Abstract
Misuse of pesticides in farming activities leads to contamination of drinking water sources and is responsible for animal and human health problems. The biobeds are practicable option to minimize contamination by pesticides during preparation, use and washing of equipment for pesticide treatments. This research aimed at testing substrate mixtures to optimize biobed efficiency to remove pesticides under the climate of the Yucatan (México). Agricultural soil and 11 mixtures adding vegetable compost, sisal pulp, corn stover and seaweed were tested under controlled conditions. Each biomixture was exposed to a mixture of five pesticides (2,4-diclorophenoxyacetic acid “2,4-D” [1.08 mg cm−3], atrazine [2.50 mg cm−3], carbofuran [0.23 mg cm−3], diazinon [0.34 mg cm−3], and glyphosate [0.36 mg cm−3]) in a period of 41 days. Monitoring of the dissipation of pesticide residues showed that pesticides were quickly dissipated in soil at microcosm level experiment, while at two critical times of 20 and 41 days, all mixtures of substrates (biomixtures) were efficient in dissipation of high concentrations of pesticide in a short time (>99%). Time, biomixture and type of pesticide were shown to be the main parameters influencing pesticide dissipation (P < 0.05). Several other physicochemical parameters of the biomixtures, such as organic matter (OM), lignin, water holding capacity (WHC), and pH, were also significant on pesticide dissipation (P < 0.05), being pH the most significant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to FAO, in the DECLARATION OF THE WORLD SUMMIT ON FOOD SECURITY Rome in 2009, it is important to increase crop yields by 70% for 2050 to address the food crisis (FAO 2009). This implies a constant increase in crop yield which will be affordable by the use of technologies including the sustainable of agrochemicals, which represents the most viable way to protect and guarantee crops. Without the use of pesticides, feed costs could double; food shortages pose a serious problem for third-world countries due that about 40% of agricultural production could be lost (Wu and Sardo 2010).
Improper handling of pesticides may result in contamination of both surface and groundwater, which represents a potential risk to the environment and health. Water pollution by pesticide originates either from nonpoint or point sources contamination. Percolation and runoff are the processes leading the dispersion of nonpoint sources contamination in the environment (De Wilde et al. 2010). Point sources contamination resulting from various activities such as spills during filling operations, leakages of spray equipment, spray leftovers, and spills of rinsing water from internal and external cleaning of the spraying equipment appear to be responsible for 40–90% of the surface water and groundwater contamination (De Wilde et al. 2010).
The regulations of the European Community (2006/118/EC, 2008/105/EC) sets a limit reference value for the presence of any pesticide of 0.1 μg L−1 in drinking water (European Community 2006, 2008). This infers that 1 g of active substance can potentially contaminate up to 10,000 m3 of water. Other regulations such as EPA in its maximum contamination level (MCL) of pesticides in water establish values of 70 μg L−1 (2,4-D), 3 μg L−1 (atrazine), 40 μg L−1 (carbofuran), 3 μg L−1 (drinking water equivalent for diazinon), and 700 μg L−1 (glyphosate) (Hamilton et al. 2009). The Mexican regulation NOM-127-SSA1–1994 reports maximum limits in water of 0.03 to 50.00 μg L−1 for many pesticides such as 2,4-D. This shows important differences between regulations in the world.
The implementation of good agricultural practices prevents and controls contamination by pesticide residues. For example, it is recommended to collect agricultural effluents produced during pesticide application (filling, applying, and washing steps, as well as pesticide leftovers). The nature and concentration of the effluent depends on the type and amount of pesticide employed and the extent of agricultural fields.
Biobed is a treatment system of agricultural effluents developed in Sweden to solve the problem caused by point source pesticide contamination of water resources (Torstensson and Castillo 1996). In its original form, it is a simple construction designed to confine and degrade pesticides in agricultural effluents. It consists in a hole of 60 cm deep in the ground, which is isolated from the soil by a layer of clay to prevent leaching of contaminants, as well as a biomixture made of straw, soil, and peat in proportion 2:1:1 v/v, respectively, and a final layer of grass (Castillo et al. 2008). Since the first biobeds in Sweden in 1993, 5055 biobeds have been implemented in Europe (Sweden, France, Italy, UK, Belgium, etc.), 1520 in American Continent, 29 in Africa, and 1 in Japan (Husby 2013). In Latin America, several countries such as Peru, Guatemala, Costa Rica, and Chile are already using biobed to control and prevent pesticide contamination of water sources and soil (Castillo et al. 2008).
The efficiency of biobed systems to dissipate pesticides residues lies on abiotic (adsorption, chemical degradation) and biotic (microbial degradation) processes occurring in the biomixture (Torstensson and Castillo 1997). For example, pesticide degradation by white rot fungi activity has a relationship with substrates with high content of lignocellulose (Castillo et al. 2008).
In designing a biobed besides basic engineering aspects, the substrates used to constitute the biomixture are a key component conditioning the performances of abiotic and biotic processes responsible for pesticide dissipation (Karanasios et al. 2010a; Diez et al. 2013a, b). Each substrate entering in the composition of the biomixture plays an important role: (i) Soil provides microorganisms and sorption sites; (ii) peat increases the adsorption capacity and regulates the pH and moisture; (iii) straw is conceived as the main substrate because of its microbial activity, especially of white rot fungus (Torstensson and Castillo 1996; Castillo and Torstensson 2007; Castillo et al. 2008). For soil, physicochemical parameters such as pH, organic matter content, moisture, temperature, and others are important in pesticide dissipation by degrading (Shahgholi and Ahangar 2014; Aparicio et al. 2015). Recently, it was proven that straw can be partially or totally replaced by other lignocellulosic materials such as sunflower, crop residues, olive leaves, grape stalks, orange peels, corn cobs, spent mushroom substrate, cork, vine branch, and others, recording good pesticide dissipation in biobeds (Karanasios et al. 2010b; Coppola et al. 2010; Karas et al. 2016; Pinto et al. 2016). Many studies about biobeds focused in dissipation process through time taking in count pesticide types, biomixtures, and the differences in biobeds (Fogg et al. 2003a, b; Castillo et al. 2008; Dakhel et al. 2013); however, physicochemical parameters of biomixtures and their effect on pesticide dissipation have been studied poorly or their importance in the process has been inferred. Castillo and Torstensson (2007) studied the effects of WHC and temperature on pesticide degradation confirming the importance of both.
