Abstract
Soil washing is an effective approach to remove soil heavy metals, and the washing agent is generally regarded as one of the primary factors in the process, but there is still a lack of efficient and eco-friendly agents for this technique. Here, we showed that four plant washing agents—from water extracts of Coriaria nepalensis (CN), Clematis brevicaudata (CB), Pistacia weinmannifolia (PW), and Ricinus communis (RC)—could be feasible agents for the removal of soil lead (Pb), zinc (Zn), and cadmium (Cd). The metal removal efficiencies of the agents increased with their concentrations from 20 to 80 g L−1, decreased with the increasing solution pH, and presented different trends with the reaction time increasing. CN among the four agents had the highest removal efficiencies of soil Pb (62.02%) and Zn (29.18%) but owned the relatively low Cd removal efficiencies (21.59%). The Fourier transform infrared spectroscopy showed that the abilities of plant washing agents for the removal of soil heavy metals may result from bioactive substances with specific functional groups such as –COOH, −NH2, and −OH. Our study provided CN as the best washing agents for the remediation of contaminated soil by heavy metals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil contamination with heavy metals has become a worldwide concern due to the intensification of human activities such as mining, smelting, and electroplating in recent years (Jho et al. 2015; Morcillo et al. 2016; Tai et al. 2013). Some heavy metals like lead (Pb) and cadmium (Cd) have high toxicity to human health (Nogawa et al. 2004; Sone et al. 2009), while others such as zinc (Zn) are indispensable at low concentrations but harmful in excessive quantities for creatures (Touceda-González et al. 2015). In addition, these heavy metals are hazardous and persistent in soils (Han et al. 2016; Wu et al. 2015). Consequently, there is an urgent need to remediate the soils contaminated by Pb, Zn, and Cd.
Several remediation techniques including stabilization (Bosio et al. 2014), electrodialysis (Gherasim et al. 2014), and phytoremediation (Zhang et al. 2013) have been widely applied to repair heavy metal-polluted soils. Although these methods could be useful, they have been demonstrated to be costly or time-consuming (Wang et al. 2014). To the contrary, soil washing is considered as one of the most promising methods to remove soil heavy metals due to its time-saving and cost-efficient properties and being applicable to multiple contaminants (Im et al. 2015; Satyro et al. 2016; Yi and Sung 2015).
Soil washing is a chemical extraction technique based on the desorption of pollutants from contaminated soils via various agents (Kulikowska et al. 2015). Many researchers have investigated the effects of different types of washing agents on the removal efficiency of soil heavy metals. For example, Moon et al. (2012) reported that 56.7% Zn was removed by hydrochloric acid (HCl). Nevertheless, strong acids can result in soil matrix destruction due to excessive acidification (Ko et al. 2005). Although several studies have shown that ethylenediaminetetraacetic acid (EDTA) is effective in removing heavy metals from contaminated soil (Cui et al. 2015; Pociecha and Lestan 2010; Voglar and Lestan 2012, 2013; Zupanc et al. 2014), it may cause a secondary pollution for its toxicity and the long persistence in soil environment (González et al. 2011). Therefore, it is essential to search new feasible eco-friendly washing agents for the remediation of metal-contaminated soils.
The plant material is cost-effective and eco-friendly due to extensive sources and strong biodegradability. Currently, although many lines of research have devoted to studying the saponin derived from soapberry as natural plant-based surfactant for the removal of soil heavy metals (Gusiatin and Klimiuk 2012; Maity et al. 2013a; Maity et al. 2013b), rather, less attention has been paid to investigating washing agents extracted from other plants to remove heavy metals. Hence, screening new plant material may be of great significance.
Coriaria nepalensis, Clematis brevicaudata, Pistacia weinmannifolia, and Ricinus communis are all perennial plants and extensively distributed in China (Zeng et al. 2007; Zhao et al. 2005; Zhao et al. 2012). Additionally, Hao et al. (2013), Ohishi et al. (2014), Yang et al. (2011), and Zhao et al. (2005) have reported that they have carboxyl or hydroxyl in their components. Therefore, we may speculate that they may chelate with heavy metal ions in soils. The results of our preliminary experiment indicated that they could remove heavy metals from contaminated soils to some extent, but a systemic study is lacking. The objectives of this study were (1) to test the effects of concentration, pH, and washing time on soil Pb, Zn, and Cd removal and (2) to identify the participant functional groups on the plant washing agents during soil washing.
