Abstract
The contamination of soil with heavy metals is a major environmental problem worldwide. The combined use of plants and their associated microbes has gained popularity in recent years for their potential to remediate heavy metal-contaminated soil. In the current study, the effect that augmentation of soil with plant growth-promoting endophytes has on the phytostabilization of chromium (Cr)-contaminated soil was investigated. Three potential endophytic bacterial strains (Enterobacter sp. HU38, Microbacterium arborescens HU33, and Pantoea stewartii ASI11) were inoculated individually as well as in combination to Leptochloa fusca and Brachiaria mutica vegetated in Cr-contaminated soil. The accumulation of Cr in the root and shoot of the plants was determined. Moreover, bacterial persistence in the rhizosphere and endosphere was determined. Augmentation with potential endophytes significantly increased root length (24–45%), shoot height (39–64%), chlorophyll content (20–55%), and the overall biomass (32–61%) of the plants. Although L. fusca and B. mutica showed potential to accumulate Cr in their root and shoot, endophytic augmentation increased uptake, translocation, and accumulation of Cr in the roots and shoots of both plant species. However, L. fusca showed more potential to phytostabilize Cr as compared to B. mutica. Furthermore, the potential endophytes showed more survival and persistence within the roots than in the rhizosphere and shoot interior. This study provides useful evidence of endophyte-assisted phytoremediation to be the most sustainable and affordable approach for in situ remediation of Cr-contaminated soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal (HM) pollution of soil and water has become a serious environmental problem worldwide. Environmental pollution due to heavy metals accelerated dramatically since the beginning of the industrial revolution. Heavy metals are non-biodegradable, and thus, persist indefinitely in the soil and water (Sessitsch et al. 2013, Khan et al. 2015, Ashraf et al. 2017). Among HMs, Cr is one of the most toxic elements in the environment arising from discharged effluents of industrial operations such as leather tanning, electroplating, alloy preparation, fabric printing, and crude oil extraction and processing (Ali et al. 2013, Afzal et al. 2014a, Shehzadi et al. 2016). Chromium is the second most common HM contaminating ground water and soil; hence, it poses a serious environmental concern (Lotfy and Mostafa 2014, Nordberg et al. 2014, Song et al. 2017).
To remediate HM-contaminated soil and water, microbe-assisted phytoremediation has proven to be an excellent strategy (Rajkumar et al. 2009, Sessitsch et al. 2013, Khan et al. 2015, Ijaz et al. 2016a). It is an economical and environment friendly approach as compared to conventional remediation techniques (Shanker et al. 2005, Yousaf et al. 2014, Ijaz et al. 2016b, Arslan et al. 2017). Phytostabilization is a mode of action for phytoremediation in which plants immobilize metals in order to minimize the transportation and leaching of contaminants while allowing metal accumulation in their roots with only small amounts of HM being translocated to the shoots (Dary et al. 2010, Korzeniowska et al. 2011, Karczewska et al. 2013). Microbial population is known to affect HM mobility and availability to the plant through release of chelating agents, acidification, phosphate solubilization, redox changes, and N2 fixation (Abou-Shanab et al. 2008, Khan et al. 2013a, Ahemad 2015, Ijaz et al. 2015, Fatima et al. 2016).