Efficiency of biobeds using different substrates has been proved by several studies. Dissipations of pesticides in range of 60–99.9% at different time periods (1–24 months) using lignocellulosic substrates in conventional biobed systems (Karanasios et al. 2010a; Diez et al. 2013a; Coppola et al. 2010; Cooper et al. 2016) and adding inoculum to accelerate the pesticide degradation (Karas et al. 2016; Pinto et al. 2016) have been reported; this is despite important differences in physicochemical parameters of biomixtures. Influencing of factors such as pH and organic matter content (OM) is evident. Highest efficiencies are reported in biobed with pH around 7 (Karanasios et al. 2010a; Coppola et al. 2010; Cooper et al. 2016; Karas et al. 2016; Pinto et al. 2016). About OM, some studies reported highest pesticide dissipation in biomixtures with the highest OM content (8.82–39.4%) (Coppola et al. 2010; Karas et al. 2016).
To be successfully implemented to treat agricultural effluents, biobed has to be adapted to regional constraint and opportunities. Having this in mind, its design and the composition of its biomixture can vary from one region to other one. Indeed, various construction materials can be used, clay layer can be substituted with concrete or plastic materials, and original substrates can be used with other equivalent available materials (Castillo et al. 2008). In order to implement biobed systems in the Yucatan area, a study to optimize the composition of the biomixture and the importance of their physicochemical parameters by testing the efficiency of different biomixtures made of various local materials including soil, vegetal compost, sisal bagasse, corn stover, and seaweed, to treat pesticide effluents under controlled conditions of temperature and humidity using miniaturized biobeds at two critical times, was carried out. During this experiment atrazine, carbofuran, diazinon, glyphosate, and 2,4-D were applied as a mixture in all biomixtures.
Materials and methods
Substrates
The substrates used in this research were agricultural soil, sisal pulp, vegetable compost, corn stover, and seaweed. The different substrates were selected because of their easy availability in Yucatan Peninsula, Mexico. The soil used was collected from a production plot of vegetables with pesticide application record. The sisal pulp was obtained from a shredder company. The vegetable compost came from a vermicompost process using plant-based substrates. Corn stover was obtained from an agricultural plot. Finally, the mixture of seaweed was collected from the coastal line from Chuburna Puerto to Telchac Puerto in the Yucatan, Mexico. All substrates were dried at environmental temperature for 24 h. Vegetable substrates were chopped using an electric device, and soil was sieved through 0.5-mm sieve.
Physicochemical characterization of substrates
The physicochemical characteristics of the different substrates are shown in Table 1. Parameters such as bulk density (BD) were determined by the core method (Blake and Hargue 1986), real density (RD) by the method of volumetric flask (Estrada and Alvarez 2011), organic matter (OM) and total organic carbon (OC) by wet digestion and colorimetry (Okalebo et al. 1993), total nitrogen (N) by Kjeldahl method (Okalebo et al. 1993), lignin by Ankon method (Van Soest 1968), the pH according to the Official Mexican Standards NOM-021-SEMARNAT-2000 (2002) (soil and compost) and NMX-F-317-S-1978 (1978) (remaining substrates), water holding capacity (%WHC) according to the method of BIOTECHNOLOGY-IPN (Juárez et al. 2009), and texture by the method of Bouyocus (Estrada and Alvarez 2011).
Biomixtures
The biomixtures (M1–M12) were prepared and their ability to dissipate pesticides was evaluated. The composition of the biomixtures is presented in Table 1; M1 refers to agricultural soil used in this research. The sandy loam soil (fine gravel [8.6%], very coarse sand [8.8%], coarse sand [21.5%], medium sand [20.1%], fine sand [19.9%], very fine sand [10.4%], coarse silt [8.5%], and clay [2.2%]) was collected from a depth not greater than 20 cm. Soil had been previously exposed to pesticides (6 months previous the research). Initial pesticide remaining in agricultural soil was measured; results showed that any pesticide studied was detected. It therefore constitutes not only a habitat for microorganisms but also a possible source of pesticide-degrading microorganisms, selected by repeated exposure to pesticides (Udikovic-Kolic et al. 2012). The soil is the dominant substrate (i.e., representing 50% of the biomixture) in the 11 biomixtures (M2 to M12) which also comprising various amounts of organic components (Table 1). Soil was used as control (M1). All the biomixtures were physicochemical characterized. Organic matter content (OM), total organic carbon, (OC), total nitrogen (N), lignin content (Lig), water holding capacity (WHC), pH, and the C/N ratio were determined (Table 1).
Pesticide dissipation kinetic in soil
Prior to biobed implementation, a first experiment to determine the ability of soil to dissipate pesticides at microcosm level using high pesticide concentration was performed; this is to know the pesticide dissipation and time effect to select the monitoring times in pilot biobeds. Systems consisted in a glass container with capacity of 100 mL containing 5 cm3 of soil (1.66 g dry weight) sealed with parafilm and incubated at temperature and humidity of 29.6 ± 2 °C and 58.5 ± 5.8%, respectively. Half milliliter of a pesticide solution at concentrations of 10,800 mg L−1 for 2,4-D, 25,000 mg L−1 for atrazine, 2290 mg L−1 for carbofuran, 3436 mg L−1 for diazinon, and 3600 mg L−1 for glyphosate was used. The mixture of pesticides was prepared in 0.01 M solution of CaCl2. Final concentration of pesticides in systems was 1.08 mg cm−3 (2,4-D), 2.50 mg cm−3 (atrazine), 0.23 mg cm−3 (carbofuran), 0.34 mg cm−3 (diazinon), and 0.36 mg cm−3 (glyphosate). All systems were adjusted at 90% of WHC. Concentrations used in this experiment were selected because experimental design considers the real concentrations used by farmers.
Dissipation was monitored in a time period of 41 days (1, 3, 7, 10, 14, 18, 22, and 41 days) with five replicates per each time. Kinetic of dissipation using a first-order kinetic model (C = C 0 e −kt) was developed. Time to dissipate the 50% of pesticides (DT 50 = ln (2)/k) and to dissipate 90% of pesticides (DT 90 = ln (10)/k) was determined for all pesticides.
Biobed systems implementation
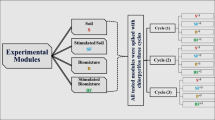
A second experiment to know the pesticide behavior in biobed systems using novel biomixtures was performed. Seventy-two miniaturized biobeds were implemented in the laboratory with six replicates per biomixture. Each miniaturized biobed contained a total volume of 500 cm3 of biomixture in plastic containers. The biobeds were implemented for 41 days under controlled temperature of 29.6 ± 2 °C and humidity of 58.5 ± 5.8%, this according with previous experiment. The systems were regularly watered to maintain water holding capacity at 90% (w/v). Times of 20 and 41 days were selected to monitoring remaining pesticides in biobeds because at this time periods, pesticide remaining in previous analyses in soil showed an asymptotic behavior (plateau), and to know if substrate addition reduce or not pesticide concentrations to similar times is relevant. Biobed scheme is given in Online Resource 1.