Materials and methods
Soil sampling and characterization
The contaminated soil was collected from the surface layer (0–20 cm) of a waste farmland in Tangjia Pb-Zn Mine in Hanyuan, Sichuan (29° 24′ N, 102° 37′ E). Samples were air-dried and sieved to 2 mm. Soil pH was measured in a 1:2.5 (w/v) soil/deionized water suspension. Soil samples were analyzed for organic carbon by Walkley-Black titrations (Nelson and Sommers 1996). Total nitrogen in soil was determined by the Kjeldahl method (Bremner and Sparks 1996), and available phosphorus was extracted using 0.5 M NaHCO3 (pH = 8.5) and measured by the molybdenum antimony colorimetry method (Olsen and Sommers 1982). Soil texture was analyzed by the pipette method (Gee and Bauder 1986). The contaminated soil was digested in a solution mixed with HNO3-HCl-HClO4 in a 1:2:2 ratio (v/v/v). Concentrations of Pb, Zn, and Cd in the contaminated soils were measured by flame atomic absorption spectrometry (AAS, Thermo Solaar M6, Thermo Fisher Scientific Ltd., USA).
Preparation of plant washing solutions
The overground part of plants including C. nepalensis (CN), C. brevicaudata (CB), P. weinmannifolia (PW), and R. communis (RC) was selected based on our preliminary experiments and obtained in Hanyuan, Sichuan. The plant samples were collected and washed thoroughly with running tap water followed by deionized water before being dried at 65 °C for 72 h and then ground by a grinder (DFY-500, Wenling Linda Machinery Co., China) and passed the 2-mm sieve. The plant powders were oscillated for 24 h by distilled water at 25 °C under 150 rpm and thereafter filtered for plant washing agents. The agent concentrations were expressed by the initial mass of plant powder and the volume of the distilled water. Concentrations of Pb, Zn, and Cd in the plant materials were determined by AAS (Thermo Solaar M6, Thermo Fisher Scientific Ltd., USA).
Soil washing experiments
Batch experiments about the four plant agents were carried out in different washing conditions including the concentrations, solution pH, and washing time. All the soil washing experiments were conducted in 50-mL-capacity polyethylene tubes with soils (2.00 g) to washing solution (40 mL) at a solid/liquid ratio of 1:20 (w/v). The samples were then shaken in a mechanical shaker at 150 rpm at room temperature (25 °C) for a period. At the end of the extraction, the suspensions were immediately filtered through a 0.45 μm filter membrane. The concentrations of Pb, Zn, and Cd in the filtrate were measured by AAS. All tests were conducted in triplicate.
Concentrations
Each plant washing agent was prepared in concentrations of 20, 35, 50, 65, and 80 g L−1. Then, the washing solutions were pipetted into soils in 50-mL-capacity polyethylene tubes and the intermixture with HNO3 or NaOH was adjusted at pH 4.0. The samples were oscillated at 150 rpm for 2 h at 25 °C in a shaker. Afterwards, the suspensions were immediately filtered for determination of heavy metals. At this pH condition, the soil values after washing were 6.1–6.7.
pH
Each plant washing agent at the concentrations of 50 g L−1 was added into soils in 50-mL-capacity polyethylene tubes, and the pH values of the reaction systems were adjusted at 3.0, 4.0, 6.0, 7.0, and 9.0. Next, the samples were shaken on a shaker at 150 rpm for 2 h at 25 °C. The suspensions were immediately filtered for determination of heavy metals.
Washing time
Kinetic experiments were conducted at the time of 0.5, 1.0, 1.5, 2.0, and 4 h in the concentrations of each reagent with 50 g L−1 at pH 4.0 in 50-mL-capacity polyethylene tubes. The samples were stirred at 150 rpm at 25 °C in a shaker. The suspensions were immediately filtered for determination of heavy metals.
FTIR analysis of plant agents before and after soil washing
In order to identify the participant functional groups on the plant washing agents during the soil washing process, the plant agents before and after washing were dried at 40 °C and analyzed by an FTIR spectrophotometer (Spectrum Two, PerkinElmer Inc., USA). The agents after soil washing were collected at concentrations of 50 g L−1 at pH 4.0 for 2 h. The materials were ground with KBr sufficiently in the agate mortar at a ratio of 1:200 and pressed into a disk under high pressure. The spectra were recorded from 400 to 4000 cm−1 at a resolution of 4 cm−1.