Despite the effectiveness of phytoremediation, Cr is a phytotoxic element and may reduce plant growth, induce chlorosis, harm the roots, disrupt photosynthesis, and eventually lead to plant death (Gill et al. 2015, Ashraf et al. 2017). Bacterial endophytes have considerable potential to reverse such adverse effects on plants, thus ensuring unhindered and effective phytoremediation of HM-polluted soils (Wu et al. 2006, Dell’Amico et al. 2008, Lebeau et al. 2008, Sessitsch et al. 2013, Khan et al. 2015). Endophytic bacteria make metals bioavailable and provide protection against toxic effects of HMs using a variety of processes including biotransformation, biosorption, and bioaccumulation (Sessitsch et al. 2013, Wang et al. 2013, Khan et al. 2015). In addition, endophytic bacteria have the potential to fix atmospheric nitrogen, solubilize inorganic phosphate, and release plant growth regulators like gibberellic acid, 1-aminocyclopropane-1-carboxylic acid, siderophores, cytokinin, and indole acetic acid which stimulate plant growth processes and enhance metal accumulation in plants (Lugtenberg and Kamilova 2009, Afzal et al. 2014b, Vejan et al. 2016). Recently, the augmentation of plant growth-promoting endophytes in constructed wetlands improved plant growth and enhanced the removal of Cr and other heavy metals from tannery wastewater (Ashraf et al. 2017). Leptochloa fusca (L.) Kunth (L. fusca) and Brachiaria mutica (Forssk) Stapf (B. mutica) are well-known plant species having well-developed fibrous root systems, large biomass, and long-term growth cycles; they have been used extensively around the world for remediation of stressed soils (Akhter et al. 2004, Mohanty and Patra 2012, Fatima et al. 2016). However, the potential of these plants has not been evaluated for phytoremediation of Cr-contaminated soil. Moreover, the effects of endophytic augmentation on the growth and phytoremediation potential of the plants have not been observed. Therefore, the present investigation aimed to study the effect of endophytic augmentation on phytoremediation of Cr-contaminated soil as well as the survival and persistence of inoculated endophytes were observed in the rhizosphere and endosphere of plants.
Materials and methods
Preparation of Cr-contaminated soil
The soil used in this experiment was an agricultural soil collected from the National Institute for Biotechnology and Genetic Engineering (NIBGE), Faisalabad, Pakistan (soil properties are shown in Table 1). The soil was air dried, milled, and passed through a 2-mm mesh. The sieved soil was mixed with compost (10% w/w) and sieved again for homogeneous mixing.
The soil was artificially contaminated using salt solutions of K2CrO4 in varying concentrations of 50, 100, 200, 400, and 600 mg Cr kg−1. Plastic pots were then filled with the contaminated soil (1.5 kg/pot), whereas one set of pots with uncontaminated soil was kept as control.
Preparation of bacterial strains for soil augmentation
Three bacterial strains, Enterobacter sp. HU38 (NCBI accession number KJ933404), Microbacterium arborescens HU33 (NCBI accession number KJ933403), and Pantoea stewartii ASI11 (NCBI accession number KJ933399) that were isolated from the shoots of Prosopis juliflora (Khan et al. 2015), were used in the present investigation. Based on metal-resistance and plant growth-promoting activities, these bacteria were selected to exploit their potential for phytoremediation of Cr-contaminated soil. All these strains have the ability to produce 1-aminocyclopropane-1-carboxylate (ACC) deaminase, indole-3-acetic acid (IAA), and siderophores along with the ability to solubilize inorganic phosphate (Khan et al. 2015). The bacterial strains were grown in Luria Bertani (LB) broth for 24 h at 28 °C and 150 rpm. Following incubation, bacterial cells were harvested by centrifugation, washed with 0.9% NaCl solution, and re-suspended in the same solution.
Experimental design
Pot experiments were conducted in plastic containers with each treatment and control in triplicate. The experimental design consisted of two plant species used to test five levels of Cr contamination (50, 100, 200, 400, and 600 mg Cr kg−1 in soil) with and without bacterial augmentation and a control. Surface-sterilized cuttings of L. fusca and B. mutica with equal size, length, and weight were planted in agricultural and contaminated soil as per the treatments/control (10 cuttings per pot). Following this, 50 ml bacterial suspension (app. 1010 CFU ml−1) of each strain was used to augment the soil as described earlier (Khan et al. 2013b). The bacterial strains were applied separately and in combination (16.66 ml of each strain) to check their individual as well as combined effects on plant growth and metal accumulation. In the control, 50 ml of 0.9% NaCl solution was added instead of the cell suspension. Distilled water was used for watering the plants on alternate days. Once grown for a week, the plants were thinned to six plants per pot. The pots were placed randomly in the premises of NIBGE, Faisalabad (31° 25′ 45″ N 73° 4′ 44″ E), Pakistan, for a period of 3 months (March–May, 2016) under natural climatic conditions (the average day/night temperatures were 43/33 °C).
Plant and soil analyses
Plants were harvested after 90 days of sowing. Different physiological parameters, such as root and shoot length and biomass, were determined. The harvested plants were brought to the laboratory, washed thoroughly with tap water to remove soil particles, and then rinsed thrice with double distilled water (Qiu et al. 2014, Adediran et al. 2015, Cheng et al. 2015). Plant biomass was dried in an oven at 70 °C for 48 h and dry weight was determined.