Pesticide mixtures
Pesticides mixture used in biobeds mimic pesticide effluents generated by farmers in Yucatan area, this according to a survey that studied the phytosanitary practices of farmers (Cardeña-Echalaz 2015). Mixture of pesticides was composed of formulated atrazine, carbofuran, diazinon, glyphosate, and 2,4-D (Table 2). The solution of pesticides was prepared in 0.01-M solution of CaCl2. One hundred milliliters of this pesticide mixture was applied evenly to each of the 11 biomixtures and agricultural soil using a small manual sprinkler.
Pesticide analysis
Samples (3 g) were taken randomly from three of the six replicates for each biomixture 20 and 41 days after pesticide mixtures application. Prior to the collection of the samples, biomixtures were homogenized. Systems were discarded after taking samples, this to ensure the integrity of samples and avoid pesticide lost by continuous handling. Samples were poured into Teflon tubes with equal amount of anhydrous sodium sulfate pre-activated at 440 °C for 4 h (Marmolejo 1999); then 25 mL of a hexane-acetone solution 1:1 (v/v) was added. A microwave-assisted extraction of method 3546 EPA (EPA 2000) was applied at power of 800 W and temperature of 110 °C maintained for 15 min using a microwave (MARSR). Two milliliters of the extract obtained was taken, filtered (fluorisil sieve with 0.45-μm pore), transferred into a glass vial, and stored under refrigeration until analysis. The extraction efficiency of the method was validated by recovery tests for the five commercial products. The results showed recovery rates of 88 ± 6% for 2,4-D, 91 ± 7% for atrazine, 91 ± 2% for carbofuran, 94 ± 4% for el diazinon, and 96 ± 7% for glyphosate.
Biomixture extracts were analyzed to quantify pesticide residues using a gas chromatography coupled to a mass spectrometry (Thermo Scientific Trace GC Ultra 1300), equipped with an electron capture detector (ECD) in tandem with a nitrogen-phosphorus detector (NPD). Chromatographic separation was done on with capillary column (PerkinElmerR Elite-17, 30 m × 0.25 mm × 0.25 μm) using helium (BIPR) as carrier gas at a constant pressure of 100 kPa. The furnace operated using a temperature ramp starting at 70 °C keeping 1 min and subsequently performing increments of 9 °C min−1 to 200 °C. The temperature was maintained for 2 min, and subsequent increases of 1.5 °C min−1 up to 220 °C were conducted maintaining this temperature for 1 min. Finally, the temperature increased 3 °C min−1 to reach 290 °C; this temperature was maintained for 1 min. The chromatograph has a temperature-programmed vaporization injector (PTV), splitless mode with initial temperature of 80 °C maintained for 0.3 min. During the evaporation, phase is increased to 260 °C with increases of 10 °C s−1 maintained for 2 min. The transfer phase increased up to 300 °C with increases of 13.5 °C s−1 and a cleansing phase to 320 °C with increases of 15.5 °C s−1 maintained for 5 min. Five microliters of each biomixture extraction was injected to GC-MS using an auto-sampler (Thermo Scientific TRIPLUS). The ECD detector had a base temperature of 300 °C and detector temperature of 330 °C with a current of 0.5 nA, pulse width 50 V, pulse bad of 1 μs, and nitrogen as make up with flow of 15 mL min−1. The NPD worked at temperature of 330 °C with air flow of 50 mL min−1 and nitrogen as make up to 50 mL min−1, with bias voltage of 50 V and current of 4 A.
For the quantification of pesticides, the equipment was calibrated using pattern solutions of 50, 500, 1000, 1500, and 2000 μg L−1 prepared from high purity pesticide standards (Sigma-Aldrich; 99.9% of purity) diluted in toluene and hexane in 1:1 ratio.
Limit of detection (LOD) and limit of quantification (LOQ) expressed in μg L−1 were 2,4-D (LOD 0.071 ± 0.045 and LOQ 0.103 ± 0.059), atrazine (LOD 0.163 ± 0.071 and LOQ 0.174 ± 0.069), carbofuran (LOD 0.086 ± 0.003 and LOQ 0.140 ± 0.19), diazinon (LOD 0.044 ± 0.025 and LOQ 0.076 ± 0.048), and glyphosate (LOD 0.212 ± 0.034 and LOQ 0.162 ± 0.047).
Data statistical analyses
Statistical analyses were developed using multifactorial analysis of variance (ANOVA) to determine the significance of factors (time, type of biomixture and of pesticide) on pesticide dissipation. Simple ANOVA analysis and a multiple range study to determine homogeneous groups between mixtures and to compare the results of the chromatographic analysis for times of 20 and 41 days were developed. Simple ANOVA analysis to determine the significance of time period on dissipation rate of pesticide was performed. Similarly, Kruskal-Wallis analysis to determine the significance of each physicochemical parameter on pesticide dissipation in each biomixture was performed. Correlations between physicochemical parameters were analyzed using Spearman’s correlation matrix. Principal component analysis (PCA) to know the most important components on pesticide dissipation and the most significant physicochemical parameters in each component was performed. The statistical analyses were done using Statgraphics Centurion XVI (StatPoint Technologies) and Primer 6 version 6.1.16 & Permanova + version 1.0.6 (Primer – ELtd).
Results
Pesticide dissipation kinetic in soil
First experiment prior to biobed implementation showed that all pesticides were quickly dissipated in soil at 41 days (Fig. 1). Results show 98% (2% of pesticide remaining) and 100% (0% of pesticide remaining) of dissipation at 18 and 41 days, respectively, for all pesticide except for diazinon which had a dissipation of 96% (4% remaining pesticide) at 18 days. Minimum changes between 18 and 22 days were observed.
The first-order kinetic model employed showed a good fit for the dissipation data for all pesticides: 2,4-D (r 2 = 94.2), atrazine (r 2 = 86.5), carbofuran (r 2 = 97), diazinon (r 2 = 94.1), and glyphosate (r 2 = 98.8). DT50 and DT90 in days were calculated to know more detailed the pesticide behavior; these values for all pesticides were DT50 = 5.50 and DT90 = 18.26 for 2,4-D, DT50 = 1.6 and DT90 = 5.2 for atrazine, DT50 = 5.7 and DT90 = 18.8 for carbofuran, DT50 = 5.88 and DT90 = 19.52 for diazinon, and finally, DT50 = 3.5 and DT90 = 11.6 for glyphosate.
