Abstract
In situ stabilization of Cd, Pb, and Zn in an Austrian agricultural soil contaminated by atmospheric depositions from a smelter plant was assessed with a pine bark chip-derived biochar, alone and in combination with either compost or iron grit. Biochar amendment was also trialed in an uncontaminated soil to detect any detrimental effect. The pot experiment consisted in ten soil treatments (% w/w): untreated contaminated soil (Unt); Unt soil amended with biochar alone (1%: B1; 2.5%: B2.5) and in combination: B1 and B2.5 + 5% compost (B1C and B2.5C), B1 and B2.5 + 1% iron grit (B1Z and B2.5Z); uncontaminated soil (Ctrl); Ctrl soil amended with 1 or 2.5% biochar (CtrlB1, CtrlB2.5). After a 3-month reaction period, the soil pore water (SPW) was sampled in potted soils and dwarf beans were grown for a 2-week period. The SPW Cd, Pb, and Zn concentrations decreased in all amended-contaminated soils. The biochar effects increased with its addition rate and its combination with either compost or iron grit. Shoot Cd and Zn removals by beans were reduced and shoot Cd, Pb, and Zn concentrations decreased to common values in all amended soils except the B1 soil. Decreases in the SPW Cd/Pb/Zn concentrations did not improve the root and shoot yields of plants as compared to the Ctrl soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Several hundred years of smelting and processing of mining ores have caused widespread pollution of field areas around the industrial site of Arnoldstein in Carinthia, Austria (Asami 1988), where the Zn/Cd/Ge smelter closed in 1992. The surrounding soils used for housing (playgrounds), horticulture, forestry, and alpine grassland agriculture with pastures and feed production are contaminated by Pb, Cd, and Zn and, to a lesser extent, Cu and As (Friesl et al. 2006; Friesl et al. 2009). Such soil contamination by trace elements (TE) generated detrimental effects on the ecosystems with TE transfer from the soil to the environment. Although Cu and Zn concentrations were under homeostatic control, high Pb and Cd concentrations were measured in arthropods species (Rabitsch 1995). High Pb concentration occurred in blood and teeth of inhabitants living nearby the smelter (Kasperowski 1993). Metal concentrations (mg kg−1) were high in Zea mays L. shoot, i.e., Pb 54, Zn 286, and Cd 2.73 (Friesl et al. 2006). Based on the Austrian Federal Environmental Agency, the restoration of such contaminated soils was needed (Kasperowski 1993; Rabitsch 1995).
To phytomanage agricultural TE-contaminated soils, in situ stabilization of the labile TE pools in excess in the soil combined with the use of TE excluder cultivars of high yielding crops is one of the gentle remediation options (GROs; Kidd et al. 2015). It relies on soil amendments to (1) improve substrate bio-physicochemical properties (e.g., organic matter and nutrient contents), (2) immobilize TE in the solid phases preventing water-soluble TE migration from the root zone, (3) limit exposure to contaminants and detrimental effects on living organisms, (4) promote safe crops and other vegetation covers, and (5) stimulate ecological restoration of soil processes and functions in line with ecosystem services (Mench et al. 2010; Bolan et al. 2014). Soil amendments can lead to immobilize Pb, Cd, and Zn in the solid phase by one or more of the following processes, i.e., sorption, precipitation, complexation, ion exchange, and redox process, thereby decreasing their mobility and bioavailability (Kumpiene et al. 2008). Input of conventional organic matter (OM) such as compost, paper mill waste and sewage sludges can (1) form immobilized complexes between organic ligands and metals, (2) improve soil texture and structure, (3) increase nutrient status and water retention, and (4) change soil pH. Inorganic soil amendments such as clays, liming materials, phosphate minerals, and Fe-, Mn-, and Al oxides are effective for immobilizing Cd, Pb, and Zn (Kumpiene et al. 2008). Newly Fe/Mn (hydr)oxides formed after iron grit (Fe(0)) corroded in the soil can reduce the available fraction of metal(loid)s and lower the risks associated with their leaching and ecotoxicity (Komarek et al. 2013; Tiberg et al. 2016).
Several GROs have been tested in the Pb/Zn-contaminated soils of Arnoldstein. Apatite and a commercial mixture of dolomite, diatomite, smectite basaltic tuff, bentonite, alginate, and zeolite (Slovakite) efficiently stabilized and decreased the bioavailability of Pb, Zn, Cu, and Cd (Tica et al. 2011). This amendment also improved microbial activities and the functional status of the contaminated soil. In a field trial, gravel sludge and red mud were effective after 5 years for immobilizing Cd, Pb, and Zn (1 M NH4NO3-extractable fractions reduced up to 99%) and to limit contaminant uptake by barley (Hordeum vulgare. L., spp. distichon; Friesl et al. 2009). Gravel sludge and siderite bearing material reduced extractable (NH4NO3) Zn and Pb concentrations and maize uptake (Touceda-González et al. 2015).
Biochar amendment is another option for in situ stabilization of TE-contaminated soils (Beesley and Marmiroli 2011; Beesley et al. 2011; Oustriere et al. 2016a). Biochar is the solid product derived from waste biomass pyrolysis, under mid to low oxygen supply and high temperatures (Lehmann 2007; Ahmad et al. 2014). Amending soils with biochar has gained attention as: (1) it replenishes C stocks and improves long-term C sequestration in soil (Sohi et al. 2010; Atkinson et al. 2010; Kookana et al. 2011); (2) it can increase soil fertility, plant growth and root proliferation by improving soil structure, porosity and physicochemical properties, nutrient and water availability, and microbial communities able to degrade xenobiotics (Rizwan et al. 2016); and (3) it can reduce leaching and phytoavailability of TE in contaminated soils (Park et al. 2011). However, all these potential gains depend on the biochar quality (Oustriere et al. 2016a).
Several experiments explained the decrease in Cd, Pb, and Zn mobility and phytoavailability in biochar-amended soils by increase in soil pH and CEC, adsorption of metal-complexing DOM, and electrostatic interactions between the positively charged metal ions and negative charges associated with delocalized π-electrons on aromatic structures of biochar (Beesley and Marmiroli 2011; Uchimiya et al. 2010a; Karami et al. 2011): e.g., sugarcane biochar, Cd/Pb/Zn, Mucuna aterrima (Piper & Tracy) Holland, Zn-contaminated mine soil (Puga et al. 2015a); bamboo and rice straw biochars, Cd/Cu/Pb/Zn, Sedum plumbizincicola L. (Lu et al. 2014). Biochar amendment reduced the extractability and bioavailability of Cd, Zn, and Pb in a soil contaminated by atmospheric depositions (Houben et al. 2013a, b), but root-induced acidification of the rhizosphere counteracted the liming effect of biochar and, in turn, suppressed short-term metal immobilization (Houben and Sonnet 2015). Miscanthus-derived biochar increased soil pH of a contaminated sewage field and reduced Zn and Cd concentrations in the soil solution whereas those of Pb and Cu increased due to soluble complexes with dissolved organic matter (DOM) (Wagner and Kaupenjohann 2015). In the Arnoldstein soil, addition of poplar-derived biochar decreased the (NH4NO3) extractable fraction of Pb, Zn, and Cd but metal concentrations in the shoots of Lolium multiflorum Lam. 1779 did not decrease (Karer et al. 2015).