Changes of heavy metal fractions by soil washing
The soils washed with 50 g L−1 plant washing agents after 2 h of reaction at 1:20 w/v and an initial pH 4.0 were collected. The distribution of metal fractions including exchangeable, reducible, oxidizable, and residual fractions in the contaminated soil before and after washing was determined by the modified Community Bureau of Reference (BCR) three-step sequential extraction procedure (Pueyo et al. 2008).
Quality control and statistical analysis
Standard reference material (GBW07405) was used for analyzing the accuracy and precision of the analytical method (accuracies within ±5%) during the digestion procedure. SPSS Version 19.0 (SPSS Inc., Chicago, Ill, USA) was used for data statistical analysis. Differences in Pb, Zn, and Cd removal efficiency among different experimental conditions were identified by one-way ANOVA. Mean values were compared by least significant difference and if p < 0.05 were considered to indicate significance.
Results and discussion
Characters of soil sample and plant agent
The texture of metal-contaminated soil was sandy clay with 63.7% sand, 11.2% silt, and 25.1% clay, respectively. Soil organic carbon, total nitrogen, available phosphorus, and pH were 19.7 g kg−1, 1.1 g kg−1, 21.9 mg kg−1, and 7.23, respectively. The concentrations of soil Pb, Zn, and Cd were 698.72, 1587.27, and 15.05 mg kg−1, respectively, and substantially exceed the threshold of farmland (The China Environmental Quality Standard for soils, GB 15618-1995). The pH values of plant agents were 5.8–6.6, and the concentrations of Pb and Zn in plant agents (80 g L−1) were 0.04 and 0.07 mg L−1, respectively (Cd was not detected).
Effect of concentrations on the removal of soil Pb, Zn, and Cd
The concentration of washing agent is essential to soil washing, as it determines the amount of substance which will involve in the reaction (Chen et al. 2016). In order to investigate the effect of concentrations of the agents on removal of soil heavy metals, four plant agents with different concentrations were used in the experiments. As shown in Fig. 1, their removal efficiencies of soil Pb, Zn, and Cd presented a logarithmic increase with increasing concentrations from 20 to 80 g L−1. The results suggest that higher concentrations of plant agents lead to more effective removal of heavy metals. This may be ascribed that the agents derived from plants may have functional groups such as carboxyl or hydroxyl, which can form complex with heavy metals (Cao et al. 2013; Song et al. 2008). Therefore, the higher concentration of agents could bind to more heavy metal ions due to more active substances. Similar results were observed with saponin derived from plant for the washing of soil heavy metals at different concentrations (Maity et al. 2013b). Among the agents, CN had the highest removal efficiencies for soil Pb (62.02%) and Zn (29.18%) at the concentration of 80 g L−1 (Fig. 1a, b), but the maximum Cd removal efficiency by CN was only 21.60% and significantly lower than those of CB and RC at the same concentration (p < 0.05). In contrast, the latter two agents had the higher Cd removal efficiencies with 25.89 and 25.24%, respectively (Fig. 1c). However, PW had the lowest removal efficiencies for the metals from the soil. Kim et al. (2016) have reported that the removal of soil heavy metals not only depends on the type of metals and their concentrations, but also depends on the type of washing agents. Previous studies indicated that CN has the characteristic bioactive constituents of sesquiterpene lactones such as coriamyrtin, tutin, and hydroxycoriatin (Yang et al. 2011; Zhao et al. 2012), and these components may be resulted in that the metal removal efficiencies by CN were higher than those of others. CB has triterpenoid saponins (Hao et al. 2013), n-hexadecanoic acid, and (Z, Z)-9,12-octadecadienoic acid (Zeng et al. 2007), and RC contains ricinoleic acid, linoleic acid, stearic acid, and sitosterol (Tyagi et al. 2013). Additionally, phytochemical studies revealed that PW is rich in gallotannins and related phenolic compounds (Zhao et al. 2005). Thus, the different components of plants might lead to the different washing efficiencies via the strength of their interactions with soil metals during chelating or ion exchange reactions.