Pesticide dissipation in biomixtures
Second experiment to know the effect of biomixture in pesticide dissipation at two critical times previously selected was performed. All the different biomixtures tested allowed to dissipate quickly and efficiently the five pesticides, proving the affectivity of biobeds; in a real situation, this could prevent the contamination of water by pesticides in farms. Pesticide dissipation was monitored at 20 and 41 days after pesticide treatment in each of the 11 biomixtures and agricultural soil. It was observed that at 20 days, less than 2% of the pesticide was detected (Fig. 2a) and, at 41 days, less than 1% was detected (Fig. 2b). These elevated dissipations averaging 98% (20 days) and 99% (41 days).
Table 3 shows the percentages of pesticide dissipation in function of initial concentration of pesticides applied to each biobed and dissipation rates for each pesticide.
Chemical analysis showed that 20 days after application, the highest 2,4-D dissipation was observed in the biomixtures M5 and M12 (i.e., approximately 99.98% (∼540 mg) of dissipation). Biomixture M5 was the most efficient in dissipating carbofuran, diazinon, and atrazine, with dissipations of 99.72% (114.18 mg), 99.56% (171.04 mg), and 99.90% (1248.75 mg), respectively. Biomixture M7 was the most efficient regarding the dissipation of glyphosate (99.61%, 179.30 mg). In total, 2253.14 mg of pesticides were dissipated in the biobeds. 41 days after their application, most pesticides were entirely dissipated, several of them being not anymore detected. The most striking aspect is glyphosate, which was completely dissipated compared with its initial concentration in all biomixtures. Other pesticides such as 2,4-D and diazinon were also dissipated almost 100% in many biomixtures.
An important aspect to note is that time periods from 0 to 20 and 20 to 41 days (F = 114.22; P < 0.05) and type of pesticide (F = 25.74; P < 0.05) were statistically significant on rate of pesticide dissipation, this despite minimum differences between them for all pesticides and biomixtures. All pesticides show a very important slowdown in pesticide rate between the two periods of time; however, to the different biomixtures, the rate of dissipation was similar to each time period. Pesticide dissipation rates in the different biomixtures had values from 26.94 to 27.00 mg day−1 (2,4-D), 62.29 to 62.44 mg day−1 (atrazine), 5.71 to 5.76 mg day−1 (carbofuran), 8.42 to 8.54 mg day−1 (diazinon), and 8.84 to 8.97 mg day−1 (glyphosate) in all biomixtures at time period of 0–20 days; at time period of 20–40 days, the dissipation rates had values from 0.00 to 0.05 mg day−1 (2,4-D), 0.03 to 0.10 mg day−1 (atrazine), 0.01 to 0.04 mg day−1 (carbofuran), 0.03 to 0.16 mg day−1 (diazinon), and 0.03 to 0.15 mg day−1 (glyphosate) in all biomixtures. M1 had the lowest dissipation rates of 2,4-D, atrazine, diazinon, and glyphosate, and M2 of carbofuran in time period from 0 to 20 days. For time period from 20 to 41 days, M1 had the lowest dissipation rate of 2,4-D, M5 of atrazine and diazinon, M6 and M7 of carbofuran, and M7 of glyphosate. The most evident case was M6 and M7 for carbofuran; dissipation rate of this pesticide was 99.97% for 0–20 days and 27.12% for 20–40 days.
Effect of biomixture, time, and pesticide type on dissipation
Multifactorial ANOVA analysis at 95% confidence for percentage of pesticide remnants and the factors of time, biomixture, and type of pesticide showed that the three factors and their interactions were statistically significant over the remaining concentration of pesticides in biomixtures under study (P < 0.05) (Table 4).
The ANOVA analysis at 95% confidence for all biomixtures showed significant differences in the percentage of remaining pesticides according to the type of biomixture (P < 0.05). Multiple range test at 95% confidence at 21 days led to the identification of four homogeneous groups G1 to G4 ranked in accordance to their ability to dissipate pesticides (least significant difference (LSD) from Fisher): group 1 (made of M3, M5, M6, M7, M8, and M10) > group 2 (made of M3, M6, M7, M8, M10, and M11) > group 3 (made of M2, M3, M4, M6, M9, and M11) > group 4 (made of M1, M2, M9, and M12). At 41 days, multiple range test at 95% confidence two homogeneous groups G1 and G2 ranked in accordance to their ability to dissipate pesticides (least significant difference (LSD) from Fisher) was identified, group 1 (made of M3, M4, M5, M6, M7, M8, M9, M10, M11, and M12) > group 2 (made of M1, M2, M4, M6, and M9). It is noteworthy that soil (M1) was part of group 4 at 20 days and of group 2 at 41 days both characterized by the lowest ability to dissipate pesticides.
Having in mind that physicochemical parameters such as soil pH and moisture, lignin content, and OM content are known to influence pesticide dissipation, statistical analyses (Kruskal-Wallis test; K = test statistic) were carried out on our data set to verify their importance in pesticide dissipation observed in the miniaturized biobed. Results showed that OM (K = 21.24; P(0.031) <0.05), lignin (K = 21.24; P(0.031) <0.05), WHC (K = 21.24; P(0.031) <0.05), and soil pH (K = 21.10; P(0.020) <0.05) were significantly influencing pesticide dissipation. According to P values, these parameters could be ranked as follows pH > lignin = WHC = OM for the importance of their influence on pesticide dissipation.
Figure 3 shows the Spearman’s correlation between each physicochemical parameter; this correlation analysis was selected because some factors had standardized kurtosis out of range. Values of r near to −1 and +1 imply a strong relationship between the two factors. The matrix shows that %OM has a better positive correlation with %N. Lignin has a good positive correlation with the C/N ratio; like lignin, the %WHC has the best correlation (positive) with C/N ratio. Finally, %N has a moderate negative correlation with C/N ratio and a low correlation (negative) with pH, being this the best correlation of pH with some parameter. The percentage of remaining pesticide (%RP) showed poor correlations with the parameters; however, pH, WHC, C/N ratio, and lignin had negative correlation; this implies that %RP decreases, while these factors increase. However, the unique statistically significant correlation of %RP was with pH; this coincides with ANOVA analyses where this parameter was the most significant.
Spearman’s correlation matrix for all physicochemical parameters of biomixtures involved in pesticide dissipation process. Organic matter (a), lignin (b), water holding capacity (c), total nitrogen (d), carbon-nitrogen ratio (e), hydrogen potential (f), and percentage of remaining pesticide (g). (+) Mean; (−) median; all correlations at confidence of 95%. *Statistically significant (P < 0.05)
Table 5 presents the principal component analysis. The importance of physicochemical parameters is evident and has relationship with ANOVA analyses where these parameters were significant on pesticide dissipation.
