 ± 9.6 cm, 55.1 ± 15.6 cm, 45.3 ± 6.1 cm, and 69.3 ± 11.8 cm, respectively. Also, Prussian carp, barbel, bream, carp, pike perch, and catfish were caught with average weights of 938 ± 226.3 g, 3842 ± 1379.5 g, 3028.4 ± 1985 g, 3906 ± 1292.5 g, 851 ± 300.6 g, and 2124.5 ± 1126.5 g, respectively. We opted to determine heavy metals in muscle, since this tissue is of the greatest interest as it is used in human nutrition. We tested 120 muscle samples of the six fish species; ten samples of each species from the Danube River at the two locations.
Fish were euthanized with an overdose of MS 222 (ethyl ester of p-amino benzoic acid, Sigma Aldrich), and then transported to the laboratory in refrigerated truck. Fish were dissected in a laboratory, and muscle samples were excised from below the dorsal fin and dispensed in polypropylene bottles (which were pre-treated (10%) nitric acid and washed three times with deionized water), and then quickly frozen and stored at −20 °C.
Heavy metal analyses
Samples of fish muscle were thawed at room temperature and mechanically homogenized. For the determination of heavy metals, fish muscle portions weighing about 1 ± 0.001 g were prepared in a mixture of nitric acid and hydrogen peroxide and subjected to microwave digestion for 30 min. After cooling to room temperature, the digested samples were quantitatively transferred to volumetric flasks. For the determination of iron, copper, zinc, cadmium, lead, and mercury, digested samples were diluted with deionized water. For determination of the arsenic content, the digested samples were quantitatively transferred with the appropriate addition of hydrochloric acid (HCl) and potassium iodide (KI), and then diluted with deionized water. Metal concentrations are expressed in milligram per kilogram fresh fish. The limits of detection were 0.015, 0.005, 0.02, 0.01, 1.0, 0.5, and 1.0 mg kg−1 for Pb, Cd, Hg, As, Fe, Cu, and Zn, respectively. Quality of analyses was controlled using the certified reference material (ERMBB 422) fish muscle. The concentrations determined in the reference material were within the tolerances specified in the delivered certificate.
Determined concentrations of heavy metals in fish muscle tissue were compared to the maximum allowed concentrations (MAC) in fish meat used for human diets, established by the Europe Union (European Commission Regulation 2006) and the Official Gazette of the Republic of Serbia (2014). According to EU legislation (European Commission Regulation 2006), MACs for Pb, Cd, and Hg are 0.30, 0.05, and 0.5 mg kg−1, respectively, while the As MAC is not determined by this regulation. The national legislation (Official Gazette of RS 2014) is in accordance with the regulation of the European Commission (2006), and the amounts of Pb, Cd, Hg, and As are 0.30, 0.05, 0.5, and 2.0 mg kg−1, respectively.
Statistical analysis
All samples were collected and analyzed in duplicate, and the results are expressed as mean ± standard deviation. Statistical analysis was elaborated using GraphPad Prisma version 6.00 software. Statistical analysis was performed using Student’s t test to determine the significance of differences between means. A level of p < 0.01 was considered significant.
Results and discussion
The presence of heavy metals/metalloids in the edible parts of fish (meat) is an indicator of environmental pollution and also a health hazard due to their consumption. In many previous studies (Ivanović et al. 2016; Trbović et al. 2011; Pantelica et al. 2012), the contents of heavy metals in the muscle of fish with different dietary habits were examined; the fish were caught from similar sites in the Danube River.
Concentrations of heavy metals (Pb, Cd, Hg, As) in the muscle tissue of the examined fish species are given in Table 4.
In Zemun, concentrations of lead (Pb) in the muscle tissues of the six fish species were in the range of 0.019 ± 0.002 mg kg−1 (bream) to 0.059 ± 0.002 mg kg−1 (carp), and in Grocka, levels ranged from 0.028 ± 0.002 mg kg−1 (bream) to 0.084 ± 0.004 mg kg−1 (carp). Extreme levels of heavy metals in both locations were found in omnivorous fish (bream, carp).