Effect of pH on the removal of soil Pb, Zn, and Cd
The pH is an important factor for the washing efficiency by affecting the metal adsorption and desorption from soil colloids (Zou et al. 2009). As shown in Fig. 2, the washing efficiencies of soil Pb, Zn, and Cd by the four agents decreased with increasing solution pH. As the pH from 3.0 to 6.0, their removal efficiencies by the agents sharply decreased (p < 0.05). This trend was similar to the washing of Pb by low molecular weight organic acid combined with nanoscale zero-valent iron (Wang et al. 2014). At pH 3.0, four plant washing agents for the removal of soil Pb, Zn, and Cd were relatively high. Under acidic conditions, the metal ions were easily desorbed from soil colloids for the solubility of carbonate-bound metals increased or exchanged with H+ from the soil surface functional groups (Begum et al. 2012). As the pH beyond 6.0, the soil Pb, Zn, and Cd removal showed either a stable or an increasing trend (Fig. 2). At neutral or alkaline conditions, the lower removal abilities of heavy metals in soil might result from the low solubility of metal oxides or precipitate by the formation of metal hydroxyl complexes (Begum et al. 2013).
Effect of washing time on the removal of soil Pb, Zn, and Cd
Removal of heavy metals from contaminated soil is a dynamic equilibrium of adsorption and desorption process (Bradl 2004; Sajadi Tabar and Jalali 2013), and the process needs to take some time. Thus, the washing time is another key parameter that influences the metal removal efficiency. In the current study, the effects of reaction time on soil Pb, Zn, and Cd removal were investigated at the concentrations of the reagents of 50 g L−1 at pH 4.0. As shown in Fig. 3, soil Pb, Zn, and Cd removal efficiencies of the washing solutions presented different trends with increasing washing time. When the time was ≤1.5 h, CN for the metal removal efficiencies significantly increased with increasing washing time (p < 0.05). When the time was >1.5 h , the efficiencies were close to equilibrium and remained almost constant (p > 0.05). These change trends of Pb, Zn, and Cd removal by CN with reaction durations were similar to the experiments of the combination of EDTA and dithionite on the Pb and Zn removal at various periods (Kim et al. 2016). Currently, some researchers have pointed out that chemical reagents for the removal of soil heavy metals with time variation could be divided into two processes: the first process washed quickly, and the second process washed slowly (Bermond and Ghestem 2001). However, the trends of CB, PW, and RC for the removal of Pb were close to equilibrium and the removal of Zn increased gradually with the washing time from 0.5 to 4.0 h, while the trends of Cd removal efficiencies by them were different with increasing reaction time. These results may be ascribed to the complicated components among different plants.
Effect of the washing time on the removal of Pb (a), Zn (b), and Cd (c) with plant washing agents from soil. CN Coriaria nepalensis, CB Clematis brevicaudata, PW Pistacia weinmannifolia, RC Ricinus communis, DW distilled water. Different letters in the same line indicate significant difference (p < 0.05)
FTIR analysis
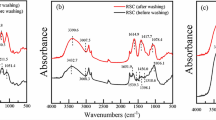
Fourier transform infrared spectroscopy (FTIR) analysis is important to identify some characteristic functional groups, especially those groups in binding of metal ions (Farooq et al. 2010; Kostić et al. 2014). The obtained spectra of all plant agents before and after washing were measured by an FTIR spectrometer (Fig. 4, Table 1). Based on the assignment of peaks in Table 1, the four agents contain many functional groups such as –COOH, −OH, −NH2, C–O–C, and C–O–P. These functional groups appeared in the phytochemical components, such as coriamyrtin, hydroxycoriatin, n-hexadecanoic acid, ricinoleic acid, or related phenolic, and have been identified as potential sites to be responsible for binding metallic ions to the biomass (Chakravarty et al. 2010).
Comparing the spectral changes of each agent before and after washing, the FTIR peaks might shift in position, change in intensity, and increase or decrease in number, indicating that there existed interaction between the functional groups of plant agents and metal ions in soil colloid (Fig. 4). The first change was the typical and intense shift of bands in the agents, which were shifted to another bands at 3388–3410 and 1595–1626 cm−1 for the increasing bond strength of hydroxyl and carboxyl groups during the soil washing process (Gutha et al. 2015), respectively. The second change was the disappeared peaks at 1431 cm−1 in CN, 1403 cm−1 in CB, 1693 and 1037 cm−1 in PW, and 1415 cm−1 in RC, indicating that hydroxyl and carboxyl groups participated in the soil washing process by ion exchange or complexation. The final change was new peaks at 1384 cm−1 in CN, 1384 and 1045 cm−1 in CB, and 1280 cm−1 in RC after soil washing. These results might be ascribed by acidic solution, and H+ ions as donors in solution could be bound to the sites on the surface of organic molecule structure of CN, CB, and RC and form functional groups (−COOH, −NH2, and –OH) (Chen et al. 2010). Besides, the intensities of these new peaks were in the order of CN > CB > RC, indicating that CN had more functional groups and led to the highest removal efficiencies for the removal of soil heavy metals. Therefore, FTIR analysis demonstrated that the ability of plant washing agents to remove heavy metals may result from bioactive substances with specific functional groups such as –COOH, −NH2, and –OH.
