Abstract
World recent concerns about the shortage of water resources and contamination of groundwater supplies have motivated scientists seeking for more efficient techniques in irrigation and fertilization of farms while taking the advantage of models. The objective of this study is to address how water and nitrogen (N) dynamics are affected by efficient management strategies and to evaluate the application of HYDRUS-1D model in these conditions. In terms of using management policies, different irrigation strategies, planting methods, and different N fertilization rates applied on safflower (Carthamus tinctorius L.) in volumetric field lysimeters. The irrigation regimes were ordinary furrow irrigation (OFI) and variable alternate furrow irrigation (VAFI) as a partial root drying (PRD) technique. The planting methods were on-ridge planting (P1) and in-furrow planting (P2) methods. The fertilizer levels were 0 (N0), 100 (N1), and 200 (N2) kg ha−1 of urea as 0, 46, and 92 kg N ha−1. Results showed that VAFI regime and in-furrow planting method favorably reduced the amount of drainage water below safflower root zone in comparison with the ordinary methods. Furthermore, VAFI regime satisfactorily decreased the seasonal nitrate (NO3-N) leaching below the root zone, whereas differences between the leached NO3-N in in-furrow and on-ridge planting methods were not significant. Moreover, VAFI regime did not show any negative effects on total N uptake in safflower seed and straw, whereas in-furrow planting accumulated higher N in comparison with the on-ridge planting method. In addition, safflower nitrogen (N) uptake was responsive to application of nitrogen, although the rate of increase in N accumulation was not significant between the application rates of 46 and 92 kg N ha−1. The soil nitrate concentration decreased during the growing season indicating that safflower root system has a great ability in absorption of NO3-N from soil N supplies. HYDRUS-1D model favorably predicted the drainage water, nitrate concentration of drainage water, crop N uptake, and residual soil NO3-N concentration for safflower field. Therefore, it can be an applicable model for prediction of water and nitrogen dynamics, despite of two-dimensional flow conditions in furrow irrigation. It was concluded that VAFI strategy and in-furrow planting method are suitable alternatives helping farmers produce food while conserving water and preserving the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
World agricultural production has been challenged by water shortage and nitrogen (N) pollutions in the recent decades. Despite the limited eligible land for cultivation and scarce water resources, the growth of human population needs to bring more land and water resources for food production. Therefore, increasing the production capacity of the cultivated lands and enhancement of the water use efficiency are the necessary issues that may be achieved by application of fertilizers, efficient irrigation strategies, and planting methods (Shahrokhnia and Sepaskhah 2017a).
Nitrogen (N) is one of the essential nutrients to produce more crop and yield per unit area that is provided by using nitrogen fertilizers in farm. Urea is the main source of nitrogen fertilizers which is quite soluble in water and thus is very popular among farmers (Ravikumar et al. 2011). On the other hand, intensive fertilizer use and excessive use of N have become serious threats for contamination of various water resources in the last decade. The risk of groundwater contamination by N is related to the high solubility of urea in water. Urea immediately reacts after its dissolution in water and nitrified into ammonium (NH4-N) and subsequently into nitrate form (NO3-N). Hence, ammonium ions are adsorbed to the clay particles, and leaching of ammonium ions from the soil profile is significantly restricted. Adversely, nitrate does not adsorb to the soil clay particles, and thus, the leaching of nitrate to groundwater is highly probable (Ravikumar et al. 2011). Consequently, nitrate is recognized as the most mobile form of N in ecosystems (Yau and Ryan 2010). Nitrate leaching is influenced by different factors such as soil properties, crops and crop rotation, irrigation methods, management practices, and climatic parameters. Therefore, application of appropriate amount of water and fertilizer along with better management strategies should be taken into consideration to maximize the application efficiency and minimize water and fertilizer loss through leaching (Ajdary et al. 2007). Regarding nitrogen requirement, the recommended N fertilizer rate for safflower has reported from 40 to 120 kg N ha−1 for various situations (Nasr et al. 1978; Cazzato et al. 1997; Elfadl et al. 2009; Yau and Ryan 2010; Bassam 2013; Shahrokhnia and Sepaskhah 2016).
In the case of drought and limited water supply, alternate furrow irrigation is considered as an appropriate irrigation strategy to reach the higher water saving and water productivity. In this type of irrigation, water is delivered to one of the two furrows; thus, only half of the furrows in a set will be irrigated. Since lateral infiltration may increase the irrigated water in the wet furrows due to expansion of water into dry neighboring furrows, this technique does not necessarily reduce water use to the half (Ebrahimian et al. 2013). The variable alternate furrow irrigation (VAFI) that is an approach of partial root drying (PRD) irrigation strategy is a proper irrigation method that can save applied irrigation water without having significant yield loss (Sepaskhah and Ahmadi 2012; Davies et al. 2002). The environmental features of PRD irrigation may be related to lower deep percolation and nitrate leaching into underground water resources that is resulted from less irrigation water use.
Saving of irrigation water in drought condition and arid regions also may be obtained by using a different planting method. On the other hand, it has been observed that more appropriate conditions for plant growth are provided in in-furrow planting due to higher soil water content and temperature (Shabani et al. 2013; Yarami and Sepaskhah 2015; Shahrokhnia and Sepaskhah 2017a). The in-furrow planting method was recognized as a more preferable method for regions with drought or saline stress risk by the study of Yarami and Sepaskhah (2015).
Simulation of water flow and solute transport in the vadose zone, along with the root water and nutrient uptake, has facilitated the understanding of dynamic processes in the soil profile. Models have proved to be useful for simulation of conditions that are economically or technically impossible to carry out in field experiments (Li and Liu 2011; Shahrokhnia and Sepaskhah 2017b). Therefore, using a calibrated and validated model on this issue will help to an optimal scheduling of nutrient applications for any time period (Ravikumar et al. 2011). HYDRUS model developed by Simunek et al. (2008) can be used to characterize water, nutrients, and pesticide transport processes at the field scale (Pang et al. 2000; Rassam and Cook 2002; Phillips 2006). HYDRUS-1D and HYDRUS (2D/3D) are models that have often been used by many researchers to simulate water flow in agricultural fields with different crops and various irrigation schemes (Kandelous et al. 2012; Ramos et al. 2012; Siyal et al. 2012; Tafteh and Sepaskhah 2012).
Water and nitrogen dynamics in safflower fields have not been investigated before, specifically in condition of partial root drying irrigation, in-furrow planting method, and different nitrogen fertilization rates. Therefore, the objective of this study was to address the behavior of deep percolation, nitrate leaching, nitrogen uptake, and soil nitrogen content under different management strategies in field lysimeters. Application and evaluation of HYDRUS-1D model by this approach can be additionally advantageous for reaching more appropriate farm management over the areas with limited water resources.