According to Beesley and Marmiroli (2011), the combination of compost and iron oxides with biochar may be more suitable than biochar alone to promote TE immobilization and buffer nutrient depletion in contaminated soils. Compost combined with biochar may improve total soil C, N, and P; stabilize soil aggregates; and stimulate microorganisms (Beesley et al. 2010; Sizmur et al. 2011; Schulz et al. 2013; Rodríguez-Vila et al. 2015). Depending on ligand types, pH, surface properties of the oxides and ligand/metal(loid) ratio, Fe/Mn/Al (hydr)oxides can promote metal(loid) adsorption (Komarek et al. 2013; Tiberg et al. 2016). Biochar combined with iron grit could reduce the water-soluble soil fraction of metals and thus the pollutant linkages associated with their leaching and ecotoxicity (Wagner and Kaupenjohann 2015). The potential gains of adding biochar with either compost or iron grit as compared to biochar alone in metal-contaminated soils remain poorly documented. This pot experiment aimed at assessing the efficiency of a biochar derived from pine bark chips, alone and in combination with either compost or iron grit, to stabilize Cd, Pb, and Zn in an agricultural, contaminated soil from the Arnoldstein area. Metals in the soil pore water and soil phytotoxicity on dwarf beans (Phaseolus vulgaris L.), reported as root and shoot dry weight (DW) yields and Pb, Cd, and Zn uptake by plants, were determined.
Material and methods
Soils and amendments
The soil was sampled nearby (300 m) the former metal smelter located at Arnoldstein, Carinthia, Austria (latitude 46° 33′ 13.74″; longitude 13° 41′ 23.70″, Table 1). This smelting activity goes back to 500 years, and the site has continuously experienced Cd, Zn, and Pb (and in a lesser extent Cu and As) atmospheric depositions. Topsoil (Unt, 0–15 cm; Leptosol, 100 kg) was collected from grassland at the subsite ARN-D (Touceda-González et al. 2015), air-dried for 2 weeks at ambient temperature, sieved to 5 mm, and manually homogenized. Its texture is sandy, i.e., 43% sand, 22% clay, and 35% silt, with 3.5% organic C and slightly acidic pH (i.e., 6; Table 1). Based on guideline values for agricultural production (Austrian Standard S 2088-2), it is mainly contaminated by Pb, Zn, and Cd, and in a lesser extent by Cu and As (Table 1). An uncontaminated sandy topsoil (Ctrl, pH 7.9, 0–20 cm) was collected in a kitchen garden, Gradignan, Gironde, France. The biochar (B) was a commercial product (Florentaise, Saint-Mars-du-Désert, France; pyrolysis 180 s at 420 °C) derived from pine bark chips. It was crushed, sieved to 2 mm and manually homogenized. In our experiments, soluble salts were not removed from the biochar. Commercial grade compost (C), made of green wastes for 9 to 12 months, was obtained from Gonzales frères, Martignas sur Jalle, France. Zerovalent iron grit (Z, GH120, particle size <0.1 mm) was obtained from Wheelabrator Allevard, France (Bes and Mench 2008). Amended soils were thoroughly homogenized in large plastic containers and individually prepared prior to use. Elemental composition, carbon content and PAH concentrations of biochar and compost were determined at the INRA Laboratoire d’Analyses des Sols (LAS, Arras, France) with standard methods (INRA LAS, 2011) (Table 2).
Experimental setup
The contaminated soil was mixed by rotation in a plastic flask with 1 or 2.5% (w/w) of biochar, alone and in combination with either compost or iron grit, based on Bes and Mench (2008) and Oustriere et al. (2016a). The uncontaminated soil was similarly mixed only with 1 or 2.5% (w/w) of biochar. Therefore, the pot experiment included ten treatments:
-
1.
Untreated contaminated soil (Unt)
-
2.
Unt soil + 1% B (B1)
-
3.
Unt soil + 1% B + 5% C (B1C)
-
4.
Unt soil + 1% B + 1% Z (B1Z)
-
5.
Unt soil + 2.5% B (B2.5)
-
6.
Unt soil + 2.5% B + 5% C (B2.5C)
-
7.
Unt soil + 2.5% B + 1% Z (B2.5Z)
-
8.
Uncontaminated soil (Ctrl)
-
9.
Ctrl soil + 1% B (CtrlB1)
-
10.
Ctrl soil + 2.5% B (CtrlB2.5)
Untreated and amended soils were potted in plastic pots (1 kg, 11 × 11 × 11 cm3, 1.3 L, in triplicates) and randomly placed in a greenhouse under controlled conditions. One Rhizon MOM moister samplers (Eijkelkamp Agrisearch Equipment, The Netherlands) was inserted with a 45° angle into each potted soil. Before sowing, amendments were allowed to react for a 3-month period with the soils, pots being manually maintained five times a week at 65% of the water holding capacity (WHC) with deionized water.
The plant test was adapted from Ruttens et al. (2006) (modified protocol from ISO 11269-2, ISO 2012). In June, four seeds of dwarf beans (P. vulgaris L. cv. Skipper, Vilmorin) were sown in all pots. Plants were cultivated during 15 days with controlled conditions (16/8-h light/darkness; 65 ± 5% relative humidity; 25 ± 2 °C) in the greenhouse. Potted soils were daily watered (deionized water) to maintain a 65% WHC rate. After 2 weeks, the shoots and roots were harvested, washed twice with deionized water, blotted with filter paper, placed in paper bags and oven dried at 60 °C to constant weight for 72 h, and then weighed for determining the shoot and root DW yields.
Plant and soil pore water analysis
For each pot, dried shoots of the four plants were pooled, ground (<1-mm particle size, Retsch MM200) then weighed aliquots (0.5 g DW) were wet digested under microwaves (CEM Marsxpress 1200 W) with 5 mL supra-pure 14 M HNO3, 2 mL 30% (v/v) H2O2 not stabilized by phosphates and 1 mL Milli-Q water. Certified reference material (BIPEA maize V463) and blank reagents were included in all series. Mineral composition (Al, As, B, Cd, Ca, Cr, Co, Cu, Fe, Mg, Mn, Mo, Ni, P, Pb, K, Na, and Zn) in digests was determined by ICP-MS (Thermo X series 200, INRA USRAVE laboratory, Villenave d’Ornon, France). All elements were recovered (>95%) according to the standard values and standard deviation for replicates was <5%. All element concentrations in plant parts are expressed in mg or g DW kg−1. The shoot metal (Me) removal was calculated as follows, using the mean shoot value of the four plants: Me (μg plant−1) = shoot DW yield (g plant−1) × shoot Me concentration (μg g−1 DW).