Effect of washing on soil metal fractions
The fractions of metals within the soil solid matrix could explain the different removal efficiencies for various metals (Barona et al. 2001). The mobility and distribution of Pb, Zn, and Cd fractions in soils before and after washing with four plant washing agents at pH 4.0 were determined by the BCR sequential extraction procedure. The results revealed that 90.93, 80.04, and 89.23% of the total Pb, Zn, and Cd contents in the contaminated soil were bound in the non-detrital fractions. The non-detrital fraction represents the metal amounts partitioned cumulatively in the exchangeable, reducible, and oxidizable fractions other than those associated with the residual fraction and could be removed easily by washing (Begum et al. 2013). As shown in Fig. 5, a significant proportion of the Pb (59.11%) was partitioned in the reducible fraction, while the Zn and Cd were equivalently distributed in all fractions approximately. The metal contents of exchangeable fraction for soil Pb, Zn, and Cd were reduced by 10.24–20.51% after washing with the four washing agents except for Pb with CB and PW (about 4%). The reductions of reducible, oxidizable, and residual fractions of heavy metals washed with CN were higher than those of the other three washing agents except for residual fraction of Cd. To the contrary, the reduction of residual fraction for Cd washed by CN was lower than that of the other three agents. The oxidizable fraction represents the metal ions bound to the organic matter, humic acids, and sulfides, which is the less labile forms, while the residual fraction, which is incorporated into the crystalline lattice of the soil minerals, is unlikely to be extracted easily (Pueyo et al. 2008; Tandy et al. 2004). The various removal rates among different fractions in soils may be attributed to the different functional groups in the four agents.
Conclusions
This study investigated four plant materials—CN, CB, PW, and RC—as eco-friendly plant washing agents to remove Pb, Zn, and Cd from contaminated soil. The concentrations of the agents, pH, and reaction time significantly affected the Pb, Zn, and Cd removal (p < 0.05). The removal efficiencies increased in response to the increasing concentrations of the agents, decreased with the increasing pH, and presented different trends with the time increasing. Results showed that four plant washing agents could be used for removing soil heavy metals, and CN was the best washing agent among the four reagents generally. The highest Pb, Zn, and Cd removal efficiencies by CN were 62.02, 29.18, and 21.60% at the concentrations of 80 g L−1, respectively. Therefore, these plant materials could be potential eco-friendly plant washing agents to remove soil heavy metals, because they contain many functional groups such as –OH, −NH2, and −COOH and may react with heavy metal ions by ion exchange, complexation, and chemical reactions with surface sites. Hence, the various removal efficiencies for heavy metals by the reagents may be in accordance with the functional groups on different plant chemical compositions and the types of metals.
Although the plant washing agents have demonstrated to be efficient in removing heavy metals, their washing mechanisms for the removal of soil metals were required for further study.