Chlorophyll content was estimated in leaves by following Arnon’s method (Arnon 1949) using double beam UV/visible spectrophotometer (Hitachi 557, Hitachi Ltd. Tokyo, Japan). The plant and soil samples were dried in an oven, ground to obtain homogenous mixture, passed through a sieve of 0.5 mm, and digested in a microwave digestion system (Multiwave3000, Anton Paar GmbH Graz, Austria) as described earlier (Brunetti et al. 2012). The metal concentration was quantified by inductively coupled plasma optical emission spectrometer (iCAP6500 ICP-OES, Thermo Scientific, UK). Electrical conductivity and pH of soil were measured using conductivity meter (XL 30, Fisher Scientific Pte Ltd. Singapore) and digital pH meter (781 pH/ion meter, Metrohm Herisau, Switzerland), respectively.
All chemicals used for sample preparation and standard dilutions were of analytical reagent grade. Reagent blank and analytical duplicates were used to ensure precision in the analysis (Jiang et al. 2008). Laboratory NIST certified standards and spikes were used in each batch for validation of ICP-OES results as described earlier (Brunetti et al. 2012).
Enumeration of bacteria
The bacterial population in the endosphere and rhizosphere of L. fusca and B. mutica was estimated by plate count method on LB agar medium having 50 mg L−1 Cr (Afzal et al. 2012). Briefly, the roots and shoots of different treatments were surface sterilized using freshly prepared 70% ethanol and 2% sodium hypochlorite solutions. Surface-sterilized roots and shoots as well as rhizosphere soil were homogenized in 0.9% NaCl solution and shaken at 180 rpm for 30 min. Serial dilutions were prepared after settlement of plant and soil particles, and aliquots (100 μl) were spread on LB agar medium containing 50 mg L−1 Cr. The plates were incubated at 37 °C for 2 days to determine CFU g−1 rhizosphere soil or plant material. Bacterial colonies were picked randomly from all treatments and identity of the isolates with inoculant strains was confirmed by restriction fragment length polymorphism (RFLP) analysis (Andria et al. 2009).
Statistical analysis
For analyzing experimental data, OriginPro 2016 (OriginLab, Northampton, MA) software was used. The ANOVA was applied after conducting Shapiro-Wilk test. The statistical significance was determined using 2-way analysis of variance (ANOVA) and the Bonferroni post hoc test for multiple comparisons between treatments with p ≤ 0.05 was considered significant.
Results
Effect of Cr contamination and bacterial augmentation on plant growth
Plant growth parameters (root and shoot length), chlorophyll content, and dry weight were determined to evaluate the effect of Cr contamination and the effectiveness of bacterial augmentation on the growth and development of L. fusca and B. mutica (Table 2; Figs. 1, 2, 3, 4). As compared to the plants grown in uncontaminated soil, a gradual decrease in growth and development as well as chlorophyll content of both plant species was observed with the increase in Cr concentration in soil (Table 2). Bacterial augmentation increased plant growth and chlorophyll content with maximum plant growth obtained with the application of all three bacterial strains together. In comparison to uninoculated controls, augmentation with bacterial consortium (mixture of Pantoea stewartii ASI11, Microbacterium arborescens HU33, and Enterobacter sp. HU38) increased root length by 34–41% and 24– 45%; shoot height by 42–64% and 39–61%; chlorophyll content by 20–31% and 43–55%; and plant dry weight by 45–61% and 32–49% in L. fusca and B. mutica grown in soil contaminated with 50, 100, and 200 mg Cr kg−1, respectively (Figs. 3 and 4). Both, L. fusca and B. mutica, showed no growth at 400 and 600 mg Cr kg−1.