The soil pore water (SPW) was collected after plant harvest (day 14) in all potted soils (two times 10 mL with a 3-day interval) using the Rhizon samplers and samples kept at 4 °C prior to their analysis. The pH (Hanna instruments, pH 210, combined electrode Ag/AgCl – 34), redox potential (Eh) and electrical conductivity (EC) (Tetracon 325 WTW), and Cu2+ concentration (Cupric ion electrode, Fischer Bioblock, USA) of SPW samples were measured, and their element composition (same elements as for plant ionome) analyzed by ICP-MS (Thermo X series 200) or ICP-AES (Varian Liberty 200) at the INRA USRAVE laboratory, Villenave-d’Ornon, France.
Statistical analysis
Influence of soil treatments on SPW parameters, shoot DW yields, shoot ionome, and element removals of plants were tested using one-way analysis of variance (ANOVA). Normality and homoscedasticity of residuals were met for all tests. When significant differences occurred between treatments, multiple comparisons of mean values were made using post hoc Tukey HSD tests. Differences were considered statistically significant at p < 0.05. When element concentrations were below the detection limits in the UNT samples, influence of soil treatments were not statistically tested. All statistical analyses were performed using R software (version 3.0.3, Foundation for Statistical computing, Vienna, Austria).
Results and discussion
Soil pore water
EC, pH, and nutrient concentrations (Table 3)
Except for the B1 treatment, the SPW EC significantly decreased in all amended soils, with lowest values in the B2.5Z and B2.5 soils. These lower EC values likely arose from (co-)precipitation or sorption of divalent cations on biochar and compost surface, as suggested by the significant decrease of SPW Ca and Mg concentrations in the B2.5 and B2.5Z soils (i.e., 2- and 3-fold as compared to the Unt soil). In previous studies, decrease in leachate concentrations of divalent cations was attributed to the increased CEC of biochar-amended soils (Lehmann et al. 2003; Ding et al. 2010; Bakshi et al. 2014). In contrast, the SPW concentrations of K and Na increased with the biochar and compost loading rate. The SPW K concentrations were 3–4-fold higher in amended soils than in the Unt soil. The SPW Na concentration was slightly enhanced in all amended soils albeit not significantly. Gain in K+, Na+, and NH4 + concentrations following biochar addition to contaminated sandy soils was previously reported (Bakshi et al. 2014). The SPW P concentration was below the detection limit (<0.2 mg L−1) in all treatments. The SPW pH increased significantly from 6.2 (Unt) to 6.9 (B2.5C) in the increasing order: Unt < B1 = B1Z < B2.5Z = B2.5 ≤ B1C = B2.5C. Such increase was likely due to the biochar alkalinity (Table 2). Biochar combined with compost led to the highest SPW pH value, in line with Beesley et al. (2010, 2014).
As, B, and Mo concentrations (Table 3)
Increase in soil pH after biochar and compost addition was correlated with enhanced SPW As, B, and Mo concentrations in the B1C, B2.5, and B2.5C soils, i.e., from 0.8 (Unt) to 1.8 (B2.5C) μg As L−1, 42 (Unt) to 56 (B1C) μg B L−1, and <0.4 (Unt) to 1.3 (B2.5C) μg Mo L−1, these elements forming oxyanions. However, these concentrations remained low compared to their values in the Ctrl soil. In the Arnoldstein soil, increased As concentration in the labile pool correlated with increasing soil pH following red mud and triple superphosphate amendment (Friesl et al. 2004, 2006). Beesley et al. (2011) found also an enhanced As mobility with increasing soil pH after biochar amendment. Authors postulated that other oxyanions would behave similarly. Riedel et al. (2015) reported a higher release of U, W, and Mo oxyanions in soil leachate of biochar-amended soils with increasing pH. Biochar combined with Z, despite increased soil pH did not enhance the SPW As, B, and Mo concentrations. The As, B, and Mo oxyanions have high affinity for Fe (hydr)oxides and may have been sorbed by the newly formed Fe and Mn oxyhydroxides after iron grit corroded in the Z-amended soils (Kumpiene et al. 2008; Komárek et al. 2013).
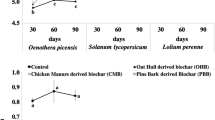
Cd, Pb, and Zn concentrations (Fig. 1A, C, E)
The SPW Cd, Pb, and Zn concentrations dropped in all amended soils, ranging (μg L−1) from 12 to 0.7 for Cd, 7.4 to <0.8 (dl) for Pb, and 600 to 7.6 for Zn in the Unt and B2.5Z soils, respectively. These decreases after biochar addition agreed with previous studies: incorporation of sugarcane straw-derived biochar decreased the DTPA-extractable concentrations of Cd, Pb, and Zn in a Zn mining soil, Vazante (Minas Gerais, Brazil, Puga et al. 2015a, b). A sewage sludge-derived biochar reduced the 0.1 M CaCl2-extractable Cu, Ni, Zn, Cd, and Pb concentrations in a sandy Mediterranean agricultural Cambisol (Méndez et al. 2012). Here, reductions of SPW Cd, Pb, and Zn concentrations were not significant for 1% biochar addition but decreases became significant for the C and Z combinations at both application rates of biochar. Decrease in 0.01 M CaCl2-extractable Cd, Zn, and Pb concentrations with increasing concentrations of Miscanthus-derived biochar (1, 5, and 10%) was reported for a metal-contaminated soil nearby Zn and Pb smelters (Houben et al. 2013 a, b). Regarding additional benefit of compost on metal sorption in biochar-amended soils, incorporation of hardwood-derived biochar combined with greenwaste compost into a multicontaminated soil decreased more Cd, Pb, and Zn concentrations in the SPW than biochar alone (Beesley et al. 2014). Compost combined with biochar reduced more the Cu, Ni, Pb, and Zn mobility in a contaminated mine soil than biochar alone (Rodríguez-Vila et al. 2015).
(a, b) Cd, (c, d) Pb, and (e, f) Zn concentrations (μg L−1) in the soil pore water (a, c, e) and in the shoots of dwarf beans (mg kg−1) (b, d, f) after the 15-day growth period in the Arnoldstein-contaminated soil (Unt, black hatch), amended with 1 or 2.5% of biochar, alone (light gray), and in combination with either compost (dark gray) or iron grit (black). Dashed lines indicate the SPW element concentration in the control soil. Mean values per treatment (n = 3; n = 6 for Unt). Values with different letters differ significantly (one-way ANOVA, p value <0.05)
Interactions between biochar-amended soils and metals are complex, and the possible mechanisms are as follows: (1) electrostatic interactions with negatively charged surfaces on soil particles activated by the pH increase, (2) specific metal-ligand complexation involving surface functional groups of biochars (in particular O, P, S, and N functional groups) that may or not involve cation exchange, and (3) sorptive interactions between cations and aromatic π electronic systems from C=C bounds of biochars (Uchimiya et al. 2010b; Zhang et al. 2013).