The principal component analysis shows that both component 1 and component 2 explain the 60.4% of variability, with 37.2 and 23.2% for components 1 and 2, respectively. From the previous table can be interpreted that component 1 corresponds to physicochemical characteristics of substrates being carbon-nitrogen ratio the main parameter; this infers that microorganism activity is very important in pesticide dissipation process. Component 2 presents as main factors %RP and pH, what supports the relationship between these factors being pH more important.
Figure 4 shows the behavior of biomixtures according with remaining pesticide and their relationship with the different factors. Perpendicular vectors mean poor or bad correlation between the two factors. The behavior of biomixtures coincides with chemical and statistical analyses of remaining pesticides, which show that M1 at the left of axis has the greatest variation for all pesticides and the lowest pesticide dissipation, this is because M1 data are in the same direction of %RP; this means that %RP is larger in this biomixture. The figure shows that most biomixtures are on the right of axis, and these are more related with lignin, WHC, C/N ratio, pH, and of course percentage of remaining pesticide (%RP) because of the opposite direction with %RP vector. Percentage of remaining pesticide in M5 according to Fig. 4 is very well related with C/N ratio and WHC and in less degree with pH and lignin content.
Bi-graphic of component weight and relationship of remaining pesticides and physicochemical parameters with biomixtures. Organic matter (a), lignin (b), water holding capacity (c), total nitrogen (d), carbon-nitrogen ratio (e), hydrogen potential (f), and percentage of remaining pesticide (g). M1 to M12 are the different biomixture tested. Clusters to biomixtures (Euclidean distance =2.1) (broken lines)
Figure 4 shows that %RP has a negative relationship with pH, lignin, C/N ratio, and WHC because of the opposite direction of their respective vectors, which implies that %RP decreases, while pH, lignin, C/N ratio, and WHC increase coinciding with correlation analysis. Relationship with OM and N was less significant.
Discussions
Pesticide dissipation
Experiment performed in soil at microcosm level showed that pesticides were dissipated quickly at 18 days (pesticide remaining < 2%) with exception of diazinon (pesticide remaining = 4%); however, differences between 18 and 22 days for all pesticides were minimum and total dissipation was observed for all pesticides at 41 days. According to DT50 and DT90 2,4-D, carbofuran and diazinon had a similar behavior being the least dissipated pesticides; moreover, DT50 and DT90 for atrazine and glyphosate showed that these pesticides were the best dissipated. Bibliography reports similar results in soil for pesticides such as 2,4-D with dissipation above 95% after 14 days (Ferrera-Cerrato et al. 2006) similar to 92% in this research in the same time; DT50 = 3.5 days for glyphosate is very similar to DT50 of 2–3 days reported by Burger and Fernández (2004). DT50 for atrazine in this research has a value of 1.6 days; this value is in range of 0.99–35 days using surface soils reported by Guillén-Garcés (2007). About carbofuran, Pimmata et al. (2013) report DT50 of 7.08–9.37 days in soil with low organic carbon content (0.89%) using mixture cultures as inoculum for high carbofuran concentrations; in this research, DT50 = 5.7 days for carbofuran was reported, and this difference can be attributed to record of pesticide applied in agricultural soil used and that organic carbon content of soil in this research is much higher (20.6%). For diazinon, Marín-Benito et al. (2014) report dissipation between 77 and 97% at 35 days using a sandy loam soil with organic carbon content of 0.47%; although soil type is the same in this research dissipation of diazinon near 97% was recorded at 22 days at microcosm level, this can be due to high organic carbon content of soil.
For miniaturized biobed systems, pesticide dissipation at 20 and 41 days above of 98 and 99% in this research was in accordance with dissipations averaging 90% for various pesticides (including atrazine) in biobed devices (Ponce-Caballero 2005; Castillo et al. 2008; de Roffignac et al. 2008; Dakhel et al. 2013) which support that new materials in biobed increase the dissipation even shorter times.
For this research, glyphosate was completely dissipated at 41 days in all biomixtures, de Roffignac et al. (2008) reported that 99% of glyphosate was degraded at 6 months using bagasse of sugar cane and soil in proportion 3:1 (v/v), M3 was a similar biomixture (1:1; v/v) used, and 41 days was enough to dissipate the total quantity of glyphosate applied (180 mg); this short time is perhaps by the differences in physicochemical parameters and the proportion of soil in biomixtures (50%) which implies that microorganisms present in soil are very important in pesticide dissipation.
Other researchers reported that 50% of atrazine was dissipated at 28.6 days replacing partially straw by oat husk in biobed (Urrutia et al. 2013); this is lower than reported in this research with atrazine dissipation above 98% in biomixtures without straw. This could be attributed to differences in operating temperature, WHC, and the substrates used, which implies that biomixture composition is very important and can be inferred that anaerobic process had place in biomixtures because of the high WHC (90%), this according with Castillo and Torstensson (2007). It is important to note that three of the best biomixtures in atrazine dissipation at 41 days (M6, M7, and M8) have in their composition compost (25%); this coincides with reported by Castillo et al. (2008) who established that biomixture with 30% of compost increase atrazine mineralization and its degradation.
A very important aspect is that 2253.14 mg of pesticides at 20 days were dissipated; this in biobeds working in farms in a real situation means prevention of the possible contamination of 22.53 × 106 L of water (2006/118/EC, 2008/105/EC) if released as point-source contamination, this according to European Community (2006, 2008).
These results show the importance of biomixtures in pesticide dissipation, this is because although pesticide remaining are between 0 and 2% at 20 days and less than 1% at 41 days with respect to the initial concentration for all biomixtures, minimum differences were significant, and remaining less than 0.1% of initial concentration implies a risk for water according to international regulations. It is very important to note that treatment systems of agricultural effluent type biobed have a lifespan of about 8–10 years (Fournier et al. 2006). After its lifespan, the biomixture remains 2 years without the application of agricultural effluents and sufficient time to dissipate any pesticide remaining.
One aspect to take into account is the temperature. Temperature used in this research (29.6 ± 2 °C) is highest compared with temperatures used in other research with values from 15 to 25 °C (Ponce-Caballero 2005; Castillo et al. 2008; Karanasios et al. 2010b; Urrutia et al. 2013; Ruíz-Hidalgo et al. 2014). Castillo and Torstensson (2007) report highest dissipations of some pesticides at 20 °C against lower temperatures. Temperature could be one of the main reasons of quickly dissipations of pesticides in this research. Castillo et al. (2008) support the fact that high temperature has an effect on microbial activity, on degradation of the organic matter, and on solubility of the pesticides. According to CONAGUA (2015), the average temperature in Yucatan peninsula has values from 30 to 35 °C; this can directly influence the dissipation of pesticides in biobeds operating in the agricultural fields.