Effect of chromium (Cr) contamination and endophytes augmentation on growth of Leptochloa fusca vegetated in Cr-contaminated soil. a uncontaminated soil; b soil contaminated with Cr (50 mg kg−1 soil); c soil contaminated with Cr (50 mg kg−1 soil) and augmented with bacterial consortium; d soil contaminated with Cr (100 mg kg−1 soil); e soil contaminated with Cr (100 mg kg−1 soil) and augmented with bacterial consortium; f soil contaminated with Cr (200 mg kg−1 soil); and g soil contaminated with Cr (200 mg kg−1 soil) and augmented with bacterial consortium
Effect of chromium (Cr) contamination and endophytes augmentation on growth of Brachiaria mutica vegetated in Cr-contaminated soil. a uncontaminated soil; b soil contaminated with Cr (50 mg kg−1 soil); c soil contaminated with Cr (50 mg kg−1 soil) and augmented with bacterial consortium; d soil contaminated with Cr (100 mg kg−1 soil); e soil contaminated with Cr (100 mg kg−1 soil) and augmented with bacterial consortium; f soil contaminated with Cr (200 mg kg−1 soil); and g soil contaminated with Cr (200 mg kg−1 soil) and augmented with bacterial consortium
Effect of bacterial augmentation on shoot and root biomass of Leptochloa fusca grown in Cr-amended soils at different contamination level. Significant differences (p < 0.05) were determined using a two-way ANOVA followed by a Bonferroni post hoc test. Treatment groups with at least one common letter are not significantly different from each other. Each error bar represents the standard deviation (SD) of the measurements. Soil was inoculated with 50 ml bacterial suspension (app. 1010 CFU ml−1) of strains S1 (Pantoea stewartii ASI11), S2 (Microbacterium arborescens HU33), and S3 (Enterobacter sp. HU38), and consortium (mixture of these three strains)
Effect of bacterial augmentation on shoot and root biomass of Brachiaria mutica grown in Cr-amended soils at different contamination level. Significant differences (p < 0.05) were determined using a two-way ANOVA followed by a Bonferroni post hoc test. Treatment groups with at least one common letter are not significantly different from each other. Each error bar represents the standard deviation (SD) of the measurements. Soil was inoculated with 50 ml bacterial suspension (app. 1010 CFU ml−1) of strains S1 (Pantoea stewartii ASI11), S2 (Microbacterium arborescens HU33), and S3 (Enterobacter sp. HU38), and consortium (mixture of these three strains)
Effect of endophytic augmentation on Cr accumulation in plant tissues
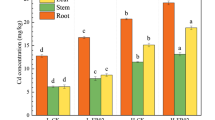
The concentration of Cr in plant tissues (roots, shoots, and leaves) was determined to evaluate the effect of inoculated plant growth-promoting endophytes on Cr uptake, translocation, and accumulation in plant tissues (Table 3). Both plant species showed the potential to take up and accumulate Cr in their roots and shoots. Augmentation with potential endophytes further enhanced metal uptake, translocation, and accumulation in the plants. Among different augmentation treatments, bacterial consortium was found more effective than the application of a single bacterial strain. In contrast to uninoculated plants, plants inoculated with bacterial consortium had increased Cr concentrations in different plant tissues; amounts increased by 34–54% and 30–43% in roots; amounts increased by 31–48% and 26–38% in shoot and by 23–43% and 33–56% in leaves of L. fusca and B. mutica grown in soil containing 50, 100, and 200 mg Cr kg−1 soil, respectively. Plants grown at a higher contamination level of 200 mg Cr kg−1 showed maximum accumulation of Cr 493 and 325 mg kg−1 in roots, 47 and 54 mg kg−1 in shoots, and 27 and 3.2 mg kg−1 in leaves of L. fusca and B. mutica, respectively. The overall accumulation of Cr in plant biomass was found in the order of roots > shoots > leaves.
Bioconcentration and translocation factor
Bioconcentration factor (BCF) and translocation factor (TF) were calculated to estimate Cr accumulation in plant tissues. Although bacterial augmentation increased BCF and TF values, most pronounced effect was observed when endophytes were used in consortium (Table 4). L. fusca and B. mutica augmented with bacterial consortium showed significant (p < 0.05) increase in root BCF values from 3.68 to 5.66 and 1.12 to 1.60; 2.26 to 3.17 and 0.78 to 1.13; and 1.84 to 2.47 and 0.77 to 1.07 at Cr contamination levels of 50, 100, and 200 mg kg−1, respectively. The shoot BCF and TF values were found to be < 1 mg kg−1 in all treatments even after bacterial inoculation. Both plants showed high values for root BCF (> 1), low shoot BCF (< 1), and low TF with least value for TF being < 0.5.