Despite high total soil Pb (Table 1), the SPW Pb concentrations remained low (i.e., 7.4 μg L−1, Unt) in all treatments (Fig. 1C), below the Ctrl value (i.e., 7.6 μg L−1) and in the low concentration range reported in the literature (Table 3). Above pH 6, Pb may form hydroxide and oxide precipitates, e.g., Pb3(OH)4 2+, Pb6O(OH)6 4+, and PbO, controlling soil Pb solubility (Hale et al. 2012). Chelation by organic matter (OM), sorption on Fe, Al, and Mn (hydr)oxides or precipitation of metal hydroxides can immobilize Pb in the soil (Bolan et al. 2014). For this Arnoldstein soil, Friesl et al. (2006, 2009) reported increased soil pH can promote Pb retention in the soil solid phase thus decreasing NH4NO3-extractable Pb. Here, the SPW Pb concentration decreased as SPW pH raised up to 6.7 (Fig. 2B) and with the biochar addition rate (Fig. 1C). A part of water-soluble Pb may precipitate as metal oxy(hydr)oxides or form soluble complexes with the dissolved organic matter (DOM) provided by the biochar and compost addition into the Unt soil (Beesley et al. 2014) as Pb can strongly associate with oxygen-containing functional groups of DOM (Kargar et al. 2015; Wagner and Kaupenjohann 2015). Such Pb oxy(hydr)oxides and Pb-DOM complexes can be retained on biochar surface (Zhang et al. 2013). The SPW Pb concentration slightly increased in the B2.5C treatments (i.e., 2.6 μg Pb L−1) compared to the B1C treatments (i.e., <0.8 μg Pb L−1, Fig. 1C). Formation of DOM increases when pH is higher than 5.5 (Bravin et al. 2012). Due to increase in SPW pH and high OM input by biochar (2.5%) and compost (5%), organic complexes might increasingly dissolve which may lead to a competition between DOM and Pb hydroxides or DOM-Pb complexes for retention on biochar surfaces (Beesley et al. 2014). The DOM may cloak the biochar pores preventing the sorption of elements (Bolan et al. 2010; Cao et al. 2011). This effect may depend on the soil ability to retain DOM, which is important to preserve the long term efficiency of amendments and avoid Pb leaching out of the root zone. The lower Pb mobility in soils amended by biochar (2.5%) plus iron grit compared to biochar (2.5%), alone or with compost, may be attributed to the sorption of inorganic Pb- or DOM-Pb complexes by newly formed Fe and Mn oxyhydroxides (Kumpiene et al. 2008). A strong Pb sorption onto ferrihydrite has been reported in the Arnoldstein soil (Friesl et al. 2006).
Relationships between (a) Cd, (b) Pb, and (c) Zn concentrations (μg L−1) in the soil pore water of the Arnoldstein-contaminated soil (Unt, black dots), amended with 1% (stars) or 2.5% (squares) of biochar, alone (light gray), and in combination with either compost (dark gray) or iron grit (black) and pH in the soil pore water. The correlation coefficient was donated by the R software
High SPW Cd and Zn concentrations (Fig. 1) reflected their total concentrations in the Unt soil (Table 1). Their values exceeded those of the Ctrl soil in all treatments except for the SPW Zn concentration in the B2.5Z soil. In the amended soils except B1, values were in the low range as compared to the literature (Table 3). Potential mechanisms for explaining decreased SPW Cd and Zn concentrations in the amended soils are as follows: surface complexation of Cd and Zn on biochar functional groups in line with increase in soil pH, coprecipitation, inner-sphere complexation of metals (Cd, Zn), and trace elements exchange with Ca2+ and Mg2+ (Chen et al. 2007; Sohi et al. 2010; Uchimiya et al. 2010b; Zhang et al. 2013; Mohamed et al. 2015). Surface complexation of Cd/Zn through –OH groups or delocalized π electrons of biochars was considered as a minor contribution (<25%; Xu et al. 2013). The liming effect of biochar and compost addition was suggested (R 2 0.79 and 0.78, respectively; Fig. 2A, C), confirming Beesley et al. (2010). In the Arnoldstein soil, decreases in water-soluble and/or exchangeable Cd/Zn fractions were partly attributed to pH increase after addition of Slovakite and apatite (Tica et al. 2011), synthetic zeolite and ferrihydrite-bearing amendments (Friesl et al. 2006), poplar derived-biochar, gravel sludge with siderite-bearing material and lime (Karer et al. 2015). Increase in SPW pH may result in Cd and Zn hydrolysis species (CdOH+ and ZnOH+), which may precipitate as hydroxides (Cd (OH)2) and (Zn (OH)2) (Melo et al. 2016). Here, such mechanisms may occur to a limited extent as the soil pH was below 7 and such species are mainly formed at alkaline pH (Uchimiya et al. 2010b). Friesl et al. (2006) found a Cd and Pb immobilization in the Arnoldstein soil after gravel sludge with ferrihydrite addition due to their chemisorption onto the Fe oxides. Accordingly, additional decrease of SPW Cd, Pb, and Zn concentrations was expected in the B1Z and B2.5Z soils as compared to biochar alone to mirror the potential metal sorption on Fe/Mn oxyhydroxides (Kumpiene et al. 2011; Komarek et al. 2013), but this was only validated for Cd in B1Z and Pb in B2.5Z (Fig. 1A, E).
Al, Cr, Co, Cu, Fe, Mn, and Ni concentrations (Table 3)
The SPW concentrations (μg L−1) decreased from 68 (Unt) to <20 in all amended soils for Mn and from 4 (Unt) to 1 (B2.5 and B2.5C) for Ni, in line with increased in SPW pH. In all soils, SPW Al, Cr, Co, Cu, and Fe concentrations were below their detection limit of <0.05, <0.2, <0.2, <8, and <20 μg L−1, respectively. All concentrations of these elements were similar or below the values in the Ctrl soil.
Plants
Plant growth parameters (Fig. 3)
Root and shoot DW yields of the Unt plants were lower as compared to the Ctrl plants albeit not significantly. The amendments did not significantly influence the plant yield, despite they reduced bean exposure to Cd and Zn (Fig. 1). Root DW yield decreased in the biochar-amended Ctrl soil albeit not significantly. The stimulation lack on biomass production after amendment of Cd-, Pb-, and Zn-contaminated soils was previously reported regardless the type of plants, biochar and application rate: straw-derived biochar (1.5–5% w/w) did not increase the shoot DW yield of M. aterrima in a Cd/Pb/Zn-contaminated mining soil (Puga et al. 2015a,b); wood biochar (0.5–1.5%) did not influence the DW yield of maize plants, in a (As, Cd, Cu, Pb, and Zn)-spiked soil (Namgay et al. 2010); wheat chaff or oil mallee plant-derived biochar (0.5 and 5%) did not improve the growth of emergent wetland species Juncus subsecundus N.A. Wakef. in a Cd-contaminated soil (Zhang et al. 2013b). Miscanthus-derived biochar amendment (1, 5, and 10%) did not promote DW yield of Italian ryegrass (L. multiflorum) grown in a soil contaminated nearby Zn and Pb smelters (Houben et al. 2013a, b).