Material and methods
Field experiment
Site description and experimental design
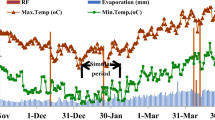
Field experiments were carried out during 2012–2013 and 2013–2014 growing seasons at the Experimental Research Station of the Agricultural College, Shiraz University in Iran. The station is located at 29° 56′ N latitude, 52° 02′ E longitude and 1810 m above mean sea level that is classified as a semi-arid region. The daily climatic parameters including rainfall, temperatures, relative humidity, wind velocity, and sunshine hours were recorded at weather station located near the site. The average of monthly climatic data is reported in Fig. 1. The annual rainfall was 433 and 276 mm for the first and second year, respectively, which mostly occurring from November to May. The long-term precipitation (from 1972 to 2013) is averagely 388 mm in the region. Comparably, the monthly precipitation was higher in November, December, and April of 2012–2013 and November and January of 2013–2014. The long-term mean values of daily temperature and relative humidity were 13.3 °C and 50%, respectively. The minimum temperature was averagely below zero from December to February of both years of study; however, they were in lower values in year 2013 due to having snowfalls compared with year 2012. The required irrigation water provided from a well located near the field with electrical conductivity (EC) of 0.72 dS m−1. Periodic analyses made on the irrigation water samples during the experiment and showed steady characteristics over the time. The soil is clay loam consisted of averagely 27.3% sand, 37.3% silt, and 36.4% clay in the layers. Soil depth is 0.90 m in the lysimeters with field capacity of 0.34 m3 m−3 and wilting point of 0.14 m3 m−3. The properties of soil and irrigation water are shown in Table 1.
The three factors studied in this experiment were included as application of different irrigation strategies, planting methods, and nitrogen fertilization rates. The design of experiment is considered as split-split plot that was arranged as randomized complete blocks in three replications. The main plot assigned for two different irrigation regimes including full irrigation by ordinary furrow irrigation (OFI) and variable alternate furrow irrigation (VAFI) as a partial root drying (PRD) strategy. The sub-plot received two various planting methods for safflower seeds as on-ridge planting (P1) and in-furrow planting (P2) methods. Three levels of nitrogen application rates were considered as N0 = 0, N1 = 100, and N2 = 200 kg ha−1 of urea fertilizer (46 and 92 kg N ha−1) that were assigned for the sub-sub-plots.
Field and irrigation practices
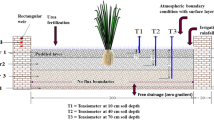
Safflower seed as local cultivar of Isfahan was planted on 25 and 10 October of 2012 and 2013, respectively, in 36 non-weighing/drainage-type lysimeters with barley as the preceding crop. The dimension of each lysimeter is 1.5 m × 1.5 m × 1.1 m with a layer of 0.05-m gravel at the bottom and a soil layer of 0.90-m height on it. Lysimeters were established by concrete materials with square designing in which different crops have been cultivated for more than 8 years before the experiment. Therefore, soil layers of the lysimeters were naturally packed as well as the surrounding field with same soil levels. The drainage water depleted from the bottom of each lysimeter is collected in individual containers. At field preparation stage in 2 weeks before planting, the triple superphosphate (46% P2O5) at rate of 100 kg P2O5 ha−1 and cattle manure (2.5 Mg ha−1) were mixed with the soil. Afterward, three furrows with 0.5-m spacing and four ridges with 0.5-m spacing and 0.15-m height were made in each lysimeter. Safflower seeds were hand-planted in three rows in in-furrow planting (P2) with distance of 0.10 m on each row. However, the distance between plants in the two side rows in on-ridge planting (P1) was 0.2 m in order to reach equal number of plants in each lysimeter (45 plants per lysimeter). Based on the experimental application rates of nitrogen, the required fertilizer in the form of urea was applied to soil at two stages of growing season. The first half of the fertilizer was applied at late winter when stem elongation began, and the second half was applied in spring before flowering. In regard to the risk of frost in December, January, and February of both years, lysimeters were covered with a plastic sheet in some freezing nights in order to prevent plants from frost damage. Additionally, weeds were removed by hand several times in the growing season, and aphids were controlled by using appropriate pesticides.
In order to determine the crop irrigation requirement, the soil water status was monitored by neutron scattering method in 7 to 10-day interval. The access tube of neutron meter was installed in the bottom of middle furrow in OFI and in the bottom of the middle and side furrows of VAFI treatments. The soil water content was measured before each irrigation event using the neutron meter at depths of 0.30, 0.60, and 0.90 m and by gravimetric sampling method at depth of 0–0.15 m. Afterward, the required irrigation water depth was determined by using the following equation:
where d is the irrigation water depth (m), θ fci and θ i are the volumetric soil water content (m3 m−3) in layer i at field capacity and before irrigation, respectively, Δz i is the soil layer thickness (m), and n is the number of soil layers. The crop root depth during the crop growing season was estimated by equation of Borg and Grimes (1986).
The gross irrigation water depth was obtained by considering an application efficiency of 75% that is commonly used by farmers for surface irrigation systems. The gross irrigation water depth was fully applied in OFI regimes in all three furrows, whereas only two thirds of gross irrigation water was applied to VAFI that were dry in the preceding irrigation cycle. A volumetric flow meter was used to measure the amount of applied irrigation water to each lysimeter. In order to provide uniform seed germination and plant stands, the three initial irrigation events were imposed as full irrigation (OFI) in all treatments. Afterward, the crop water requirement was mostly provided by precipitation before safflower elongation stage that various irrigation strategies were started.
Field measurements and analysis
The volume of the collected drainage water from the lysimeters was measured by a volumetric container following each irrigation and rainfall event. The samples of drainage water taken from each lysimeter were kept at 4 °C, and its NO3-N concentration was determined in less than 48 h by a spectrophotometer (DR5000, HACH Co.). Thus, nitrate leaching was obtained by multiplication of drainage water volume and NO3-N concentration. Soil samples at three depths of 0.30, 0.60, and 0.90 m were taken before and after each growing season using a tube auger for nitrate analysis. The samples were air dried, crushed to pass through a 2-mm sieve, and extracted in KCl 2 M. Finally, the soil samples were analyzed by cadmium reduction method (Keeney and Nelson 1982), and the NO3-N concentration was determined.
Safflower plants reached maturity in July of both experimental years. They were cut at ground level from two middle rows of lysimeters and then dried at 80 °C. The nitrogen concentration in seed and straw (mixture of stems and leaves) were separately measured in each plot using Kjeldahl method. Therefore, seed nitrogen uptake and straw nitrogen uptake were determined by multiplying the nitrogen concentration fraction in seed and straw individually by the relevant produced yield (kg ha−1). The total crop nitrogen uptake was determined by summation of nitrogen uptake by seed and straw.
Selection of model for simulating water and nitrogen transport
HYDRUS software is a finite element numerical model that simulates the movement of water, heat, and multiple solutes in variably saturated media (Simunek et al. 2008). Furrow irrigation is governed by a two-dimensional water transfer process, and it is expected that soil water contents, pressure heads, and solute concentration distribution are considered as two-dimensional in the soil surface layer. However, water transport tends to vertical movements (one-dimensional) in deeper depth from the soil surface. In this study, the bottom water flux and solute concentration beneath the lysimeters were far away from the soil surface; thus, water and solute transport can be averagely considered as one-dimensional in whole soil profile. The application of HYDRUS-1D instead of HYDRUS-2D can be justified in this situation (Tafteh and Sepaskhah 2012). Similarly, Ebrahimian et al. (2013) studied the application of both one- and two-dimensional HYDRUS models in which HYDRUS-1D model could successfully simulate fertigation in the case of alternative furrow irrigation, despite the two-dimensional conditions in water and fertilizer movement. Therefore, HYDRUS-1D (version 4.16) was used in this study to simulate water flow, nitrate leaching, and soil nitrate content in safflower root zone and crop N uptake.