References
Barka N, Abdennouri M, El Makhfouk M, Qourzal S (2013) Biosorption characteristics of cadmium and lead onto eco-friendly dried cactus (Opuntia ficus indica) cladodes. J Environ Chem Eng 1:144–149
Barona A, Aranguiz I, Elıas A (2001) Metal associations in soils before and after EDTA extractive decontamination: implications for the effectiveness of further clean-up procedures. Environ Pollut 113:79–85
Begum ZA, Rahman IMM, Tate Y, Sawai H, Maki T, Hasegawa H (2012) Remediation of toxic metal contaminated soil by washing with biodegradable aminopolycarboxylate chelants. Chemosphere 87:1161–1170
Begum ZA, Rahman IMM, Sawai H, Mizutani S, Maki T, Hasegawa H (2013) Effect of extraction variables on the biodegradable chelant-assisted removal of toxic metals from artificially contaminated European reference soils. Water Air Soil Pollut 224:1381–1402
Bermond A, Ghestem JP (2001) Kinetic study of trace metal EDTA-desorption from contaminated soils. In: Selim HM, Sparks DL (eds) Heavy metals release in soils. Lewis, Boca Raton, pp 131–147
Bosio A, Zacco A, Borgese L, Rodella N, Colombi P, Benassi L, Depero LE, Bontempi E (2014) A sustainable technology for Pb and Zn stabilization based on the use of only waste materials: a green chemistry approach to avoid chemicals and promote CO2 sequestration. Chem Eng J 253:377–384
Bradl HB (2004) Adsorption of heavy metal ions on soils and soils constituents. J Colloid Interf Sci 277:1–18
Bremner JM, Sparks DL (1996) Nitrogen-total. In: Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Sumner ME (eds) Methods of soil analysis. ASA/SSSA, Madison, pp 1085–1121
Calero M, Pérez A, Blázquez G, Ronda A, Martín-Lara MA (2013) Characterization of chemically modified biosorbents from olive tree pruning for the biosorption of lead. Ecol Eng 58:344–354
Cao M, Hu Y, Sun Q, Wang L, Chen J, Lu X (2013) Enhanced desorption of PCB and trace metal elements (Pb and Cu) from contaminated soils by saponin and EDDS mixed solution. Environ Pollut 174:93–99
Chakravarty P, Sarma NS, Sarma HP (2010) Removal of lead (II) from aqueous solution using heartwood of Areca catechu powder. Desalination 256:16–21
Chen H, Dai G, Zhao J, Zhong A, Wu J, Yan H (2010) Removal of copper (II) ions by a biosorbent—Cinnamomum camphora leaves powder. J Hazard Mater 177:228–236
Chen Y, Zhang S, Xu X, Yao P, Li T, Wang G, Gong G, Li Y, Deng O (2016) Effects of surfactants on low-molecular-weight organic acids to wash soil zinc. Environ Sci Pollut R 23:4629–4638
Cui L, Wang Y, Gao L, Hu L, Yan L, Wei Q, Du B (2015) EDTA functionalized magnetic graphene oxide for removal of Pb (II), Hg (II) and Cu (II) in water treatment: adsorption mechanism and separation property. Chem Eng J 281:1–10
Farooq U, Kozinski JA, Khan MA, Athar M (2010) Biosorption of heavy metal ions using wheat based biosorbents—a review of the recent literature. Bioresour Technol 101:5043–5053
Gee GW, Bauder JW (1986) Particle-size analysis. In: Klute A (ed) Methods of soil analysis. ASA/SSSA, Madison, pp 383–411
Gherasim C, Křivčík J, Mikulášek P (2014) Investigation of batch electrodialysis process for removal of lead ions from aqueous solutions. Chem Eng J 256:324–334
González I, Cortes A, Neaman A, Rubio P (2011) Biodegradable chelate enhances the phytoextraction of copper by Oenothera picensis grown in copper-contaminated acid soils. Chemosphere 84:490–496
Gusiatin ZM, Klimiuk E (2012) Metal (Cu, Cd and Zn) removal and stabilization during multiple soil washing by saponin. Chemosphere 86:383–391
Gutha Y, Munagapati VS, Naushad M, Abburi K (2015) Removal of Ni (II) from aqueous solution by Lycopersicum esculentum (Tomato) leaf powder as a low-cost biosorbent. Desalin Water Treat 54:200–208
Han W, Fu F, Cheng Z, Tang B, Wu S (2016) Studies on the optimum conditions using acid-washed zero-valent iron/aluminum mixtures in permeable reactive barriers for the removal of different heavy metal ions from wastewater. J Hazard Mater 302:437–446