The lowest concentrations of lead (Pb) were observed in muscle tissue of omnivorous fish (bream) in Zemun, while significantly higher concentrations of lead (Pb) were identified in the muscle tissue of carp (omnivore) from Grocka. These results are in agreement with another study (Ivanović et al. 2016), which found the muscle tissue of carp harvested from the Danube in the region of Belgrade harbored lead concentrations from 0.05 to 0.06 mg kg−1. In contrast, another study (Andreji et al. 2005) reported higher levels of lead (Pb) (0.40 to 5.81 mg kg−1 and 0.24 to 0.89 mg kg−1) in muscle tissue of carnivorous (perch) and omnivorous (barbel) fish species in relation to the our results. The lead (Pb) concentration in river fish muscle tissue was similar to ours in another study, with the proviso that carp showed a tendency to accumulate the heavy metal in all tissues except in the gonads (Has-Schön et al. 2006). The highest content of lead (Pb) in muscle tissue of carp, an omnivorous fish species, could be related to the type of feeding, as this fish resides at the bottom and feeds on benthic organisms (Wei et al. 2014). Therefore, the fish is in contact with sediment and accumulates relatively high levels of heavy metals, and thus, reliably reflects the ecological state of the (aquatic) environment. According to Serbian Regulations, the MAC prescribed for Pb in fish flesh is 0.3 mg kg−1 (Official Gazette of RS 2014). In all examined samples of fish from both Danube River locations, the determined concentrations of lead (Pb) (0.019 to 0.084 mg kg−1) did not exceed this prescribed value.
Cadmium (Cd) levels observed in muscle tissue of carp (0.082 ± 0.003 mg kg−1) from Grocka were significantly higher (p < 0.01) than in other fish muscle tissues. This heavy metal, at both locations (Zemun, Grocka), was deposited in the lowest levels in the muscle tissue of bream (omnivore) (0.021 to 0.027 mg kg−1). In aquatic ecosystems, cadmium (Cd) easily bioaccumulates in all fish tissues, although the target organ for deposition is the kidneys (Squadrone et al. 2013). Cadmium (Cd) in fish muscle from Zemun ranged from 0.021 ± 0.002 mg kg−1 in omnivorous species (bream) to 0.068 ± 0.002 mg kg−1 in carnivorous species (catfish). A significantly higher cadmium (Cd) content (0.082 ± 0.003 mg kg−1) in relation to all other fish species was recorded in the muscle tissue of omnivorous fish (carp) from Grocka. Similar results were reported by others (Has-Schön et al. 2006), who determined higher content of cadmium (Cd) (0.016 to 0.155 mg kg−1) in carp muscle in comparison to other tested fish species. In the current study, fish muscle from both locations contained a higher amount of cadmium than is specified in the Official Gazette of the Republic of Serbia (2014) and in the European Union Regulation (0.05 mg kg−1), with the exception of bream and pike perch muscle. Increased cadmium (Cd) content in muscle tissue can be derived from fertilizer (especially phosphate) and fungicides used in agriculture, which are washed by rain into waterways. Such intensive agricultural activities (use of fertilizer in spring) near the Danube River occur especially at Grocka, where we verified a higher concentration of cadmium (Cd). Also, this heavy metal can accumulate via sewage sludge from urban waste water (Velev et al. 2009).
In omnivorous fish muscle (carp), significantly higher concentrations of mercury (Hg) (0.393 to 0.466 mg kg−1) were found in relation to all other types of fish from both examined locations. On the other hand, concentrations of mercury (Hg) in the muscle tissue of an omnivorous species (Prussian carp) were significantly lower (p < 0.01) in relation to all other types and were in the range of 0.094 to 0.139 mg kg−1. The results are in accordance with other studies (Trbović et al. 2011), and a higher mercury content was determined in carnivorous fish muscle (pike, 0.484 mg kg−1) in relation to omnivorous fish muscle (bream, 0.288 mg kg−1, barbel 0.218 mg kg−1, sturgeon, 0.146 mg kg−1, carp 0.099 mg kg−1, respectively). Some higher concentrations of mercury (0.327 ± 0.110 mg kg−1) were observed in carnivorous fish muscle (catfish) caught in a similar location in the Danube River near Belgrade (Milanov et al. 2016). Mercury (Hg) easily attaches to the thiol groups of proteins in muscle tissue, which may explain its increased concentration in carp (Castro-Gonzalez and Mendez-Armenta 2008). Some studies have emphasized that fish age, size, and diet are the main reasons for the increased content of mercury in fish muscle (Zrnčić et al. 2013). In the present study, three specimens weighted more than 4.500 g, of which two were carp, with a mercury concentration of 0.486 and 0.476 mg kg−1, and one was barbel with a mercury concentration of 0.365 mg kg−1. Importantly, high mercury levels can also be caused by migration of mercury from sludge to fish, because carp dive into river sediment mud in search of food. According to Serbian and European Union Regulation, the permitted amount of mercury in fish flesh is 0.5 mg kg−1. In all tested fish from both locations, the average mercury content did not exceed the MAC prescribed, although determined concentrations in carp muscle (0.466 ± 0.006 mg kg−1) from Grocka were very close to the MAC. These values can be explained by the influence of industry as well as waste water, because Grocka is downstream from Belgrade.