HYDRUS-1D model description
In HYDRUS-1D model, the one-dimensional uniform water movement in a partially saturated rigid porous medium is described by a modified form of the Richards equation using the assumptions that the air phase plays an insignificant role in the liquid flow process and that water flow due to thermal gradients can be neglected by the following equation (Simunek et al. 2013):
where θ is the soil volumetric water content (cm3 cm−3), h is the water pressure head (cm), t is the time (day), K is the unsaturated hydraulic conductivity (cm day−1), z is the vertical axis (upward positive) depending on the origin of the surface flux, and α is the angle between the flow direction and the vertical axis (i.e., α = 0 for vertical flow). The sink term, S, is defined as the volume of water removed from a unit volume of soil per unit time due to plant water uptake. The rate of root water uptake (S) is determined by Feddes et al. (1978) equation as follows:
where the water stress response function α(h) is a prescribed dimensionless function of the soil water pressure head (0 ≤ α ≤ 1) and S p is the potential water uptake rate (day−1). The variable S p is equal to the water uptake rate during periods of no water stress when α(h) = 1.
HYDRUS-1D assumes that the actual root depth (L R ) is the product of the maximum rooting depth, L m (cm), and a root growth coefficient, f r (t) (Simunek and Suarez 1993) as follows:
The model uses the classical Verhulst–Pearl logistic growth function for the root growth coefficient, f r (t) by Eq. (5).
where L0 is the initial value of the rooting depth at the beginning of the growing season (cm) and r the growth rate (day−1). The growth rate is calculated either from the assumption that 50% of the rooting depth will be reached after 50% of the growing season has elapsed.
HYDRUS implements the soil hydraulic functions of van Genuchten (1980) who used the statistical pore size distribution model of Mualem (1976) to obtain a predictive equation for the unsaturated hydraulic conductivity function in terms of soil water retention parameters. The soil water retention, θ(h), and hydraulic conductivity, K(h), functions by van Genuchten (1980) are given as
where θ s is the soil saturated water content (cm3 cm−3); θ r is the residual water content (cm3 cm−3); m and n are empirical shape factors in the water retention function, m = 1 – 1 / n; K s is the soil saturated hydraulic conductivity (cm day−1) measured using soil column experiments; l is the pore connectivity parameter in the hydraulic conductivity function that was estimated by (Mualem 1976) to be about 0.5 as an average for many soils; and S e is the relative saturation, that is defined as follows:
The partial differential equation governing one-dimensional non-equilibrium chemical transport of nitrate in the soil profile presented by Simunek and van Genuchten (1995) that was considered in this study as the following equation:
where c is solute concentrations in the liquid phase (mg cm−3), q is the volumetric flux density (cm day−1), r a is the root nutrient uptake term (mg cm−3 day−1) that for passive uptake is equal to the product of the sink term S in the water flow equation and the concentration of the sink term C r (mg cm−3), and Dw is the dispersion coefficient (cm2 day−1) for the liquid phase. In concept, the rhizosphere dynamics of water and nutrient uptake is very complex in root zone (Bar-Yosef 1999). In order to avoid the complications involved in active N uptake, N mineralization, and denitrification, this study makes the typical assumption that root nitrate uptake is strictly passive and assumes that the above mechanisms are not occurring (Hanson et al. 2006; Tafteh and Sepaskhah 2012).
The root solute uptake models implemented in HYDRUS-1D was developed by Simunek and Hopmans (2009). Passive nitrate uptake is simulated by multiplying root water uptake with the dissolved nitrate concentration, for concentration values below the maximum concentration (c max ), or
where c is the dissolved nutrient concentration (mg cm−3) and c max is the maximum allowed solution concentration (mg cm−3) that can be taken up by plant roots during passive root uptake. Passive actual root nitrate uptake for the whole root domain, P a (mg cm−2 day−1), is calculated by integrating the local passive root nutrient uptake rate, p a , over the entire root zone.
Input parameters and boundary condition
Water movement
Soil profile was considered as three layers based on the variability of its physical properties. The organic matter content in the layers 1 and 2 is higher than that in the layer 3 due to manure application in the previous cropping season (Bazegari et al. 2017). The inverse analysis of soil hydraulic parameters was accomplished by HYDRUS-1D model including residual water content (WCR), saturated water content (WCS), saturated hydraulic conductivity (K s ), and alpha and n parameters (Table 2). Initial values of soil hydraulic parameters were used from a local study by Mahbod and Zand-Parsa (2010) and then optimized by inverse solution results. Time variable boundary conditions were activated in daily time steps for whole safflower growing season. The initial condition was given in water content for different soil layers according to experimental field measurements. Safflower potential evaporation and transpiration was calculated according to Allen et al. (1998) by dual coefficient method that was reported as more accurate method by the local investigation of Shahrokhnia and Sepaskhah (2013). The daily ETo was determined by using modified Penman–Monteith equation (Razzaghi and Sepaskhah 2012) and daily measured climatic data from the adjacent weather station. The proposed model by Feddes et al. (1978) was used to describe the root water uptake using parameter values reported by Tafteh and Sepaskhah (2012) for rapeseed as the following: P0 = 0, POpt = − 1 cm, P2H = − 500 cm, P2L = − 900 cm, P3 = − 16,000 cm, r2H = 0.5 cm day−1, r2L = 0.1 cm day−1. Safflower may be known as a lengthy root crop reaches up to 1.9-m depth (Merrill et al. 1994, 2002). However, the maximum rooting depth of 0.90 m was considered in this study with regard to the limited soil depth in the lysimeters. The root growth rate was predicted based on the assumption that 50% of the rooting depth is reached after 50% of the growing season is elapsed.
Solute transport
Leaching of nitrogen occurs mostly in the nitrate form (Ajdary et al. 2007) and ammonium (NH4-N) is assumed to be adsorbed to the solid phase (Ramos et al. 2012); therefore, the leaching of ammonium was not simulated in this study (Tafteh and Sepaskhah 2012). Furthermore, ammonia volatilization was ignored due to the prompt irrigating of soil after application of nitrogen fertilizer (Tafteh and Sepaskhah 2012). Besides, denitrification was neglected in this study because in most of the safflower growing season, soil was not in saturation conditions (Ravikumar et al. 2011). Furthermore, N mineralization was not considered in this investigation, and it was assumed that the most of the organic nitrogen has transformed to nitrate before planting. In other words, most of easily mineralizable N in rotted manure has converted to inorganic forms and contribution of the remaining N in mineralization is not significant. The solute transport parameters were initially calibrated by inverse analysis in HYDRUS-1D model based on the nitrate concentration observed in the drained water. The molecular diffusion coefficient in free water (Diff. w) for NO3-N was initially assumed as 1.64 cm2 day−1 (Li et al. 2015), and then, it was calibrated. The longitudinal dispersivity was initially considered as one tenth of the soil profile depth (Beven 1993; Cote et al. 2003; Phogat et al. 2012), and then, it was calibrated (Table 2). The initial soil nitrate concentrations in soil water were given to model as initial conditions according to the field data. The upper boundary condition for solute transport was concentration flux boundary condition, and the lower was zero concentration gradient. The root solute uptake was considered as passive NO3-N uptake by specifying a large value for c max parameter (e.g., 20 mg cm−3) which allowed to nitrate uptake occurred without limitation (Simunek and Hopmans 2009; Li et al. 2015).