(a) Root and (b) shoot DW yields of dwarf bean (mg DW plant−1) after the 15-day growth period in the control soil (white, Ctrl), amended with 1% (CtrlB1) or 2.5% (CtrlB2.5) of biochar, and in the Arnoldstein-contaminated soil (Unt, black hatch), amended with 1 or 2.5% of biochar, alone (light gray), and in combination with either compost (dark gray) or iron grit (black). Mean values per treatment (n = 3; n = 6 for Unt). Values with different letters differ significantly (one-way ANOVA, p value < 0.05)
Plant nutrients (Table 4)
The SPW K and Na concentrations increased in all biochar-amended soils (Table 3). Consequently, shoot Na concentrations significantly increased for the B1C and B2.5C plants, while shoot K concentration significantly increased in all amended soils, being in the upper range of common values in plants (i.e., 20–50 g K kg−1 DW). Such relationship was less evident for Ca, Mg, and P. The SPW Ca and Mg concentrations decreased in the biochar-amended soils, especially in the B2.5 and B2.5Z treatments (Table 3). Consequently, shoot Ca concentrations significantly decreased for the B1Z, B2.5, and B2.5Z plants and shoot Mg concentration significantly fell in plants from all amended soils, but all values remained in the common ranges for shoots (i.e., 1–50 g Ca and 1.5–3.5 g Mg kg−1 DW). Antagonistic and synergistic effects may alter plant ionome, as well as DW yield and development stage (Wagner and Kaupenjohann 2014). Increase of SPW and shoot K concentrations due to amendment addition (Tables 3 and 4) may contribute to decrease shoot Ca and Mg concentrations (Jakobsen 1993). The shoot P concentration did not differ across treatments, and its values remained in the common range for shoots (i.e., 1.6–6.0 g P kg−1 DW). Combination of compost or iron grit with biochar did not have an additional effect on shoot nutrient concentrations as compared to biochar alone.
Shoot As, B, and Mo concentrations (Table 4)
The SPW As, B, and Mo concentrations increased in the B1C, B2.5, and B2.5C treatments (Table 3). Changes in shoot As, B, and Mo concentrations across treatments however were mostly insignificant. Only shoot Mo concentration in the B2.5C plants was significantly higher than in the Unt plants, exceeding the common range for shoots but remaining below Mo concentrations in stressed plants (Tremel-Schaub and Feix 2005).
Shoot Pb concentrations and removals (Fig. 1D, Tables 4 and 5)
Shoot Pb concentration did not significantly change after soil amendment except a decrease for the B2.5Z plants (Fig. 1D). Shoot and SPW Pb concentrations were correlated in our soil series (Fig. 1C). For all plants, the shoot Pb concentrations were relatively close to or in their common range (Table 4, Tremel-Schaub and Feix 2005). In the B2.5 soil, the shoot Pb concentration is higher, albeit not significantly, than in the Unt soil, this trend reflecting increase in SPW Pb concentration (Fig. 1C). The shoot Pb removal (μg Pb plant−1) was only significantly lower in the B1C (i.e., 0.4) and B2.5Z (i.e., 0.3) treatments as compared to the Unt plants (i.e., 0.9).
Shoot Cd and Zn concentrations and removals (Fig. 1B, F, Tables 4 and 5)
All treatments significantly decreased the shoot Cd and Zn concentrations (mg kg−1) by up to 83 and 66%, (i.e., Cd: from 3.7 ± 0.08 to 0.6 ± 0.5; Zn: from 245 ± 9 to 80 ± 43). Shoot Cd concentrations varied (mg kg−1) between 3.5 ± 0.3 (Unt) and 0.6 ± 0.5 (B2.5Z) in the decreasing order: Unt > B1 > B1Z > B2.5 = B1C ≥ B2.5C = B2.5Z, this reduction being enhanced by the biochar addition rate and the combination with C or Z. For the Unt, B1, BIZ, and B2.5 plants, shoot Cd concentration exceeded its common values (Table 4; Tremel-Schaub and Feix 2005) and the maximum permitted concentration (MPC) in forage (0.5–1 mg kg−1; Tremel-Schaub and Feix 2005). For the B1C, B2.5C, and B2.5Z plants, shoot Cd concentration was in its common range. Shoot Cd removal peaked for the Unt plants (i.e., 0.5 μg Cd plant−1), being 2- and 6-fold higher as compared to the B1 and B1C plants, respectively (Table 5). Shoot Zn concentration (mg kg−1) significantly dropped for all amended soils from 238 ± 20 (Unt) to 80 ± 43 (B2.5Z), in the decreasing order: Unt > B1 = B1Z ≥ B2.5 ≥ B2.5C = B1C = B2.5Z. Except for the B1 plants, all values were in the common range (10–150 mg kg−1; Tremel-Schaub and Feix 2005). Shoot Zn removal peaked for the Unt plants (i.e., 34 μg Zn plant−1) and significantly decreased in all amended soils except for the B1 plants (Table 5).
Decreased in SPW and shoot Cd/Zn concentrations were correlated (R 2: 0.81 and 0.71, respectively; Supplemental material 1) (Fig. 1A, E). This confirmed previous reports on biochar-amended soils and amendment testing in the Arnoldstein soil (Friesl et al. 2006; Bolan et al. 2003; Beesley et al. 2010; Zhang et al. 2013; Kargar et al. 2015). Miscanthus-derived biochar (at 10%) reduced the shoot Cd, Zn, and Pb concentrations of Brassica napus L. by −71, −87, and −92% (Houben et al. 2013a). Bamboo and rice straw-derived biochar (1 and 5%) decreased shoot Cd, Cu, Pb, and Zn concentrations of S. plumbizincicola in a Cd, Cu, Pb, and Zn-contaminated soil nearby a Cu smelter (Lu et al. 2014). Straw-derived biochar (1.5, 3.0, and 5.0%) added to a Cd-, Pb-, and Zn-contaminated mining site decreased the shoot Cd, Pb, and Zn concentrations of M. aterrima by −56, −50, and −54%, respectively (Puga et al. 2015a, b). Authors mentioned that decreased shoot Cd and Zn concentrations may reflect metal immobilization through sorption by biochar amendment. Here, as decrease in SPW metal concentrations globally did not significantly influence the root and shoot DW yields (Fig. 3), a potential “dilution effect” in the shoot biomass would be insignificant (Park et al. 2013).