Significantly higher concentrations of arsenic (As) (0.258 to 0.333 mg kg−1) were found in omnivorous fish (carp) muscle in relation to other fish species, at both river locations. Smaller quantities of arsenic (As) were found in another omnivorous species (Prussian carp) while the lowest (0.105 ± 0.003 mg kg−1) were observed in carnivorous fish (pike perch) muscle from both locations (Zemun, Grocka). Studying the levels of arsenic in the examined fish muscles, an equal distribution of this heavy metal was observed in all fish types at both locations. Comparing omnivorous fish, arsenic (As) concentrations decreased in the order carp > barbel > bream, meaning distribution of this element is obviously not the result of diet. The results are consistent with previous studies (Lenhardt et al. 2012; Zrnčić et al. 2013), which examined the content of arsenic (As) in Danube River fish muscle, and found higher levels in omnivorous fish (carp) muscle in relation to muscle of carnivorous (pike, catfish) and herbivorous fish (silver carp). Similar results were reported by other authors (Subotić et al. 2013), which emphasized higher levels of arsenic in omnivorous fish (carp) in relation to carnivorous fish (catfish, pike perch). The amount of arsenic (As) prescribed in Serbia for freshwater fish is 2.0 mg kg−1 (Official Gazette of the Republic of Serbia 2014), so the arsenic concentrations in the muscle tissue of all investigated fish species did not exceed the MAC prescribed by national legislation.
The contents of copper, iron, and zinc in the muscle tissue of the six fish species (Prussian carp, barbel, bream, carp, catfish, and pike perch) are shown in Table 5.
The highest copper (Cu) content (1.55 to 1.62 mg kg−1) was found in carnivorous fish muscle (catfish), while the lowest content of this heavy metal (0.548 to 0.574 mg kg−1) was also found in carnivorous fish muscle of another species (pike perch). At both locations, in all cases, comparison of copper content in muscle tissue of examined different fish species showed statistically significant differences.
In a previous study, Subotić et al. (2013) observed that the distribution of copper in the muscle of fish of different dietary habits increased in the order pike perch < carp < catfish (the fish were caught in the same location of the Danube River). Barbel, bream, Prussian carp, and carp are all omnivorous and have a similar diet so it is expected that uniform accumulation of copper should be observed. The verified content of heavy metals in the tissues of carnivorous catfish, which in the ecosystem are at the top of the food chain, may well reflect the ambient concentration of the metal (Subotić et al. 2013), although there are other observations. Thus, in another study, (Zrnčić et al. 2013) pointed out that omnivorous fish (carp) are better biological indicators of environmental contamination, and provide a safer assessment of the state of the environment.
A significantly higher iron (Fe) concentration was observed in the muscle of bream and barbel (omnivorous species) compared to the carnivorous species (pike perch, catfish) and the omnivorous Prussian carp, which contained the minimum level detected (Table 5). A previous study (Lenhardt et al. 2012) identified an approximate iron content of 7.42 mg kg−1 in omnivorous fish (carp) from the Danube River in the Belgrade region, compared to results obtained at the two test locations (9.38 to 9.68 mg kg−1). On the other hand, lower iron concentrations were found in pooled samples of muscle and skin of carnivorous fish (4.98 and 6.77 mg kg−1 in pike and catfish, respectively) (Matasin et al. 2011) compared to the current results obtained for catfish muscle (8.17 and 8.32 mg kg−1 from Zemun and Grocka, respectively).