Model calibration and validation
The data obtained in the first year of study was used to calibrate the parameters of the model. The HYDRUS-1D predictions conducted on the second year of experiment, and the outputs were evaluated with the real measured data (drainage water, solute concentration in the drainage water as NO3-N, crop nitrogen uptake, and residual soil nitrate concentration). To find out the level of agreement between the model prediction outputs and field measured data, the following statistical indices were used.
where NRMSE and d are the normalized root mean square error and the index of agreement, respectively, and n is the number of observations, X is the measured value, Y is the predicted value, O is the mean value of the measured data, and O e is mean value of the predicted data. The value of NRMSE and d approaches 0.0 and 1.0, respectively, for the accurate simulation. The closer the NRMSE is to 0, the model is more accurate. The value of d varies between 0 and 1.0, and the closer its value to 1.0, the model accuracy is higher. In addition, relationship between the measured and predicted values was compared with 1:1 line, statistically.
Results and discussions
Water transport
The amount of water that is drained beneath the root zone is affected by several factors such as precipitation depth, irrigation management, soil water content, and evapotranspiration. The total water applied by irrigation and the seasonal drainage water for both years of study are demonstrated in Fig. 2. For N1 and N2 treatments, higher plant growth resulted in higher soil water uptake and higher irrigation water was applied; therefore, the summation of irrigation and rainfall in these treatments was higher than that for N0 (Fig. 2). The high applied water for safflower is due to the low irrigation efficiency, long growing season, and semi-arid climate with high ETo. In the case of longer growing season and drier weather conditions in the second year of study, higher irrigation water was also applied in comparison with the first year. In analogy, VAFI strategy significantly reduced drainage water compared with the OFI treatments by mean value of 48 and 70% in both years, respectively (Table 3). The number of irrigation events in the first year was lower than that in the second year due to more rainfall events. Therefore, the drainage water reduction in the first year (48%) was lower than that in the second year (70%). In other words, the higher rainfall events that occurred in the first year of study led to lower drainage water conservation compared with the second year because less management practices have implemented for these conditions. Meanwhile, the applied irrigation water in VAFI regime was about 30% less than that used in the full irrigation treatments (Shahrokhnia and Sepaskhah 2016); however, drainage water decreased at a higher rate in this strategy. Similarly, VAFI strategy reduced drainage water by 40 to 50% for maize (Sepaskhah and Tafteh 2012), rapeseed (Tafteh and Sepaskhah 2012), and sugarbeet (Barzegari et al. 2017). In the case of VAFI regime, water transport is greatly involved with lateral movement in soil profile and the downward transport is weaker than that in OFI. This fact is the main reason for decreasing the volume of drainage water further than the amount of irrigation water that is saved by this technique. Consequently, it is indicated that VAFI can be a favorable strategy for water conservation both on applied irrigation water and deep percolation. Furthermore, in-furrow planting method showed 8 and 18% less drainage water compared with the on-ridge planting for two consecutive years, respectively (Table 3). The root system under in-furrow planting is mostly distributed and densified below the furrows than the ridges. Therefore, it may touch the incoming water promptly and be able to absorb water faster than the on-ridge planted crops. In addition, the distributed root system in furrows may build up more resistance against water movement to the deeper soil layers compared with on-ridge planting method. The higher biomass produced by in-furrow planting (Shahrokhnia and Sepaskhah 2016) may verify this issue regarding better water availability in furrows than the ridge planting. Regarding improvement of environmental issues in farming, VAFI and in-furrow planting strategies are found to have effective influences on saving of applied water and reducing the drainage water transfer beneath the root zone. Besides, drainage water was significantly reduced by enhancement of fertilization rate due to higher plant growth and water use.
Total precipitation and deep percolation in safflower field. a 2012–2013. b 2013–2014. I1: ordinary furrow irrigation; I2: variable alternate furrow irrigation; P1: on-ridge planting; P2: in-furrow planting; N0: nitrogen application at rate of 0 kg N ha−1; N1: nitrogen application at rate of 46 kg N ha−1; N2: nitrogen application at rate of 92 kg N ha−1. DP: deep percolation; Irri + rain: the total of irrigation and rain
Soil hydraulic parameters were calibrated by the use of cumulative drained water depths in the first year of study (Table 2), and they were used to simulate water movement in the second year as validation. The results of calibration obtained by the inverse solution were in accordance with a local study by Mahbod and Zand-Parsa (2010) as 0.014 cm−1, 1.33, and 20.6 cm day−1 for alpha, n, and K s , respectively. The mean predicted and measured values of cumulative drainage water at different days after first irrigation are presented in Fig. 3. The simulated drainage water by HYDRUS-1D has accurately fitted on the field observed data. The mean value of NRMSE and d index in all treatments was 0.120 and 0.991 for the first year (calibration) and 0.108 and 0.994 for the second year (validation), respectively. This analysis demonstrated the appropriate matching of drainage water predictions and measurements for both years of calibration and validation. However, statistical analysis showed higher accuracy for predictions in OFI regime compared with the VAFI. This may be due to not considering two-dimensional water movement in VAFI regime. Moreover, a linear relationship between predicted and measured values of drainage water as cumulative and periodic scales were individually compared with 1:1 line (not presented). Indeed, the daily drainage water was gathered from beginning to end of growing season for cumulative scale, while in the case of periodic scale, the drainage water between two consecutive irrigation or rainfall events was considered. For the cumulative scale, line slopes were 1.06 and 1.03 with r2 of 0.98 and 0.99 for the calibration and validation year, respectively. Whereas, for the periodic scale, line slopes were 0.97 and 1.01 with r2 of 0.78 and 0.82 for the calibration and validation year, respectively. Therefore, it was indicated that HYDRUS-1D could predict drainage water for both cumulative and periodic scales, but higher errors (weaker r2) were observed in periodic scale prediction in comparison with the cumulative scale.
Mean values of cumulative drainage water measured and predicted by HYDRUS-1D in 2012–2013 (a calibration) and 2013–2014 (b validation). Measured (squares), predicted (lines). I1: ordinary furrow irrigation; I2: variable alternate furrow irrigation; N0: non-fertilized plots; N1,2: average of fertilized plots by N1 and N2 levels
According to Fig. 3, cumulative drainage water was increased slightly from the day after the first irrigation (DAFI) up to 205 and 208 days later for the first and second year, respectively. This duration occurred in fall and winter, and the crop water requirement was mostly provided by rainfall events. After this period, by increase in the air temperature, rapid growth of safflower was initiated and irrigation operations implemented in order to provide safflower water needs. It was observed that the cumulative drainage water was rapidly increased by starting the irrigation practices in 222 and 218 DAFI for the first and second year, respectively (Fig. 3). As it was expected, the increasing trend was much remarkable for OFI regime rather than VAFI strategy. This was due to higher irrigation water depths applied compared with the amount of previous rainfall events.
Nitrogen dynamics
NO3-N leaching
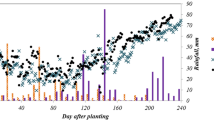
Nitrate leaching was investigated as NO3-N concentration (mg L−1) in the drainage water and the cumulative downward NO3-N transfer (kg ha−1) below the safflower root zone. The measured and simulated values of nitrate concentration in drainage water, on average, are presented in Fig. 4. It is observed that nitrate concentration was initially increased after the first irrigation and then decreased slowly till the harvest time. This is mainly due to higher amount of nitrogen in soil in regard to the presence of organic manure at soil surface and also small seedlings with undistributed roots at the beginning weeks after planting. The maximum and minimum NO3-N concentration in drainage water was 38.5 and 6.6 mg L−1 in the first year and 39.5 and 6.8 mg L−1 in the second year, respectively (Fig. 4). The highest solute concentrations can be attributed to VAFI treatments in fertilized plots and in-furrow planting method that demonstrated higher availability of nitrate in the root zone. In other words, higher NO3-N concentration in drainage water at VAFI regime was resulted from the lower amounts of deep percolation that led to higher nitrate concentrations. Indeed, by application of higher amount of water, higher amounts of drainage water and nitrate leaching are occurred. Similar findings were reported by Cameira et al. (2003), Vazquez et al. (2006), Gheysari et al. (2009), and Sepaskhah and Tafteh (2012). Based on Fig. 4, the maximum NO3-N concentration in drainage water was observed about 47 and 60 days after planting resulted from intensive rainfall events for 2 years, respectively. In contrast, the minimum nitrate concentration values observed for the last irrigation applied at the end of growing season for both years.