At microcosm level in soil, all pesticides were dissipated at 41 days, but in biobed, atrazine and carbofuran were not dissipated completely in any biomixture; this could be attributed to conditions of both experiments. Biobeds have 500 cm3 of biomixture including soil and pesticide in solution can leach to bottom of container and be retained more time.
Effect of biomixture, time, and pesticide type on dissipation
Statistical analyses ANOVA showed that biomixture was significant (P < 0.05) on pesticide dissipation, but time and pesticide type were also significant (P < 0.05), so this factors should be considered when biobeds are implemented. According to Kruskal-Wallis analyses, physicochemical parameters of biomixtures (pH, lignin, WHC, OM, OC) were significant (P < 0.05) in pesticide dissipation, being pH the most significant; this is accordance with previous studies (Ponce-Caballero 2005; Castillo and Torstensson 2007) showing the importance of these parameters for pesticide dissipation in biobed. The pH significance is important because of let to know that minimum variations (7.25–7.67) of this parameter between biomixtures are more significant than other parameters.
Lignin content is maybe the parameter more studied, even in most of research about biobeds straw was replace with other lignin-rich substrates reporting higher pesticide dissipations (Torstensson 2000; Pigeon et al. 2005; Castillo and Torstensson 2007; Coppola et al. 2010; Ruiz-Hidalgo et a. 2014; Pinto et al. 2016). These results coincide with obtained in this research where lignin was one of the most significant parameters (P < 0.05) on pesticide dissipations in biomixtures without straw, corroborating that lignin content in biomixtures is very important.
Castillo et al. (2008) establish a strong relationship between C/N ratio and lignin content of biomixtures in pesticide degradation processes. This could be proved in this research. Spearman’s correlation (Fig. 3) shows that the best positive correlation of lignin was with C/N ratio and this is consistent with principal components analysis.
Principal components analysis (Fig. 4) shows that relationship of %RP with pH, lignin, C/N ratio, and WHC is negative; this implies that %RP decreases, while pH, lignin, C/N ratio, and WHC increase coinciding with correlation analysis, but according to Castillo and Torstensson (2007), low pH values (∼5.9) increase pesticide degradation by fungi, so the pH values above 7.0 registered in this research for all biomixtures implies that dissipation by degradation cannot be attributed totally to possible fungi presence and bacteria presences should be considered (Fournier et al. 2006). Analytical results for all pesticides in all biomixtures show that M5 was the most efficient biomixture; this is supported by Fig. 4 where remaining pesticides in M5 are well related with C/N ratio, lignin content, and pH. It is important to mention that presence of fungi was observed in some biomixtures such as M5; this could confirm the biodegradation of pesticide. Dakhel et al. (2013) report best retention and dissipation of pesticides at two monitoring times (4 and 13 months) in a biomixture composed by soil and straw (1:1); this coincides with pesticide dissipation in M5 whose composition is very similar (soil/corn stover, 1:1). However, soil seems to be very important because all biomixtures (soil content of 50%) had high dissipation.
Statistical analyses show that pesticide type is significant in pesticide dissipation. In this research, five different commercial pesticides were used, all them in solution. According to Pesticide Properties DateBase (PPDB 2016), more soluble pesticides were glyphosate and 2,4-D, and they were best dissipated in this research. Gimsing et al. (2004) establish that glyphosate has relatively rapid degradation in soil, so degradation could be a factor of complete dissipation for this pesticide. Cabildo et al. (2008) establish that more soluble pesticides are more likely to be chemically degraded and the less soluble pesticides have a greater contaminant potential; this supports the glyphosate and 2,4-D behavior in all biomixtures. Contrary, carbofuran and atrazine had the lowest dissipation in all biomixtures at 41 days; according to Pesticide Properties DateBase (PPDB 2016), these pesticides are less soluble than the others, and according to Cabildo et al. (2008), this makes these pesticides more persistent in all biomixtures.
Adsorption phenomena could be an important factor in pesticide behavior in biomixtures; Pesticide Properties DateBase (PPDB 2016) reports higher values of k f for diazinon and glyphosate (0.70–2573 and 9.4–700 mL g−1) which implies that these pesticides have good affinity to soil and maybe more available for biodegradation because of their behavior in all biomixture for this research. Contrary, atrazine has the lowest values of k f (1.3–6.3 mL g−1); this means that atrazine does not have good related with soil and maybe other factors affect its dissipation. However, it is important to establish that these results consider soils with OM values lower than reported in this research, and affinity of pesticides in soil may vary. Complementary factors such as pH of biomixtures could affect degradation and adsorption of pesticides (Wang et al. 2011). However, it is important to highlight the relationship between dissipation and adsorption phenomena (Olvera-Velona et al. 2008; Aparicio et al. 2015).
It is important to highlight that all commercial pesticides used have in their composition interveners that can modify their physicochemical characteristics and maybe this is the reason of similitude in dissipation for all pesticides.
Conclusions
The research corroborates the efficiency of biobeds (even only soil) at 41 days, recording dissipation of atrazine, carbofuran, diazinon, glyphosate, and 2,4-D over 99% in all biomixtures. This time is lower than time reported in other research.
Statistical analyses showed significant differences in pesticide dissipation according to the type of biomixtures, with M5 (soil/corn stover) the most efficient on pesticide dissipation, being this strongly affected by WHC, lignin, C/N ratio, and pH proving the importance of physicochemical parameters of biomixtures. However, the difference in efficiency with other biomixtures is minimal, since all biomixtures had efficiencies greater to 98% at 20 days and greater than 99% at 41 days. Therefore, it is important to note that 0.01% of remaining pesticides according to their initial concentrations implies a risk for groundwater pollution.
In conclusion, agricultural effluent treatment systems with high concentrations of pesticides type biobed present a viable option for farmers in the region in preventing pollution of soil and water bodies by pesticides. It has been proved that adding substrates from the region never used in biobeds (sisal, seaweed) increases the efficiency in pesticide dissipation, indicating that replacement of the original substrates such as straw and peat with new substrates can be an option to adapt biobeds to other regions and for different environmental conditions, preventing groundwater and soil pollution by pesticides.
References
Aparicio V, De Gerónimo E, Hernández Guijarro K, Pérez D, Portocarrero R, Vidal C (2015) Pesticides added to the soil and its destination in the environment. National Institute of Agricultural Technology. INTA editions 2015. Argentina (in Spanish).