Practical implications
Except for 1% biochar alone, all treatments reduced the SPW Cd, Pb, and Zn concentrations to reach their low value ranges as compared to the literature. Similarly, shoot Cd, Pb, and Zn concentrations decreased in all treatments, except for 1% B. Biochar addition rate of 2.5% or a combination with either compost or iron grit was necessary to stabilize such metals in this Arnoldstein soil. Likely due to the Pb affinity for DOM, the SPW Pb concentration increased in the B2.5 and B2.5C soils; accordingly shoot Pb concentration increased in the B2.5C plants as compared to the B1C. Only the B1C, B2.5C, and B2.5Z plants had shoot metal concentration in the common ranges for Cd, Pb, and Zn. Influence of these three treatments to stabilize these metals in this Arnoldstein soil must be long term investigated, the biochar combination with compost being less costly albeit its lasting effect is questionable as compost OM would decay, and it slightly promoted the As concentration in the soil pore water (Table 3). At short-term, the biomass production of dwarf bean in the uncontaminated Ctrl and Unt soils was statistically similar, albeit influence on soil biota was not determined. No tested amendment improved the root and shoot DW yield as compared to the Ctrl soil, but N fertilization may be necessary in the biochar-amended soils. After this option appraisal, plots for testing the best combination can be implemented and compare with gravel sludge, siderite, and red muds, notably with non-food crops useable for the bioeconomy.
Conclusion
Pine bark chip-derived biochar combined with either compost or iron grit efficiently stabilized the labile Cd, Pb, and Zn pools in the Arnoldstein soil contaminated by atmospheric depositions. This lowered the potential metal leaching out of the root zone to proximal waters and dispersion through the environment. Decrease in Cd/Pb/Zn mobility was improved by the biochar loading rate and the combination with either compost or iron grit. This positive effect was mainly attributed to increase in soil pH, (co-)precipitation and various sorption mechanisms with the biochar surface. Consequently, dwarf bean exposure to Cd and Zn was reduced and shoot Cd, Pb, and Zn concentrations were in the common ranges for plants grown in all amended soils except for 1% biochar alone. However, decrease in Cd, Zn, and Pb concentrations in the soil pore water did not improve the root and shoot DW yields of dwarf bean.
References
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS (2014) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99:19–33. doi:10.1016/j.chemosphere. 2013.10.071
Asami T (1988) Soil pollution by metals from mining and smelting activities. In: Salomon W, Forstner U (eds) Chemistry and biology of solid waste: dredged material and mine tailings. Springer-Verlag, Berlin, pp 144–169
Atkinson CJ, Fitzgerald JD, Hipps NA (2010) Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 337:1–18. doi:10.1007/s11104-010-0464-5
Baize D (1997) Un Point sur les Teneurs Totales en Eléments Traces Métalliques dans les Sols, First edn. INRA Éditions, Paris, France
Baize D (2000) Guide des analyses en pédologie, Second edn. INRA Éditions, Paris, France, 257 p
Bakshi S, He ZL, Harris WG (2014) Biochar amendment affects leaching potential of copper and nutrient release behavior in contaminated sandy soils. J Environ Qual 43:1894–1902. doi:10.2134/jeq2014.05.0213
Beesley L, Marmiroli M (2011) The immobilisation and retention of soluble arsenic, cadmium and zinc by biochar. Environ Pollut 159:474–480. doi:10.1016/j.envpol. 2010.10.016
Beesley L, Inneh OS, Norton GJ, Moreno-Jimenez E, Pardo T, Clemente R, Dawson JJC (2014) Assessing the influence of compost and biochar amendments on the mobility and toxicity of metals and arsenic in a naturally contaminated mine soil. Environ Pollut 186:195–202. doi:10.1016/j.envpol. 2013.11.026
Beesley L, Moreno-Jimenez E, Gomez-Eyles JL (2010) Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ Pollut 158:2282–2287. doi:10.1016/j.envpol. 2010.02.003
Beesley L, Moreno-Jiménez E, Gomez-Eyles JL, Harris E, Robinson B, Sizmur T (2011) A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ Pollut 159:3269–3282. doi:10.1016/j.envpol. 2011.07.023
Bes C, Mench M (2008) Remediation of copper-contaminated topsoils from a wood treatment facility using in situ stabilisation. Environ Pollut 156:1128–1138. doi:10.1016/j.envpol. 2008.04.006
Bolan NS, Adriano DC, Mani S, Khan AR (2003) Adsorption, complexation and phytoavailability of copper as influenced by organic manure. Environ Toxicol Chem 22:450–456. doi:10.1002/etc. 5620220228
Bolan NS, Adriano D, Senesi N, Kunhikrishnan A, James T, McDowell R (2010) Dissolved organic carbon: biogeochemistry, dynamics and agro-environmental significance in soils. Adv Agron 110:1–67
Bolan NS, Kunhikrishnan A, Thangarajana R, Kumpiene J, Parke J, Makino T, Kirkham MB, Scheckel K (2014) Remediation of heavy metal(loid)s contaminated soils—to mobilize or to immobilize? J Hazard Mater 266:141–166. doi:10.1016/j.jhazmat. 2013.12.018
Bravin MN, Garnier C, Lenoble V, Gérard F, Dudal Y, Hinsinger P (2012) Root-induced changes in pH and dissolved organic matter binding capacity affect copper dynamic speciation in the rhizosphere. Geochim Cosmochim Acta 84:256–268
Cao X, Ma L, Liang Y, Gao B, Harris W (2011) Simultaneous immobilization of lead and atrazine in contaminated soils using dairy-manure biochar. Environ Sci Technol 45:4884–4889. doi:10.1021/es103752u
Chen J, Zhu D, Sun C (2007) Effect of heavy metals on the sorption of hydrophobic organic compounds to wood charcoal. Environ Sci Technol 41:2536–3541. doi:10.1021/es062113+
Di Bonito M (2005) Trace elements in soil pore water: a comparison of sampling methods. University of Nottingham, United Kingdom
Ding Y, Liu YX, Wu WX, Shi DZ, Yang M, Zhong ZK (2010) Evaluation of biochar effects on nitrogen retention and leaching in multilayered soil columns. Water Air Soil Pollut 213:47–55. doi:10.1007/s11270-010-0366-4
Friesl W, Friedl J, Platzer K, Horak O, Gerzabek MH (2006) Remediation of contaminated soils in the vicinity of a former Pb/Zn smelter in Austria: batch, pot, and field experiments. Environ Pollut 1441:40–50. doi:10.1016/j.envpol. 2006.01.012