Mean values of NO3-N concentration in drainage water measured and predicted by HYDRUS-1D in 2012–2013 (a calibration) and 2013–2014 (b validation). Measured (squares), predicted (lines). I1: ordinary furrow irrigation; I2: variable alternate furrow irrigation; N0: non-fertilized plots; N1,2: average of fertilized plots by N1 and N2 levels
Indeed, the major point in this study is the reduction of total leached nitrate to underground water resources which is obtained by these strategies.
In the case of groundwater that is the source of irrigation water, although nitrate concentration increases under VAFI, more underground water is also saved in the water resource. So, higher nitrate concentration with more remained underground water in VAFI is against the lower nitrate concentration with less amount of remained water resource in OFI. Therefore, the final nitrate concentration in underground water may not be a concern in VAFI condition.
Solute parameters in HYDRUS-1D were calibrated by the use of NO3-N concentration in the drainage water under the lysimeters in the first year of study, and they were used to simulate NO3-N transport in the second year (validation). The calibrated longitudinal dispersivity and the molecular diffusion coefficients in free water were finally close to the values reported by Abbasi et al. (2003) and Li et al. (2015) for 3 to 34 cm for dispersivity and 1.64 cm2 day−1 for molecular diffusion coefficients, respectively. In the case of HYDRUS-1D predictions for leached NO3-N concentration, results compared with the measured values by using the statistical indices of NRMSE and d. The NRMSE were lower than 0.1 with d indices higher than 0.94 for both years and all treatments and indicated that HYDRUS-1D predictions were more favorable for solute concentration of drainage water in comparison with the amount of drainage water. Based on the statistical indices, model predictions could accurately match with the NO3-N concentration of drainage water below the safflower root zone.
The seasonal leached nitrate under safflower field was calculated based on drainage water and NO3-N concentrations (Table 3). The lowest leached nitrate was 54.3 and 33.1 kg ha−1 for 2 years, respectively, that were observed in non-fertilized VAFI treatments with in-furrow planting method. This is mainly due to the reduction of downward water flux in VAFI regime and in-furrow planting method. Results indicated that VAFI reduced NO3-N leaching by 27 and 47% compared with full irrigation treatments for 2 years, respectively. Meanwhile, leached nitrate increased by increasing the N application rates (Table 3). The cumulative leached nitrate during safflower growing season is shown in Fig. 5 with respect to the effects of irrigation strategies and N fertilization rate on nitrate leaching. In-furrow planting method reduced N loss below the safflower root zone about 2% compared with on-ridge planting method although it was not statistically significant (Table 3). On the other hand, highest nitrate loss was 94.8 and 81.8 kg ha−1 for the first and second year, respectively, that occurred in the full irrigation plots with on-ridge planting method and 92 kg ha−1 N application rate. This issue is mainly resulted from higher volume of drainage water below the root zone and higher N application rate. Moreover, N loss was more intense at initial stages of safflower growing season coincided with autumn and winter rainfalls (Fig. 5). Therefore, initial rainfall events play an important role in increasing NO3-N leaching from farms, and it is required to consider some management policies for these situations. In this respect, Cavero et al. (1999) suggested that reducing the soil NO3-N content before fall and winter period would decrease the risk of nitrate leaching. Application of minimum N fertilization rates during the previous summer crop can be an effective instance for this approach. On the other hand, safflower N uptake is minor at initial growing stages due to the low distributed root system as reported by Jabloun et al. (2015). Therefore, it would be more proper to postpone N fertilization from planting time to the middle of growing season when root system actively absorbs nitrate.
Cumulative leached nitrate measurements below the safflower root zone. On-ridge planting method in year 2012–2013 (a1). On-ridge planting method in year 2013–2014 (a2). In-furrow planting method in year 2012–2013 (b1). In-furrow planting method in year 2013–2014 (b2). I1: ordinary furrow irrigation; I2: variable alternate furrow irrigation; N0: nitrogen application at rate of 0 kg N ha−1; N1: nitrogen application at rate of 46 kg N ha−1; N2: nitrogen application at rate of 92 kg N ha−1
Regarding of the accuracy of HYDRUS-1D predictions for leached NO3-N transfer, it was compared with the calculations based on the measured drainage water and concentration of leached nitrate values. The NRMSE were lower than 0.2 with d indices higher than 0.96 for both years and all treatments. Besides, the relationship between predicted and measured leached NO3-N compared with 1:1 line (not presented), and it was resulted in a line slope of 1.07 and r2 of 0.97. Consequently, model prediction is agreed with the measured NO3-N transfer below the root zone although it was accompanied with a minor overestimation by the model. This overestimation may be associated with consideration of one-dimensional movement by the model.
Safflower N uptake
The measured safflower N uptake is consisted of seed N uptake and aboveground straw N uptake (Table 4). The highest values of N uptake by safflower seeds were 83.7 and 87.2 kg N ha−1 for both years, respectively, that were obtained in plots with 92 kg N ha−1 and in-furrow planting method. Besides, the highest amounts of straw N uptake observed as 113.3 and 127.8 kg N ha−1 that occurred in the same plots. There were not any significant differences between the irrigation strategies for N uptake in seeds and straw. Hence, VAFI regime did not impose any limitation for safflower N uptake. The minimum N uptake by seed was measured as 36.1 and 38.0 kg N ha−1, and they were 43.7 and 43.5 kg N ha−1 for straw in the first and second year, respectively. These values occurred in N0 treatments, and they were not significantly influenced by irrigation strategies or planting methods. Generally, seed N uptake was approximately 40 to 50% of total safflower N uptake, and the rest of N uptake (50–60%) was accumulated in straw. Similarly, straw N uptake reported to be about 65% of total safflower N uptake by Yau and Ryan (2010). Total safflower N uptake reached the maximum value of 194.6 and 215 kg N ha−1 in the first and second year, respectively. Indeed, they were under 92 kg N ha−1 application rate, in-furrow planting method and VAFI strategy. In this respect, total aboveground safflower N uptake was reported as about 150 and 200 kg N ha−1 by Jones and Tucker (1968) and Bassil (2000), respectively. The lowest safflower N uptake was observed in N0 by mean value of 79.8 and 87.0 kg N ha−1 without taking significant influence from irrigation regimes or planting methods (Table 4). Statistical analysis of seed N uptake, straw N uptake, and total safflower N uptake indicated that VAFI strategy had no significant effect on reducing the N uptake (Table 4). Therefore, VAFI strategy can be an alternative for safflower in regard to the amount of N uptake. On the other hand, in-furrow planting method significantly enhanced safflower N uptake (in seed, straw, and whole crop) compared with on-ridge planting by mean amounts of 16 and 19% for first and second year, respectively. This may have resulted from the presence of better conditions in furrows in terms of water and nitrogen availability. In addition, application of 46 kg N ha−1 enriched N uptake by 61 and 54% for both years, respectively. Whereas, they were enhanced about 29% by application rate of 92 kg N ha−1 compared with 46 kg N ha−1. Similarly, application of 40 and 80 kg N ha−1 in the study of Elfadl et al. (2009) led to a significant increase in safflower seed yield compared with the control. Generally, N uptake increased as N application rate increased for all treatments. Generally, the results expressed higher N uptake in in-furrow planting and VAFI due to lower leached NO3-N and less amount of water applied for VAFI. The higher biomass production reported by Shahrokhnia and Sepaskhah (2016) in these conditions may also verify this finding.