Blake GR, Harge KH (1986). Bulk Density. In: Klute A. (Ed.) (1986) Methods of soil analysis: part 1. Physical and Mineralogical Methods, Agronomy Monograph 25, American Society of Agronomy-Soil Science Society of America. Madison Wisconsin, USA.
Burger M, Fernández S (2004) Exposure to glyphosate herbicide: clinical toxicological aspects. Medical Journal of Uruguay 20:202–207 (in Spanish)
Cabildo Miranda MP, Clamunt Villespí RM, Cornago Ramírez MP, Escolástico León C, Esteban Santos S, Farrán Morales MA, García Fernández MA, López García C, Pérez Esteban J, Pérez Torralba M, Santa María Gutierrez D, Sanz del Castillo D (2008) Recycling and waste treatment. National University of Distance Education, Madrid Spain (in Spanish)
Cardeña-Echalaz FV (2015) Diagnosis of use and management of pesticides in open area and unclosed area. MD Thesis, Autonomous University of Yucatán, Faculty of Engineering, December 2015 (in Spanish)
Castillo MP, Torstensson L (2007) Effect of biobed composition moisture and temperature on the degradation of pesticides. J Agric Food Chem 55:5725–5733. doi:10.1021/jf0707637
Castillo MP, Torstensson L, Stenstreom J (2008) Biobeds for environmental protection from pesticide uses a review. J Agric Food Chem 56:6206–6219. doi:10.1021/jf800844x
CONAGUA (2015) Mexico Weather Report “Annual Report 2015”. General Coordination of the National Meteorological Service, Meteorology and Climatology Management, Medium and Long Term Forecasting Manager http://smn1.conagua.gob.mx/climatologia/analisis/reporte/Anual2015.pdf (in Spanish)
Cooper RJ, Fitt P, Hiscock KM, Lovett AA, Gumm L, Dugdale SJ, Rambohul J, Williamson A, Noble L, Beamish J, Hovesen P (2016) Assessing the effectiveness of a three-stage on-farm biobed in treating pesticide contaminated wastewater. J Environ Manag 181:874–882
Coppola L, Castillo MP, Vischetti C (2010) Degradation of isoproturon and bentazona in peat -and compost- based biomixtures. Pest Manag Sci 67:107–113. doi:10.1002/ps.2040
Dakhel N, Guisolan N, Burdet J-P, Burgos S (2013) Efficiency of several biomix to retain and degrade pesticides in biobeds under Swiss pedo-climatic conditions, in 4th European Biobed Workshop, Wageningen, March 20–21. Wageningen, Netherlands, 2013, pp 19–20.
de Roffignac L, Cattan P, Mailloux J, Herzog D, Le Bellec F (2008) Efficiency of a bagasse substrate in a biological bed system for the degradation of glyphosate, malathion and lambda-cyhalothrin under tropical climate conditions. Pest Manag Sci 64:1303–1313. doi:10.1002/ps.1633
De Wilde T, Spanoghe P, Sniegowksi K, Ryckeboer J, Jaeken P, Springael D (2010) Transport and degradation of metalaxyl and isoproturon in biopurification columns inoculated with pesticide-primed material. Chemosphere 78:56–60. doi:10.1016/j.chemosphere.2009.10.011
Diez MC, Tortella GR, Briceño G, Castillo MP, Díaz J, Palma G, Altamirano C, Calderón C, Rubilar O (2013a) The influence of novel lignocellulosic residues in a biobed biopurification system on the degradation of pesticides applied in repeatedly high doses. Electron J Biotechnol. doi:10.2225/vol16-issue6-fulltext-17
Diez MC, Urrutia C, Gallardo F (2013b) Biopurification system for pesticides degradation: an integral study, in 4th European Biobed workshop, Wageningen, march 20-21. Wageningen, Netherlands 2013:15–16
EPA (2000) Microwave Extraction, Method 3546. US Environmental Protection Agency. February 2007.
Estrada Medina H, Álvarez Rivera OO (2011) Laboratory technique manual for analysis of Soil, Plant and Water (LASPA). Autonomous University of Yucatan, Faculty of Veterinary Medicine, México, 2011 (in Spanish)
European Union (EU) (2006) Directive 2006/118/EC of The European Parliament and of the Council of December 12, 2006, on the protection of groundwater against pollution and deterioration.
European Union (EU) (2008) Directive 2008/105/EC of The European Parliament and of the Council of December 16, 2008, on environmental quality standards in the field of water policy.
FAO (2009) Declaration of the World Summit of Food Security, Rome, November 2009. http://www.mofa.go.jp/policy/economy/fishery/wsfs0911–2.pdf
Ferrera-Cerrato R, Rojas-Avelizapa NG, Poggi-Varaldo HM, Alarcón A, Cañizares-Villanueva RO (2006) Bioremediation processes of soil and water contaminated by petroleum hydrocarbons and other organic compounds. Latin American Journal of Microbiology 48:179–187 (in Spanish)
Fogg P, Boxall AB, Walker A (2003a) Degradation of pesticides in Biobeds: the effect of concentration and pesticide mixtures. J Agric Food Chem 51:5344–5349. doi:10.1021/jf030060z
Fogg P, Boxall AB, Walker A, Jukes AA (2003b) Pesticide degradation in a ‘biobed’ composting substrate. Pest Manag Sci 59:527–537. doi:10.1002/ps.685
Fournier J-C, Martin-Laurent F, Catroux G (2006) Detoxifying plant health product effluents from farms, in Live from the labs, No.4, INRA 2006.
Gimsing AL, Borggaard OK, Bang M (2004) Influence of soil composition on adsorption of glyphosate and phosphate by contrasting Danish surface soils. Eur J Soil Sci 55:183–191. doi:10.1046/j.1365-2389.2003.00585.x
Guillén-Garcés RA, Hansen AM, Afferden van M (2007) Mineralization of atrazine in agricultural soil: inhibition by nitrogen. Environ Toxicol Chem 26:844–850
Hamilton DJ, Ambrus Á, Dieterle RM, Felsot AS, Harris CA, Holland PT, Katayama A, Kurihara N, Linders J, Unsworth J, Wong S-S (2009) Regulatory limits for pesticide residues in water (IUPAC technical report). Pure Appl Chem 75:1123–1155. doi:10.1351/pac200375081123
Husby J (2013) Biobed Workshops and Biobeds in the World, in 4th European Biobed Workshop, Wageningen, March 20–21. Wageningen, Netherlands, 2013, pp 8–9.