Friesl W, Horak O, Wenzel WW (2004) Immobilization of heavy metals in soils by the application of bauxite residues: pot experiments under field conditions. J Plant Nutr Soil Sci 167:54–59
Friesl W, Platzer K, Horak O, Gerzabek MH (2009) Immobilising of Cd, Pb, and Zn contaminated arable soils close to a former Pb/Zn smelter: a field study in Austria over 5 years. Environ Geochem Health 31:581–594. doi:10.1007/s10653-009-9256-3
Hale B, Evans L, Lambert R (2012) Effects of cement or lime on Cd, Co, Cu, Ni, Pb, Sb and Zn mobility in field-contaminated and aged soils. J Hazard Mater 199–200:119–127. doi:10.1016/j.jhazmat. 2011.10.065
Houben D, Sonnet P (2015) Impact of biochar and root-induced changes on metal dynamics in the rhizosphere of Agrostis capillaris and Lupinus albus. Chemosphere 139:644–651. doi:10.1016/j.chemosphere. 2014.12.036
Houben D, Evrard L, Sonnet P (2013a) Mobility, bioavailability and pH-dependent leaching of cadmium, zinc and lead in a contaminated soil amended with biochar. Chemosphere 92:1450–1457. doi:10.1016/j.chemosphere. 2013.03.055
Houben D, Evrard L, Sonnet P (2013b) Beneficial effects of biochar application to contaminated soils on the bioavailability of Cd, Pb and Zn and the biomass production of rapeseed Brassica napus L. Biomass Bioenerg 57:196–204. doi:10.1016/j.biombioe. 2013.07.019
INDIQUASOL (2016) Indicator of soil quality data base. INRA, Orleans. Available online at http://www.acklins.orleans.inra.fr/geoindiquasol/index. php
Jakobsen ST (1993) Interaction between plant nutrients III. Antagonism between potassium, magnesium and calcium. Acta Agric Scand Sect B 43:1–5. doi:10.1080/09064719309410223
Kabata-Pendias A, Pendias H (1984) Trace elements in soils and plants. CRC Press, Boca Raton, Florida
Karami N, Clemente R, Moreno-Jimenez E, Lepp NW, Beesley L (2011) Efficiency of green waste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass. J Hazard Mater 191:41–48. doi:10.1016/j.jhazmat. 2011.04.025
Karer J, Wawra A, Zehetner F, Dunst G, Wagner M, Pavel PB, Puschenreiter M, Friesl-Hanl W, Soja G (2015) Effects of biochars and compost mixtures and inorganic additives on immobilisation of heavy metals in contaminated soils. Water Air Soil Pollut 226:342. doi:10.1007/s11270-015-2584-2
Kargar M, Clark OG, Hendershot WH, Jutras P, Prasher SO (2015) Immobilization of trace metals in contaminated urban soil amended with compost and biochar. Water Air Soil Pollut 226:191. doi:10.1007/s11270-015-2450-2
Kasperowski E (1993) Schwermetalle in Böden im Raum Arnoldstein. Monographien Bd. 33, Umweltbundesamt UBA, Wien, Austria.
Kidd P, Mench M, Álvarez-Lopez V, Bert V, Dimitriou I, Friesl-Hanl W, Herzig R, Janssen JO, Kolbas A, Müller I, Neu S, Renella G, Ruttens A, Vangronsveld J, Puschenreiter M (2015) Agronomic practices for improving gentle remediation of trace element-contaminated soils. Int J Phytorem 17:1005–1037. doi:10.1080/15226514.2014.1003788
Komárek M, Vanek A, Ettler V (2013) Chemical stabilization of metals and arsenic in contaminated soils using oxides. A review. Environ Pollut 172:9–22. doi:10.1016/j.envpol. 2012.07.045
Kookana RS, Sarmah AK, Van Zwieten L, Krull E, Singh B (2011) Chapter three—biochar application to soil: agronomic and environmental benefits and unintended consequences. Adv Agron 112:103–143
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As. Cr, Cu, Pb and Zn in soil using amendments – A review Waste Manage 28:215–225. doi:10.1016/j.wasman. 2006.12.012
Kumpiene J, Mench M, Bes C, Fitts JP (2011) Assessment of aided phytostabilization of copper-contaminated soil by X-ray absorption spectroscopy and chemical extractions. Environ Pollut 159:1536–1542. doi:10.1016/j.envpol. 2011.03.005
Lehmann J (2007) A handful of carbon. Nature 447:143–144. doi:10.1038/447143a
Lehmann J, Silva JP, Steiner C, Nehls T, Zech W, Glaser B (2003) Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: fertilizer, manure and charcoal amendments. Plant Soil 249:343–357
Lu K, Yang X, Shen J, Robinson B, Huang H, Liu D, Bolan N, Pei J, Wang H (2014) Effect of bamboo and rice straw biochars on the bioavailability of Cd, Cu, Pb and Zn to Sedum plumbizincicola. Agric Ecosyst Environ 191:124–132. doi:10.1016/j.agee. 2014.04.010
Marchand L, Nsanganwimana F, Cook B, Vystavna Y, Huneau F, Lecoustumer P, Lamy JB, Oustrière N, Mench M (2014) Trace element transfer from soil to leaves of macrophytes along the Jalle d’Eysines River, France and their potential use as contamination biomonitors. Ecol Indic 46:425–437. doi:10.1016/j.ecolind. 2014.07.011
Melo LCA, Puga AP, Coscione AR, Beesley L, Abreu CA, Camargo OA (2016) Sorption and desorption of cadmium and zinc in two tropical soils amended with sugarcane-straw-derived biochar. J Soils Sediments 16:226–234. doi:10.1007/s11368-015-1199-y
Mench M, Lepp N, Bert V, Schwitzguébel JP, Gawronski SW, Schröder P, Vangronsveld J (2010) Successes and limitations of phytotechnologies at field scale: outcomes, assessment and outlook from COST Action 859. J Soils Sediments 16:876–900. doi:10.1007/s11368-010-0190-x
Méndez A, Gómez A, Paz-Ferreiro J, Gascó G (2012) Effects of sewage sludge biochar on plant metal availability after application to a Mediterranean soil. Chemosphere 89:1354–1359. doi:10.1016/j.chemosphere. 2012.05.092
Mohamed I, Zhang G, Li Z, Liu Y, Chen F, Dai K (2015) Ecological restoration of an acidic Cd contaminated soil using bamboo biochar application. Ecol Eng 84:67–76. doi:10.1016/j.ecoleng. 2015.07.009
Moreno-Jiménez E, Beesley L, Lepp NW, Dickinson NM, Hartley W, Clemente R (2011) Field sampling of soil pore water to evaluate trace element mobility and associated environmental risk. Environ Pollut 159:3078–3085. doi:10.1016/j.envpol. 2011.04.004
Namgay T, Singh B, Singh BP (2010) Influence of biochar application to soil on the availability of as, Cd, Cu, Pb, and Zn to maize Zea mays L. Aust J Soil Res 48:638–647. doi:10.1071/SR10049
Oustriere N, Marchand L, Bouchardon JL, Faure O, Moutte J, Mench M (2016b) Aided phytostabilization of a trace element-contaminated technosol developed on steel mill wastes. J Hazard Mater 320:458–468. doi:10.1016/j.jhazmat. 2016.08.048