The cumulative safflower N uptake predicted by HYDRUS-1D is presented in Fig. 6. It is observed that N uptake proceeded slowly after sowing up to about 130 and 160 DAFI for 2 years, respectively. This is where the stem elongation of safflower has begun, and also, the first stage of N application was used. Afterward, N uptake was increased at a higher proportion till the harvest, specifically with a higher steep trend in fertilized plots. Generally, crop N uptake had a rapid increase approximately from the second half of safflower growing period that was related to the activity of root system for N absorption and development of canopy.
The cumulative safflower N uptake predicted by HYDRUS-1D. a OFI treatments in year 2012–2013. b VAFI treatments in year 2012–2013. c OFI treatments in year 2013–2014. d VAFI treatments in year 2013–2014. P1: on-ridge planting; P2: in-furrow planting; N0: nitrogen application at rate of 0 kg N ha−1; N1: nitrogen application at rate of 46 kg N ha−1; N2: nitrogen application at rate of 92 kg N ha−1
The predicted seasonal N uptake by HYDRUS-1D model was compared with the mean measured values (n = 12) at end season. The values of NRMSE and d were 0.098 and 0.975 for the first year and 0.071 and 0.984 for the second year, respectively. These indicated that HYDRUS-1D could predict safflower N uptake with acceptable accuracy. Besides, the slope of correlation line between the measured and predicted safflower N uptake at harvest (not presented) was 0.94 and 0.99 for two consecutive years, respectively. It is shown that the predicted N uptake was negligibly lower than the measured values. This maybe resulted from not considering the processes such as organic N mineralization, direct ammonium uptake, and active uptake of nitrate by safflower although they were not much substantial in this study. According to similar investigations by Simunek and Hopmans (2009), Ramos et al. (2012), Li et al. (2015), and Deb et al. (2015), dynamics of N uptake is very complicated in root zone due to involvement of both passive and active mechanisms. Therefore, considering only passive uptake may underestimate the crop N uptake and overestimate N leaching. These hints are in agreement with the findings of present study.
Safflower nitrogen uptake approximately used 57 and 65% of available N (fertilization + NO3-N of soil and water) in both years, respectively. A simple linear relationship between safflower N uptake and total available nitrogen was obtained for irrigation regimes by Eqs. (13) and (14) where NU is the plant N uptake in kilogram per hectare and TAN is the total available N in kilogram per hectare.
According to Eqs. (13) and (14), a threshold value for TAN is obtained where safflower N uptake begins (Sepaskhah and Tafteh 2012). Therefore, the threshold values are calculated as 62.4 and 44.3 kg N ha−1 for OFI and VAFI, respectively. The fitted equations in Fig. 7a indicated that for every TAN, the safflower N uptake was higher in VAFI compared with OFI. This is in agreement with findings of Ahmadi et al. (2011) and Sepaskhah and Tafteh (2012) in which the higher N uptake was achieved by applying VAFI strategy for the potato and rapeseed, respectively.
Similar relationship between the safflower N uptake and the total available nitrogen may be obtained regarding of the planting method [(Eqs. (15) and (16)].
In analogy, the threshold values were 38.5 and 33.6 kg N ha−1 for on-ridge and in-furrow planting, respectively, which are close to each other. However, the fitted equations in Fig. 7b indicated that for every TAN, the safflower N uptake was relatively higher in in-furrow planting treatments compared with the on-ridge. Therefore, higher N uptake was occurred by application of in-furrow planting method which may be due to higher availability of water and nitrogen in furrows and lower N leaching.
Soil NO3-N concentration
The measured soil NO3-N concentration is presented in Fig. 8 at seeding and harvest. It is observed that soil NO3-N concentration of soil top layer at seeding is higher than that in the bottom layers. It can be attributed to the pre-season application of cattle manure in all treatments, and it is gradually decreased from top to bottom of the soil profile. Soil nitrate concentration at seeding was in the range of 4 to 11 mg N kg−1 soil for the first year and 1.5 to 8 mg N kg−1 soil in the second year. Soil nitrate concentration was significantly decreased to values lower than 2 mg kg−1 soil at harvest for both years of study. This indicated that safflower has greatly used the soil N content. This finding agrees with the results of Elfadl et al. (2009) in which soil N content was strongly reduced after safflower harvesting. Other studies have pointed to the importance of residual soil N and the extensive rooting habit of safflower (Jones and Tucker 1968). According to studies of Yau and Ryan (2010), safflower was able to take up accumulated N below the rooting zone of other crops and reduced nitrate leaching to the groundwater. Gilbert and Tucker (1967) and Kaffka and Kearney (1998) showed the response of safflower to pre-planting soil residual N and reported that effect of applied N fertilizer was apparent when the concentration of soil residual N was low. In contrast to seeding, soil N concentration at harvest was higher at bottom soil layers rather than that in the top layers for both years. Indeed, the N leaching has moved the nitrogen front from top of the soil to the bottom layers during the season due to the mobility of NO3-N.
The predicted residual soil NO3-N by HYDRUS-1D was compared with the measured soil nitrate after harvest. The mean values of NRMSE and d were 0.081 and 0.789 for the first year and 0.047 and 0.970 for the second year, respectively. Therefore, it is shown that model predictions are close to the observed values. Furthermore, the measured and predicted residual soil NO3-N at harvest were compared with the 1:1 line (not presented) in which the slope of this line was about 0.99 and 1.01 for two consecutive years with r2 = 0.61 and r2 = 0.93, respectively. Consequently, HYDRUS-1D could predict the end-season soil N concentration, accurately although it was accompanied by minor errors in the calibration year (weak r2).
Conclusions
In conclusion, VAFI strategy and in-furrow planting method can be considered as an alternate management practice for water conservation and environment preserving. Based on the results, VAFI strategy successfully reduced the drainage water below safflower root zone by mean value of 60% compared with the ordinary irrigation strategy. Meanwhile, drainage water was decreased by in-furrow planting method averagely by 13% compared with on-ridge planting method. The concentration of NO3-N in the drainage water percolated below the root zone was maximum in fall and winter seasons followed by rainfall events. Consequently, low available nitrogen content at initial stage of growth (at autumn) is more favorable in terms of reducing the contamination of underground water resources and preserves the environment. Furthermore, VAFI regime satisfactorily reduced the seasonal nitrate leaching below the root zone by mean value of 37%. Whereas, the reduction of leached N in in-furrow planting method was not significant in comparison with the on-ridge planting. In this respect, VAFI and in-furrow planting strategies were found to be the alternatives for reducing drainage water and contaminant transfer beneath the root zone. Application of VAFI strategy did not show any negative effects for N uptake of safflower both in seed and straw. Moreover, in-furrow planting made 19% higher N accumulation for safflower in comparison with the on-ridge planting method. Safflower N accumulation responded to each level of N application rate, although the rate of increase in crop N uptake was not remarkable between the 46 and 92 kg N ha−1. Soil nitrate concentration was decreased during the growing season indicating that safflower root system has a great ability in absorption of NO3-N from soil N supplies. One-dimensional HYDRUS model was used in this study, and it was able to describe water and nitrogen dynamics reasonably well. In conclusion, application of such a simulating model will be useful for making proper management decisions in applying water and fertilizer in a more environmental-friendly manner.