Juárez Juárez M, Franco Hernández MO, Jaens Contreras T, Ascencio Rasgado VP (2009) Manual of Laboratory Practice Environmental Chemistry II. National Polytechnic Institute, México, Enero 2009. (in Spanish)
Karanasios E, Tsiropoulos NG, Karpouzas DG, Ehaliotis C (2010a) Degradation and adsorption of pesticides in compost–based biomixtures as potential substrates for Biobeds in southern Europe. J. Agric. Food Chem 58:9147–9156. doi:10.1021/jf1011853
Karanasios E, Tsiropoulos NG, Karpouzas DG, Menkissoglu U (2010b) Novel biomixture based on local mediterranean lignocellulosic materials: evaluation for use in biobed systems. Chemosphere 80:914–921. doi:10.1016/j.chemosphere.2010.06.003
Karas PA, Makri S, Papadopoulou ES, Ehaliotis C, Menkissoglu-Spiroudi U, Karpouzas DG (2016) The potential of organic substrates based on mushroom substrate and straw to dissipate fungicides contained in effluents from the fruit-packaging industry – Is there a role for Pleurotus ostreatus? Ecotoxicol Environ Saf 124:447–454. doi:10.1016/j.ecoenv.2015.11.022
Marín-Benito JM, Herrero-Hernández E, Andrades MS, Sánchez-Martín MJ, Rodríguez-Cruz MS (2014) Effect of different organic amendments on the dissipation of linuron, diazinon and myclobutanil in an agricultural soil incubated for different time periods. Sci Total Environ 476-477:611–621. doi:10.1016/j.scitotenv.2014.01.052
Marmolejo Rodriguez AJ (1999) Determination of organochlorine pesticides in adult shrimp (Penaeus vannamei). MD Thesis, University of Colima, School of Marine Sciences, México, November 1999. (in Spanish)
Official Mexican Standard NMX-F-317-S-1978, determination of pH in food, Mexican standards. General Directorate of Standards, May 23, 1978 (in Spanish)
Official Mexican Standard NOM-021-SEMARNAT-2000, which establishes the specifications of fertility, salinity and soil classification study, sampling and analysis. Ministry of Environment and Natural Resources, México, December 31, 2002 (in Spanish)
Okalebo JR, Gathua KW, Woomer PL (1993) Laboratory methods of soil and plant analysis: a working manual. KARI, SSSEA, TSBF, UNESCO. Nairobi, Kenia.
Olvera-Velona A, Benoit P, Barriuso E, Ortiz-Hernandez L (2008) Sorption and desorption of organophosphate pesticides, parathion and cadusafos, on tropical agricultural soils. Agron Sustain Dev 28:231–238. doi:10.1051/agro:2008009
Pigeon O, Cd V, Cors F, Weickmans B, Bd R, Pussemier L, Debongnie P, Culot M (2005) Development of biofilters to treat the pesticides wastes from spraying applications. Commun Agric Appl Biol Sci 70:1003–1012
Pimmata P, Reungsang A, Plangklang P (2013) Comparative bioremediation of carbofuran contaminated soil by natural attenuation, bioaugmentation and biostimulation. Int Biodeterior Biodegrad 85:196–204. doi:10.1016/j.ibiod.2013.07.009
Pinto AP, Rodrigues SC, Caldeira AT, Teixeira DM (2016) Exploring the potential of novel biomixtures and Lentinula edodes fungus for the degradation of selected pesticides, evaluation for use in biobed systems. Sci Total Environ 541:1372–1381. doi:10.1016/j.scitotenv.2015.10.046
Ponce-Caballero C (2005) Microbial degradation of pesticide residues in rustic devices, type biobed: Biobacs. PhD Thesis University of Bourgogne, France, January 2005 (in French)
PPDB: Pesticide Properties DataBase 7.0. University of Hertfordshire, United Kingdom. http://sitem.herts.ac.uk/aeru/footprint/es/. Accessed 15 November 2016
Ruiz-Hidalgo K, Chin-Pampillo JS, Masís-Mora M, Carazo RE, Rodríguez-Rodríguez CE (2014) Degradation of carbofuran by Trametes versicolor in rice husk as apotential lignocellulosic substrate for biomixtures: from mineralization to toxicity reduction. Process Biochem 49:2266–2271. doi:10.1016/j.procbio.2014.10.006
Shahgholi H, Ahangar AG (2014) Factors controlling degradation of pesticides in the soil environment: a review. Agric Sci Dev 3:273–278
Torstensson L (2000) Experiences of biobeds in practical use in Sweden. Pesticide Outlook 11:206–211. doi:10.1039/b008025j
Torstensson L, Castillo MDP (1996) Biobeds minimize environmental risks when filling agricultural spraying equipment. Proceedings of the COST 66 Workshop, Stratford on Avon, May 13–15, 1996, U.K. pp 223–224.
Torstensson L, Castillo MP (1997) Use the Biobeds in Sweden to minimize environmental spillage from agricultural spraying equipment. Pesticide Outlook 8:24–27
Udikovic-Kolic N, Scott C, Martin-Laurent F (2012) Evolution of atrazine-degrading capabilities in the environment. Appl Microbiol Biotechnol 96:1175–1189. doi:10.1007/s00253-012-4495-0
Urrutia C, Rubilar O, Tortella GR, Diez MC (2013) Degradation of pesticide mixture on modified matrix of a biopurification system with alternatives lignocellulosic wastes. Chemosphere 92:1361–1366. doi:10.1016/j.chemosphere.2013.04.088
Van Soest PJ, Wine RH (1968) Determination of lignin and cellulose in acid detergent fiber with permanganate. Journal of the AOAC 51:780–785
Wang X, Guo X, Yang Y, Tao S, Xing B (2011) Sorption mechanisms of Phenanthrene, Lindane, and atrazine with various humic acid fractions from a single soil sample. Environ Sci Technol 45:2124–2130. doi:10.1021/es102468z
Wu J, Sardo V (2010) Sustainable versus organic agriculture, in: Sociology, organic farming, climate change and soil science. Springer, Netherlands, pp 41–76. doi:10.1007/978-90-481-3333-8_3
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Angeles Blanco
Electronic supplementary material
ESM 1
(TIFF 2833 kb)
Rights and permissions
About this article
Cite this article
Góngora-Echeverría, V.R., Martin-Laurent, F., Quintal-Franco, C. et al. Agricultural effluent treatment in biobed systems using novel substrates from southeastern Mexico: the relationship with physicochemical parameters of biomixtures. Environ Sci Pollut Res 24, 9741–9753 (2017). https://doi.org/10.1007/s11356-017-8643-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8643-z