Hao D, Gu X, Xiao P, Peng Y (2013) Chemical and biological research of Clematis medicinal resources. Chinese Sci Bull 58:1120–1129
Im J, Yang K, Jho EH, Nam K (2015) Effect of different soil washing solutions on bioavailability of residual arsenic in soils and soil properties. Chemosphere 138:253–258
Jho EH, Im J, Yang K, Kim Y, Nam K (2015) Changes in soil toxicity by phosphate-aided soil washing: effect of soil characteristics, chemical forms of arsenic, and cations in washing solutions. Chemosphere 119:1399–1405
Kim EJ, Jeon E, Baek K (2016) Role of reducing agent in extraction of arsenic and heavy metals from soils by use of EDTA. Chemosphere 152:274–283
Ko I, Chang Y, Lee C, Kim K (2005) Assessment of pilot-scale acid washing of soil contaminated with As, Zn and Ni using the BCR three-step sequential extraction. J Hazard Mater 127:1–13
Kostić M, Radović M, Mitrović J, Antonijević M, Bojić D, Petrović M, Bojić A (2014) Using xanthated Lagenaria vulgaris shell biosorbent for removal of Pb (II) ions from wastewater. J Iran Chem Soc 11:565–578
Kulikowska D, Gusiatin ZM, Bułkowska K, Kierklo K (2015) Humic substances from sewage sludge compost as washing agent effectively remove Cu and Cd from soil. Chemosphere 136:42–49
Li X, Tang Y, Cao X, Lu D, Luo F, Shao W (2008) Preparation and evaluation of orange peel cellulose adsorbents for effective removal of cadmium, zinc, cobalt and nickel. Colloids Surf A-Physicochem Eng Asp 317:512–521
Li Z, Tang X, Chen Y, Wei L, Wang Y (2009) Activation of Firmiana simplex leaf and the enhanced Pb (II) adsorption performance: equilibrium and kinetic studies. J Hazard Mater 169:386–394
Maity JP, Huang YM, Hsu C, Wu C, Chen C, Li C, Jean J, Chang Y, Chen C (2013a) Removal of Cu, Pb and Zn by foam fractionation and a soil washing process from contaminated industrial soils using soapberry-derived saponin: a comparative effectiveness assessment. Chemosphere 92:1286–1293
Maity JP, Huang YM, Fan CW, Chen CC, Li CY, Hsu CM, Chang YF, Wu CI, Chen CY, Jean JS (2013b) Evaluation of remediation process with soapberry derived saponin for removal of heavy metals from contaminated soils in Hai-Pu, Taiwan. J Environ Sci (China) 25:1180–1185
Moon DH, Lee J, Wazne M, Park J (2012) Assessment of soil washing for Zn contaminated soils using various washing solutions. J Ind Eng Chem 18:822–825
Morcillo P, Esteban MÁ, Cuesta A (2016) Heavy metals produce toxicity, oxidative stress and apoptosis in the marine teleost fish SAF-1 cell line. Chemosphere 144:225–233
Nelson DW, Sommers LE (1996) Total carbon, organic carbon, and organic matter. In: Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Sumner ME (eds) Methods of soil analysis. ASA/SSSA, Madison, pp 961–1010
Nogawa K, Kobayashi E, Okubo Y, Suwazono Y (2004) Environmental cadmium exposure, adverse effects and preventive measures in Japan. Biometals 17:581–587
Ohishi K, Toume K, Arai MA, Sadhu SK, Ahmed F, Mizoguchi T, Itoh M, Ishibashi M (2014) Ricinine: a pyridone alkaloid from Ricinus communis that activates the Wnt signaling pathway through casein kinase 1α. Bioorgan Med Chem 22(17):4597–4601
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. ASA/SSSA, Madison, Wisconsin, pp 581–893
Pociecha M, Lestan D (2010) Using electrocoagulation for metal and chelant separation from washing solution after EDTA leaching of Pb, Zn and Cd contaminated soil. J Hazard Mater 174:670–678
Pueyo M, Mateu J, Rigol A, Vidal M, López-Sánchez JF, Rauret G (2008) Use of the modified BCR three-step sequential extraction procedure for the study of trace element dynamics in contaminated soils. Environ Pollut 152:330–341
Sajadi Tabar S, Jalali M (2013) Kinetics of Cd release from some contaminated calcareous soils. Natural Res Rese 22:37–44
Satyro S, Race M, Di Natale F, Erto A, Guida M, Marotta R (2016) Simultaneous removal of heavy metals from field-polluted soils and treatment of soil washing effluents through combined adsorption and artificial sunlight-driven photocatalytic processes. Chem Eng J 283:1484–1493