References
Abbasi F, Simunek J, Feyen J, Van Genuchten MT, Shouse PJ (2003) Simultaneous inverse estimation of soil hydraulic and solute transport parameters from transient field experiments: homogeneous soil. Trans of the ASAE 46:1085–1095
Ahmadi S, Andersen MN, Lærke PE, Plauborg F, Sepaskhah A, Jensen CR, Hansen S (2011) Interaction of different irrigation strategies and soil textures on the nitrogen uptake of field grown potatoes. Inter J Plant Prod 5:263–274
Ajdary K, Singh DK, Singh AK, Khanna M (2007) Modelling of nitrogen leaching from experimental onion field under drip fertigation. Agric Water Manag 89(1-2):15–28. https://doi.org/10.1016/j.agwat.2006.12.014
Allen RG, Pereira LS, Raes D, Smith M (1998) Crop evapotranspiration—guidelines for computing crop water requirements. FAO Irrigation and Drainage Paper No. 56. FAO, Rome
Bar-Yosef B (1999) Advances in fertigation. Adv Agron 65:1–77. https://doi.org/10.1016/S0065-2113(08)60910-4
Barzegari M, Sepaskhah AR, Ahmadi SH (2017) Irrigation and drainage managements affect nitrogen leaching and root yield of sugar beet. Nutr Cycl Agroecosyst 108(2):211–230. https://doi.org/10.1007/s10705-017-9853-y
Bassam NE (2013) Energy plant species: their use and impact on environment and development. Routledge
Bassil ES (2000) Response of safflower to saline soils and irrigation and uptake of residual soil nitrogen following cotton in rotation. University of California, Davis, p 95
Beven K (1993) Prophecy, reality and uncertainty in distributed hydrological modelling. Adv Water Resou 16(1):41–51. https://doi.org/10.1016/0309-1708(93)90028-E
Borg H, Grimes DW (1986) Depth development of roots with time: an empirical description. Trans of the ASAE 29(1):194–197. https://doi.org/10.13031/2013.30125
Cameira M, Fernando R, Pereira L (2003) Monitoring water and NO 3-N in irrigated maize fields in the Sorraia Watershed, Portugal. Agric Water Manag 60(3):199–216. https://doi.org/10.1016/S0378-3774(02)00175-0
Cavero J, Plant R, Shennan C, Friedman D, Williams J, Kiniry J, Benson V (1999) Modeling nitrogen cycling in tomato–safflower and tomato–wheat rotations. Agric Syst 60(2):123–135. https://doi.org/10.1016/S0308-521X(99)00023-2
Cazzato E, Ventricelli P, Corleto A (1997) Response of hybrid and open-pollinated safflower to increasing doses of nitrogen fertilizer. Proceeding of 4th International Safflower Conference Bari, Italy, pp 98–102
Cote CM, Bristow KL, Charlesworth PB, Cook FJ, Thorburn PJ (2003) Analysis of soil wetting and solute transport in subsurface trickle irrigation. Irrig Sci 22(3-4):143–156. https://doi.org/10.1007/s00271-003-0080-8
Davies WJ, Wilkinson S, Loveys B (2002) Stomatal control by chemical signalling and the exploitation of this mechanism to increase water use efficiency in agriculture. New Phytol 153(3):449–460. https://doi.org/10.1046/j.0028-646X.2001.00345.x
Deb SK, Sharma P, Shukla MK, Ashigh J, Simunek J (2015) Numerical evaluation of nitrate distributions in the onion root zone under conventional furrow fertigation. J Hydrol Eng 21(2):05015026
Ebrahimian H, Liaghat A, Parsinejad M, Playán E, Abbasi F, Navabian M (2013) Simulation of 1D surface and 2D subsurface water flow and nitrate transport in alternate and conventional furrow fertigation. Irrig Sci 31(3):301–316. https://doi.org/10.1007/s00271-011-0303-3
Elfadl E, Reinbrecht C, Frick C, Claupein W (2009) Optimization of nitrogen rate and seed density for safflower (Carthamus tinctorius L.) production under low-input farming conditions in temperate climate. Field Crops Res 114(1):2–13. https://doi.org/10.1016/j.fcr.2009.06.012
Feddes RA, Kowalik PJ, Zaradny H (1978) Simulation of field water use and crop yield. Centre for Agricultural Publishing and Documentation, Wageningen
Gheysari M, Mirlatifi SM, Homaee M, Asadi MS, Hoogenboom G (2009) Nitrate leaching in a silage maize field under different irrigation and nitrogen fertilizer rates. Agric Water Manag 96(6):946–954. https://doi.org/10.1016/j.agwat.2009.01.005
Gilbert NW, Tucker T (1967) Growth, yields, and yield components of safflower as affected by source, rate, and time of application of nitrogen. Agron J 59(1):54–56. https://doi.org/10.2134/agronj1967.00021962005900010016x
Hanson BR, Šimůnek J, Hopmans JW (2006) Evaluation of urea–ammonium–nitrate fertigation with drip irrigation using numerical modeling. Agric Water Manag 86(1-2):102–113. https://doi.org/10.1016/j.agwat.2006.06.013
Jabloun M, Schelde K, Tao F, Olesen JE (2015) Effect of temperature and precipitation on nitrate leaching from organic cereal cropping systems in Denmark. Eur J Agron 62:55–64. https://doi.org/10.1016/j.eja.2014.09.007
Jones J, Tucker T (1968) Effect of nitrogen fertilizer on yield, nitrogen content, and yield components of safflower. Agron J 60(4):633–634. https://doi.org/10.2134/agronj1968.00021962006000040009x
Kaffka SR, Kearney TE (1998) Safflower production in California. University of California, Davis
Kandelous MM, Kamai T, Vrugt JA, Simunek J, Hanson B, Hopmans JW (2012) Evaluation of subsurface drip irrigation design and management parameters for alfalfa. Agric Water Manag 109:81–93. https://doi.org/10.1016/j.agwat.2012.02.009
Keeney DR, Nelson DW (1982) Nitrogen-inorganic forms. In: Page AL, Miller RH, Keeney DR (eds) Methods of Soil Analysis-Part 2-Chemical and Microbiological Properties. American Society of Agronomy, Madison, WI
Li J, Liu Y (2011) Water and nitrate distributions as affected by layered-textural soil and buried dripline depth under subsurface drip fertigation. Irrig Sci 29(6):469–478. https://doi.org/10.1007/s00271-010-0255-z
Li Y, Simunek J, Zhang Z, Jing L, Ni L (2015) Evaluation of nitrogen balance in a direct-seeded-rice field experiment using Hydrus-1D. Agric Water Manag 148:213–222. https://doi.org/10.1016/j.agwat.2014.10.010
Mahbod M, Zand-Parsa S (2010) Prediction of soil hydraulic parameters by inverse method using genetic algorithm optimization under field conditions. Arch Agron Soil Sci 56(1):13–28. https://doi.org/10.1080/03650340902956652
Merrill, S. D., Tanaka, D. L., & Black, A. L. (1994). Root growth of sunflower and safflower crops affected by soil management in the Northern Great Plains. Agronomy Abstracts. ASA, Madison, WI, 366
Merrill SD, Tanaka DL, Hanson JD (2002) Root length growth of eight crop species in Haplustoll soils. Soil Sci Soc Am J 66(3):913–923. https://doi.org/10.2136/sssaj2002.9130
Mualem Y (1976) A new model for predicting the hydraulic conductivity of unsaturated porous media. Water Resou Res 12(3):513–522. https://doi.org/10.1029/WR012i003p00513