In the muscle tissue of all tested fish species, the lowest zinc levels were verified in pike perch muscle (carnivorous species), while the highest concentration of Zn in both locations were recorded in Prussian carp muscle (omnivorous species). Other studies (Pantelica et al. 2012; Lenhardt et al. 2012) confirmed a similar trend, namely that the greatest concentrations of zinc formed in the muscle tissue of omnivorous, and then herbivorous fish, and by far the lowest zinc levels were found in carnivorous fish. The Official Gazette of the Republic of Serbia (2014) does not define the MAC for Cu, Fe, or Zn in fresh fish, so the results obtained quantifying these metals can be interpreted freely. Among the same fish species, in the two different locations, similar levels of Cu, Fe, and Zn were found, which indicates the important physiological role that these metals have in the body.
In muscle tissue of omnivorous fish (bream, barbel, carp, except Prussian carp) caught downstream from Belgrade (Grocka), the contents of lead, cadmium, mercury, and arsenic were statistically significantly higher (p < 0.01) than the contents of these elements in omnivorous fish muscle (bream, barbel, carp) harvested in Zemun.
The average contents of heavy metals (lead, cadmium, mercury, copper, and arsenic) in the muscle tissue of carnivorous fish (pike perch, catfish) from Grocka were significantly higher (p < 0.01) than the average contents of these elements in muscle tissue of carnivorous fish (pike perch, catfish) harvested from Zemun, with the exception of the average cadmium content (catfish) where there was no statistical difference.
Heavy metals are serious pollutants in our natural environment due to their toxicity. When organisms are exposed to high metal levels in an aquatic environment, they can absorb the available metals directly from the environment, contaminated water and food, and thus, accumulate them in their tissues, and they can then enter the food chain and extend the problem to humans. The six fish species studied are most abundant fish of commercial interest in Serbia. For that reason, determination of heavy metals in muscle tissue is very important in terms of food safety for consumers. Also, the increasing demands of food safety have accelerated research regarding the risk associated with consumption of fish contaminated by heavy metals. Clearly, heavy metals cause undesirable effects, impairing the welfare of the environment (air, water, and soil), reducing the quality of life and may eventually cause death. Higher concentrations of non-essential heavy metals (lead, cadmium, mercury, and arsenic) found in fish from Grocka (located downstream from Belgrade and Pančevo) can be explained by anthropological influence, the development of industry, the discharge of wastewater, and the subsequent increased heavy metal content in sewage sludge. Fish and other aquatic species are good indicators of environmental conditions, so higher concentrations of heavy metals accumulated in the muscle tissue of fish directly reflects the metals’ presence in the environment. Also, future studies should examine the health consequences of metal pollution for fish and humans using biomarkers (Colin et al. 2016b).
Conclusion
The study of heavy metals in the muscle tissue of six fish species of different feeding habits from the Danube River revealed statistically significant differences in their contents in fish from two locations (Zemun and Grocka). Muscle tissue of carp, barbel, bream, and catfish from both locations had a higher quantity of cadmium than allowed in the Serbian and European regulations. On the other hand, the contents of lead, mercury, and arsenic in fish muscle were below the maximum allowable concentrations in the Republic of Serbia. The highest content of all investigated toxic elements (lead, cadmium, mercury, and arsenic) in both locations was observed in carp muscle (an omnivorous species), with exception of cadmium content in muscle of catfish from Grocka. On the other hand the highest content of microelements (copper, iron, and zinc, respectively) in fish from both locations was symmetrically arranged by species (catfish, barbel, and Prussian carp, respectively).
The results of this study show that continuous monitoring of the state of aquatic ecosystems is required, along with the introduction of efficient wastewater treatment and control of potential industrial polluters. On the territory of Belgrade and throughout Serbia, these environmental protections are minimal but are basic measures to improve the existing situation in the aquatic ecosystem of the Danube River, which is of international importance for fishing and sailing.