Oustriere N, Marchand L, Galland W, Gabon L, Lottier N, Motelica M, Mench M (2016a) Influence of biochars, compost and iron grit, singly and in combination, on copper solubility and phytotoxicity in a Cu-contaminated soil from a wood preservation site. Sci Total Environ 566–567:816–825. doi:10.1016/j.scitotenv. 2016.05.091
Park JH, Choppala GK, Bolan NS, Chung JW, Chuasavathi T (2011) Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 348:439–451. doi:10.1007/s11104-011-0948-y
Park JH, Choppala G, Lee SJ, Bolan N, Chung JW, Edraki M (2013) Comparative sorption of Pb and Cd by biochars and its implication for metal immobilization in soils. Water Air Soil Pollut 224:1711. doi:10.1007/s11270-013-1711-1
Puga AP, Abreu CA, Melo LCA, Beesley L (2015a) Biochar application to a contaminated soil reduces the availability and plant uptake of zinc, lead and cadmium. J Environ Manag 159:86–93. doi:10.1016/j.jenvman. 2015.05.036
Puga AP, Abreu CA, Melo LCA, Paz-Ferreiro J, Beesley L (2015b) Cadmium, lead, and zinc mobility and plant uptake in a mine soil amended with sugarcane straw. Environ Sci Pollut Res 22:17606–17614. doi:10.1007/s11356-015-4977-6
Rabitsch WB (1995) Metal accumulation in arthropods near a lead/zinc smelter in Arnoldstein, Austria. I. Environ Pollut 90:221–237
Riedel T, Hennessy P, Iden SC, Koschinsky A (2015) Leaching of soil-derived major and trace elements in an arable topsoil after the addition of biochar. Eur J Soil Sci 66:823–834. doi:10.1111/ejss. 12256
Rizwan M, Ali S, Farooq Qayyum M, Ibrahim M, Zia-ur-Rehman M, Abbas T, Ok YS (2016) Mechanisms of biochar-mediated alleviation of toxicity of trace elements in plants: a critical review. Environ Sci Pollut Res 23:2230–2248. doi:10.1007/s11356-015-5697-7
Rodríguez-Vila A, Asensio V, Forján R, Covelo EF (2015) Chemical fractionation of Cu, Ni, Pb and Zn in a mine soil amended with compost and biochar and vegetated with Brassica juncea L. J Geochem Explor 158:74–81. doi:10.1016/j.gexplo. 2015.07.005
Ruttens A, Mench M, Colpaert JV, Boisson J, Carleer R, Vangronsveld J (2006) Phytostabilization of a metal contaminated sandy soil. I: influence of compost and/or inorganic metal immobilizing soil amendments on phytotoxicity and plant availability of metals. Environ Pollut 144:524–532. doi:10.1016/j.envpol. 2006.01.021
Schulz H, Dunst G, Glaser B (2013) Positive effects of composted biochar on plant growth and soil fertility. Agron Sust Dev 33:817–827. doi:10.1007/s13593-013-0150-0
Sizmur T, Wingate J, Hutchings T, Hodson ME (2011) Lumbricus terrestris L. does not impact on the remediation efficiency of compost and biochar amendments. Pedobiologia 54 Suppl, S211 S216. doi: 10.1016/j.pedobi. 2011.08.008
Sohi SP, Krull E, Lopez-Capel E, Bol R (2010) A review of biochar and its use and function in soil. Adv Agron 105:47–82. doi:10.1016/S0065-211310.05002-9
Tiberg C, Kumpiene J, Gustafsson JP, Marsz A, Persson I, Mench M, Kleja DB (2016) Immobilization of Cu and As in two contaminated soils with zerovalent iron—long-term performance and mechanisms. Appl Geochem 67:144–152. doi:10.1016/j.apgeochem. 2016.02.009
Tica D, Udovic M, Lestan D (2011) Immobilization of potentially toxic metals using different soil amendments. Chemosphere 85:577–583
Touceda-González M, Brader G, Antonielli L, Balakrishnan Ravindran V, Waldner G, Friesl-Hanl W, Corretto E, Campisano A, Pancher M, Sessitsch A (2015) Combined amendment of immobilizers and the plant growth-promoting strain Burkholderia phytofirmans PsJN favours plant growth and reduces heavy metal uptake. Soil Biol Biochem 91:140–150. doi:10.1016/j.soilbio. 2015.08.038
Tremel-Schaub A, Feix I (2005) Contamination des Sols - Transferts des Sols vers les Plantes, Agence de l’Environnement et de la Maîtrise de l’Énergie, Angers, and EDP Sciences, Les Ulis.
Uchimiya M, Lima IM, Klasson KT, Wartelle LH (2010a) Contaminant immobilization and release by biochar soil amendment: roles of natural organic matter. Chemosphere 80:935–940. doi:10.1016/j.chemosphere. 2010.05.020
Uchimiya M, Lima IM, Klasson KT, Chang S, Wartelle LH, Rodgers JE (2010b) Immobilization of heavy metal ions CuII, CdII, NiII, and PbII. By broiler litter-derived biochars in water and soil. J Agric Food Chem 58:5538–5544. doi:10.1021/jf9044217
Villanneau E, Perry-Giraud C, Saby N, Jolivet C, Marot F, Maton D, Floch-Barneaud A, Antoni V, Arrouays D (2008) Détection de valeurs anomaliques d’éléments traces métalliques dans les sols à l’aide du Réseau de Mesure de la Qualité des Sols. Etude et Gestion des Sols 15(3):183–200
Wagner A, Kaupenjhann M (2014) Suitability of biochars pyro- and hydrochars for metal immobilization on former sewage-field soils. Eur J Soil Sci 65:139–148. doi:10.1111/ejss. 12090
Wagner A, Kaupenjhann M (2015) Biochar addition enhanced growth of Dactylis glomerata L. and immobilized Zn and Cd but mobilized Cu and Pb on a former sewage field soil. Eur J Soil Sci 66:505–515. doi:10.1111/ejss. 12246
Xu X, Cai X, Zhao L, Wang H, Yu H, Gao B (2013) Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ Sci Pollut Res 201:358–368. doi:10.1007/s11356-012-0873-5
Zhang X, Wang H, He L, Lu K, Sarmah A, Li J, Bolan NS, Pei J, Huang H (2013) Using biochar for remediation of soils contaminated with heavy metals and organic pollutants. Environ Sci Pollut Res 20:8472–8483. doi:10.1007/s11356-013-1659-0
Acknowledgements
This work was supported by ADEME (French Agency for the Environment and Energy) (PhD grant of N. Oustrière n° 2013-ID5081), =the French National Research Agency (ANR C2ii, program PHYTOCHEM) and the ERA-Net FACCE SURPLUS (project INTENSE, n° ANR-15-SUSF-0007-06; http://faccesurplus.org/research-projects/intense/). The UMR Biogeco is a member of the INRA Ecotoxicologist network, ECOTOX.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hailong Wang
Electronic supplementary material
ESM 1
(DOCX 63 kb)
Rights and permissions
About this article
Cite this article
Oustriere, N., Marchand, L., Rosette, G. et al. Wood-derived-biochar combined with compost or iron grit for in situ stabilization of Cd, Pb, and Zn in a contaminated soil. Environ Sci Pollut Res 24, 7468–7481 (2017). https://doi.org/10.1007/s11356-017-8361-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8361-6