Sone H, Fugetsu B, Tanaka S (2009) Selective elimination of lead (II) ions by alginate/polyurethane composite foams. J Hazard Mater 162:423–429
Song S, Zhu L, Zhou W (2008) Simultaneous removal of phenanthrene and cadmium from contaminated soils by saponin, a plant-derived biosurfactant. Environ Pollut 156:1368–1370
Tai Y, McBride MB, Li Z (2013) Evaluating specificity of sequential extraction for chemical forms of lead in artificially-contaminated and field-contaminated soils. Talanta 107:183–188
Tandy S, Bossart K, Mueller R, Ritschel J, Hauser L, Schulin R, Nowack B (2004) Extraction of heavy metals from soils using biodegradable chelating agents. Environ Sci Technol 38:937–944
Touceda-González M, Brader G, Antonielli L, Ravindran VB, Waldner G, Friesl-Hanl W, Corretto E, Campisano A, Pancher M, Sessitsch A (2015) Combined amendment of immobilizers and the plant growth-promoting strain Burkholderia phytofirmans PsJN favours plant growth and reduces heavy metal uptake. Soil Biolo Biochem 91:140–150
Tyagi K, Sharma S, Rashmi R, Kumar S (2013) Study of phyto-chemical constituents of Ricinus communis Linn. under the influence of industrial effluent. J Pharmacy Res 6:870–873
Voglar D, Lestan D (2012) Pilot-scale washing of metal contaminated garden soil using EDTA. J Hazard Mater 215-216:32–39
Voglar D, Lestan D (2013) Pilot-scale washing of Pb, Zn and Cd contaminated soil using EDTA and process water recycling. Chemosphere 91:76–82
Wang G, Zhang S, Xu X, Li T, Li Y, Deng O, Gong G (2014) Efficiency of nanoscale zero-valent iron on the enhanced low molecular weight organic acid removal Pb from contaminated soil. Chemosphere 117:617–624
Wang G, Zhang S, Yao P, Chen Y, Xu X, Li T, Gong G (2015) Removal of Pb (II) from aqueous solutions by Phytolacca americana L. biomass as a low cost biosorbent. Arab J Chem. doi:10.1016/j.arabjc.2015.06.011
Wu Q, Cui Y, Li Q, Sun J (2015) Effective removal of heavy metals from industrial sludge with the aid of a biodegradable chelating ligand GLDA. J Hazard Mater 283:748–754
Yang J, Pu J, Du X, Zhang H, Xiao W, Sun H (2011) Two new compounds from Coriaria nepalensis. Chinese Chem Lett 22:1078–1080
Yi YM, Sung K (2015) Influence of washing treatment on the qualities of heavy metal–contaminated soil. Ecol Eng 81:89–92
Zeng Y, Zhao C, Liang Y, Yang H, Fang H, Yi L, Zeng Z (2007) Comparative analysis of volatile components from Clematis species growing in China. Anal Chim Acta 595:328–339
Zhang S, Lin H, Deng L, Gong G, Jia Y, Xu X, Li T, Li Y, Chen H (2013) Cadmium tolerance and accumulation characteristics of Siegesbeckia orientalis L. Ecol Eng 51:133–139
Zhao X, Sun H, Hou A, Zhao Q, Wei T, Xin W (2005) Antioxidant properties of two gallotannins isolated from the leaves of Pistacia weinmannifolia. BBA - Gen Subjects 1725:103–110
Zhao F, Liu Y, Ma S, Qu J, Yu S, Fang Z, Li L, Si Y, Zhang J (2012) New sesquiterpenes from the roots of Coriaria nepalensis. Tetrahedron 68:6204–6210
Zou Z, Qiu R, Zhang W, Dong H, Zhao Z, Zhang T, Wei X, Cai X (2009) The study of operating variables in soil washing with EDTA. Environ Pollut 157:229–236
Zupanc V, Kastelec D, Lestan D, Grcman H (2014) Soil physical characteristics after EDTA washing and amendment with inorganic and organic additives. Environ Pollut 186:56–62
Acknowledgements
This study was supported by the Projects of Sci-tech Support, Sichuan, China (No. 2014NZ0044), and the Projects of National Sci-tech Support, China (2012BAD14B18-2). The authors also acknowledge the contribution of Ping Yao, Chuer Zhang, Qinmei Zhong, Linxian Li, Yue Chen, Yijun Wang, Rui Ma, and Guangrong Xu of Sichuan Agricultural University in supporting the investigation and research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Zhihong Xu
Rights and permissions
About this article
Cite this article
Cao, Y., Zhang, S., Wang, G. et al. Removal of Pb, Zn, and Cd from contaminated soil by new washing agent from plant material. Environ Sci Pollut Res 24, 8525–8533 (2017). https://doi.org/10.1007/s11356-017-8542-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8542-3