References
Andreji J, Stránai I, Massàyi P, Valent M (2005) Concentration of selected metal in muscle of various fish species. J Environ Sci Heal A 40:899–912
Andreji J, Stránai I, Massàyi P, Valent M (2006) Accumulation of some metals in muscles of five fish species from lower Nitra River. J Environ Sci Heal A 41:2607–2622
Balık İ, Karaşahin B, Özkök R, Çubuk H, Uysal R (2003) Diet of silver crucian carp Carasssius gibelio in Lake Eğirdir. Turkish J Fish Aquat Sci, 3(2)
Burger J, Gochfeld M (2005) Heavy metals in commercial fish in New Jersey. Environ Res 99:403–413
Castro-Gonzalez MI, Mendez-Armenta M (2008) Heavy metals: implications associated to fish consumption. Environ Toxicol Phar 26:263–271
Cole DW, Cole R, Gaydos SJ, Gray J, Hyland G, Jacques ML, Powell-Dunford N, Sawhney C, Au W (2009) Aquaculture: environmental, toxicological, and health issues. Int J Hyg Envir Heal International 212:369–377
Colin N, Maceda-Veiga A, Flor-Arnau N, Mora J, Fortuño P, Vieira C, de Sostoa A (2016a) Ecological impact and recovery of a Mediterranean river after receiving the effluent from a textile dyeing industry. Ecotoxicol Environ 132:295–303
Colin N, Porte C, Fernandes D, Barata C, Padrós F, Carrassón M, Maceda-Veiga A (2016b) Ecological relevance of biomarkers in monitoring studies of macro-invertebrates and fish in Mediterranean rivers. Sci Total Environ 540:307–323
European Commission Regulation, (2006) Setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Union, Commission Regulation No. 1881/2006/EC
Falco G, Llobet JM, Bocio A, Domingo JL (2006) Daily intake of arsenic, cadmium, mercury, and lead by consumption of edible marine species. J Agr Food Chem 54:6106–6112
Ginsberg GL, Toal BF (2009) Quantitative approach for incorporating methylmercury risks and omega-3 fatty acid benefits in developing species-specific fish consumption advice. Environ Health Perspect 117:267–275
Guallar E, Sanz-Gallardo MI, Van’t Veer P, Bode P, Aro A, Gomez-Aracena J, Kark JD, Riemersma AR, Martín-Moreno JM, Frans JK (2002) Mercury, fish oil, and the risk of myocardial infarction. New Engl J Med 347:1747–1754
Has-Schön E, Bogut I, Strelec I (2006) Heavy metal profile in five fish species included in human diet, domiciled in the end flow of River Neretva (Croatia). Arch Environ Contam Toxicol 50:545–551
Ivanović J, Janjić J, Baltić M, Milanov R, Bošković M, Marković RV, Glamočlija N (2016) Metal concentrations in water, sediment and three fish species from the Danube River, Serbia: a cause for environmental concern. Environ Sci Pollut R 23:17105–17112
Janjić J, Ivanović J, Marković R, Starčević M, Bošković M, Đorđević V, Baltić MŽ (2015) Metal concentration in muscle tissue of carp and pike from different fish ponds in Belgrade area. J Agric Sci Technol 5:429–436
Janković S, Nikolić D, Stefanović S, Radičević T, Spirić D, Petrović Z (2013) Evaluation of food intake of cadmium in Serbia. Tehnologija mesa 54:123–129
Jovičić K, Nikolić DM, Višnjić-Jeftić Ž, Đikanović V, Skorić S, Stefanović SM, Lenhardt M, Hegediš A, Krpo-Ćetković J, Jarić I (2015) Mapping differential elemental accumulation in fish tissues: assessment of metal and trace element concentrations in wels catfish (Silurus glanis) from the Danube River by ICP-MS. Environ Sci Pollut R 22:3820–3827
Kehrig HA, Seixas TG, Malm O, Di Beneditto AP, Rezende CE (2013) Mercury and selenium biomagnification in a Brazilian coastal food web using nitrogen stable isotope analysis: a case study in an area under the influence of the Paraiba do Sul River plume. Mar Pollut Bull 75:283–290
Lenhardt M, Jarić I, Višnjić-Jeftić Ž, Skorić S, Gačić Z, Pucar M, Hegediš A (2012) Concentrations of 17 elements in muscle, gills, liver and gonads of five economically important fish species from the Danube River. Knowl Manag Aquat Ec 407:1–10
Maceda-Veiga A, Monroy M, de Sostoa A (2012) Metal bioaccumulation in the Mediterranean barbel (Barbus meridionalis) in a Mediterranean River receiving effluents from urban and industrial wastewater treatment plants. Ecotoxicol Environ 76:93–101