Nasr HG, Katkhuda N, Tannir L (1978) Effects of N fertilization and population rate-spacing on safflower yield and other characteristics. Agron J 70(4):683–685. https://doi.org/10.2134/agronj1978.00021962007000040037x
Pang L, Close ME, Watt JP, Vincent KW (2000) Simulation of picloram, atrazine, and simazine leaching through two New Zealand soils and into groundwater using HYDRUS-2D. J Contam Hydrol 44(1):19–46. https://doi.org/10.1016/S0169-7722(00)00091-7
Phillips I (2006) Modelling water and chemical transport in large undisturbed soil cores using HYDRUS-2D. Soil Res 44(1):27–34. https://doi.org/10.1071/SR05109
Phogat V, Mahadevan M, Skewes M, Cox JW (2012) Modelling soil water and salt dynamics under pulsed and continuous surface drip irrigation of almond and implications of system design. Irrig Sci 30(4):315–333. https://doi.org/10.1007/s00271-011-0284-2
Ramos TB, Šimůnek J, Gonçalves MC, Martins JC, Prazeres A, Pereira LS (2012) Two-dimensional modeling of water and nitrogen fate from sweet sorghum irrigated with fresh and blended saline waters. Agric Water Manag 111:87–104. https://doi.org/10.1016/j.agwat.2012.05.007
Rassam DW, Cook FJ (2002) Numerical simulations of water flow and solute transport applied to acid sulfate soils. J Irrig Drain Eng 128(2):107–115. https://doi.org/10.1061/(ASCE)0733-9437(2002)128:2(107)
Ravikumar V, Vijayakumar G, Šimůnek J, Chellamuthu S, Santhi R, Appavu K (2011) Evaluation of fertigation scheduling for sugarcane using a vadose zone flow and transport model. Agric Water Manag 98(9):1431–1440. https://doi.org/10.1016/j.agwat.2011.04.012
Razzaghi F, Sepaskhah AR (2012) Calibration and validation of four common ETo estimation equations by lysimeter data in a semi-arid environment. Arch Agron Soil Sci 58(3):303–319. https://doi.org/10.1080/03650340.2010.518957
Sepaskhah AR, Ahmadi SH (2012) A review on partial root-zone drying irrigation. Inter J Plant Prod 4(4):241–258
Sepaskhah AR, Tafteh A (2012) Yield and nitrogen leaching in rapeseed field under different nitrogen rates and water saving irrigation. Agric Water Manag 112:55–62. https://doi.org/10.1016/j.agwat.2012.06.005
Shabani A, Sepaskhah AR, Kamgar-Haghighi AA (2013) Responses of agronomic components of rapeseed (Brassica napus L.) as influenced by deficit irrigation, water salinity and planting method. Inter J Plant Prod 7:313–340
Shahrokhnia MH, Sepaskhah AR (2013) Single and dual crop coefficients and crop evapotranspiration for wheat and maize in a semi-arid region. Theor Appl Climat 114(3-4):495–510. https://doi.org/10.1007/s00704-013-0848-6
Shahrokhnia MH, Sepaskhah AR (2016) Effects of irrigation strategies, planting methods and nitrogen fertilization on yield, water and nitrogen efficiencies of safflower. Agric Water Manag 172:18–30. https://doi.org/10.1016/j.agwat.2016.04.010
Shahrokhnia MH, Sepaskhah AR (2017a) Physiologic and agronomic traits in safflower under various irrigation strategies, planting methods and nitrogen fertilization. Indus Crops Prod 95:126–139. https://doi.org/10.1016/j.indcrop.2016.10.021
Shahrokhnia MH, Sepaskhah AR (2017b) Safflower model for simulation of growth and yield under various irrigation strategies, planting methods and nitrogen fertilization. Inter J Plant Prod 11(1):167–192
Simunek J, Hopmans JW (2009) Modeling compensated root water and nutrient uptake. Ecol Model 220(4):505–521. https://doi.org/10.1016/j.ecolmodel.2008.11.004
Simunek J, Sejna M, Saito H, Sakai M, van Genuchten M (2013) The HYDRUS-1D software package for simulating the one-dimensional movement of water, heat, and multiple solutes in variably-saturated media, HYDRUS Software Series, Version 4.16. Department of Environmental Sciences, University of California, Riverside, California
Simunek J, Sejna M, Saito H, Sakai M, van Genuchten MT (2008) The HYDRUS-1D software package for simulating the one-dimensional movement of water, heat, and multiple solutes in variably-saturated media, HYDRUS Software Series, Version 4.0. Department of Environmental Sciences, University of California, Riverside, California
Simunek J, Suarez DL (1993) Modeling of carbon dioxide transport and production in soil: 1. Model development. Water Resou Res 29(2):487–497. https://doi.org/10.1029/92WR02225
Simunek J, van Genuchten MT (1995) Numerical model for simulating multiple solute transport in variably-saturated soils, Water Pollution III: Modelling, Measurement, and Prediction, Computation Mechanics Publication, Ashurst Lodge, Ashurst, Southampton, UK, pp. 21–30
Siyal AA, Bristow KL, Simunek J (2012) Minimizing nitrogen leaching from furrow irrigation through novel fertilizer placement and soil surface management strategies. Agric Water Manag 115:242–251. https://doi.org/10.1016/j.agwat.2012.09.008
Tafteh A, Sepaskhah AR (2012) Application of HYDRUS-1D model for simulating water and nitrate leaching from continuous and alternate furrow irrigated rapeseed and maize fields. Agric Water Manag 113:19–29. https://doi.org/10.1016/j.agwat.2012.06.011
Van Genuchten MT (1980) A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci Soc Am J 44(5):892–898. https://doi.org/10.2136/sssaj1980.03615995004400050002x
Vázquez N, Pardo A, Suso M, Quemada M (2006) Drainage and nitrate leaching under processing tomato growth with drip irrigation and plastic mulching. Agric Ecosyst Environ 112(4):313–323. https://doi.org/10.1016/j.agee.2005.07.009
Yarami N, Sepaskhah AR (2015) Saffron response to irrigation water salinity, cow manure and planting method. Agric Water Manag 150:57–66. https://doi.org/10.1016/j.agwat.2014.12.004
Yau SK, Ryan J (2010) Response of rainfed safflower to nitrogen fertilization under Mediterranean conditions. Indus Crops Prod 32(3):318–323. https://doi.org/10.1016/j.indcrop.2010.05.008
Acknowledgements
This research supported in part by a research project funded by grant no. 96-GR-AGR 42 of Shiraz University Research Council, Drought National Research Institute, the Center of Excellence for On-Farm Water Management, and Iran National Science Foundation (INSF).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Marcus Schulz
Rights and permissions
About this article
Cite this article
Shahrokhnia, M.H., Sepaskhah, A.R. Water and nitrate dynamics in safflower field lysimeters under different irrigation strategies, planting methods, and nitrogen fertilization and application of HYDRUS-1D model. Environ Sci Pollut Res 25, 8563–8580 (2018). https://doi.org/10.1007/s11356-017-1184-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-1184-7