Matasin Ž, Ivanušić N, Oreščanin V, Nejedli S, Gajger IT (2011) Heavy metal concentrations in predator fish. J Anim Vet Adv 109:1214–1218
Milanov R, Krstić M, Marković R, Jovanović D, Baltić B, Ivanović J, Jovetić M, Baltić M (2016) Analysis of heavy metals concentration in tissues of three different fish species included in human diet from Danube river, in the Belgrade region, Serbia. Acta Vet (Beograd) 66:89–102
Milanović A, Kovačević-Majkić J, Milivojević M (2010) Water quality analysis of Danube River in Serbia—pollution and protection problems. Bulletin of the Serbian Geographical Society 90:47–68
Mitic VD, Stankov-Jovanovic VP, Tosic SB, Pavlovic AN, Cvetkovic JS, Dimitrijevic MV, Nikolic-Mandic SD (2015) Chemometric approach to evaluate heavy metals’ content in Daucus carota from different localities in Serbia. Hem Ind 69:643–650
Monroy M, Maceda-Veiga A, Sostoa A (2014) Metal concentration in water, sediment and four fish species from Lake Titicaca reveals a large-scale environmental concern. Sci Total Environ 487:233–244
Noël L, Chekri R, Millour S, Merlo M, Leblanc J, Guérin T (2013) Distribution and relationships of As, Cd, Pb and Hg in freshwater fish from five French fishing areas. Chemosphere 90:1900–1910
Official Gazette of RS (2014) Rules on the maximum allowable residues of pesticides in food and animal feed which is determined by the maximum allowable amounts of residues of plant protection products (“Official Gazette of RS”, no. 29/2014, 37/2014 - isp., 39/2014 and 72/2014)
Pantelica A, Ene A, Georgescu II (2012) Instrumental neutron activation analysis of some fish species from Danube River in Romania. Microchem J 103:142–147
Squadrone S, Prearo M, Brizio P, Gavinelli S, Pellegrino M, Scanzio T, Guarise S, Benedetto A, Abete M (2013) Heavy metals distribution in muscle, liver, kidney and gill of European catfish (Silurus glanis) from Italian rivers. Chemosphere 90:358–365
Storelli M, Barone M, Garofalo G, Marcotrigiano GO (2007) Metals and organochlorine compounds in eel (Anguilla anguilla) from the Lesina lagoon, Adriatic Sea (Italy). Food Chem 100:1337–1341
Subotić S, Spasić S, Višnjić-Jeftić Ž, Hegediš A, Krpo-Ćetković J, Mićković B, Skorić S, Lenhardt M (2013) Heavy metal and trace element bioaccumulation in target tissues of four edible fish species from the Danube River (Serbia). Ecotox Environ Safe 98:196–202
Trbović D, Janković S, Ćirković M, Nikolić D, Matekalo-Sverak V, Đorđević V, Spirić A (2011) Bezbednost i kvalitet mesa nekih slatkovodnih riba u Srbiji. Tehnologija mesa 52:276–282
Vallod D, Sarrazin B (2010) Water quality characteristics for draining an extensive fish farming pond. Hydrolog Sci J 55:394–402
Velev R, Krleska-Veleva N, Čupić V (2009) The poisoning of domestic animals with heavy metals. Vet Glasnik 63:393–405
Verep B, Multu C, Apaydin G, Cevik U (2012) The trace element analysis in freshwater fish species, water and sediments in Iyidere stream (Rize-Turkey). Pak J Biol Sci 15:658–665
Wei YH, Zhang JY, Zhang DW, Tu TH, Luo LG (2014) Metal concentrations in various fish organs of different fish species from Poyang Lake, China. Ecotox Environ Safe 104:182–188
Zrnčić S, Oraić D, Ćaleta M, Mihaljević Ž, Zanella D, Bilandžić N (2013) Biomonitoring of heavy metals in fish from the Danube River. Environ Monit Assess 185:1189–1198
Acknowledgments
This paper was supported by the Ministry of Education, Science and Technological Development, Republic of Serbia, via the project TR 31011.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Jovanović, D.A., Marković, R.V., Teodorović, V.B. et al. Determination of heavy metals in muscle tissue of six fish species with different feeding habits from the Danube River, Belgrade—public health and environmental risk assessment. Environ Sci Pollut Res 24, 11383–11391 (2017). https://doi.org/10.1007/s11356-017-8783-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8783-1