Effect of Cr on bacterial survival and persistence
The survival and persistence of inoculated strains were observed in the rhizosphere and endosphere of L. fusca and B. mutica (Table 5). The inoculated bacterial strains showed persistence in the rhizosphere as well as roots and shoots of both plants; higher numbers of bacteria were observed in cases where bacterial consortium was applied as compared to application of a single bacterial strain. Bacterial survival and persistence decreased with the increase in Cr contamination levels. Maximum number of CFUs was found in the roots of treatment with 50 mg Cr kg−1 soil where L. fusca yielded 894 ± 37 × 104 CFU g−1 dry biomass and B. mutica yielded 462 ± 19 × 104 CFU g−1 dry biomass. More bacterial population was observed within the roots than in shoot and rhizosphere of plants.
Discussion
Heavy metal contamination in soil inhibits plant growth and biomass production (Sessitsch et al. 2013, Khan et al. 2015). In this study, Cr contamination in soil inhibited plant growth, particularly at higher levels of contamination. Similar findings have been reported earlier, whereby HMs including Cr inhibit plant growth and development (Chen et al. 2003, Prapagdee et al. 2013, Lin et al. 2014). However, in this study, inoculated endophytic bacteria were observed to reduce inhibitory effects of Cr by significantly improving growth of plants (Table 2, Figs.1, 2, 3, 4). Bacteria can enhance plant growth and protect plants from abiotic stresses, such as the presence of contamination in soil, through a wide variety of mechanisms including nitrogen fixation, phosphate solubilization, ACC deaminase activity, and production of siderophores and phytohormones (Souza et al. 2015). In this study, the inoculated endophytes possessed several plant growth promotion activities, such as ACC deaminase, siderophore, and IAA production which allowed them to contribute positively towards plant growth and development in the presence of high Cr contamination in soil. Similar findings have already been reported (Lee et al. 2004, Singh et al. 2010, Srivastava et al. 2013). Inoculated bacteria with ACC deaminase activity reduce HM stress by enzymatic hydrolysis of ACC, thereby increasing plant growth by decreasing the amount of ACC and ethylene in contaminated soils (Dell’Amico et al. 2005, Glick et al. 2007, Ma et al. 2011a). IAA produced by inoculated PGP bacteria contributes to plant growth and development by increasing root growth along with elongation of root hair and better absorption of nutrients and metals by plant roots (Taghavi et al. 2009, Prapagdee et al. 2013, Ahemad 2015). Inoculated PGP endophytic bacteria also exhibited potential of producing siderophores (metal chelating agents) that is responsible to increase chlorophyll biosynthesis by making iron available to metal-stressed plants (Ahemad 2015). Siderophores also enhance bioavailability of metals in rhizosphere by their metal binding capacity through complexation reactions (Braud et al. 2009, Ullah et al. 2015). In addition, endophytic bacteria stimulate plant defense mechanisms against pathogens (Nunes da Silva et al. 2014) and act as biofertilizers as well (Vessey 2003, Sheng et al. 2008, Chen et al. 2010).
Bacteria reduce metal toxicity in plants grown in metal-polluted environment by increasing the uptake of trace elements (Sheng et al. 2008). In this study, the application of PGP bacterial isolates individually and in combination showed increase in plant growth and metal uptake. Plants inoculated with different PGP bacterial strains showed better growth as compared to uninoculated plants. Different inoculated bacterial strains affect plant growth and metal accumulation up to different extent, however, bacterial consortium showed maximum performance in enhancing plant growth and metal accumulation, especially in roots of both plant species grown in Cr-contaminated soils (Table 3). The significant (p < 0.05) increase in plant growth as well as metal accumulation observed here may be attributed to the different bacteria present in consortium which work synergistically promoting each other’s beneficial effect (Dary et al. 2010, Ahemad 2015, Kumar et al. 2016).
L. fusca and B. mutica showed potential for phytostabilization of Cr-polluted soils. The BCF values of roots were recorded to be > 1, and of shoots to be < 1, and TF values were < 0.5. The high root BCF values along with low shoot BCF values confirm the phytostabilization potential of both plants, whereas low TF values indicate that Cr is poorly translocated within plants. Plants ability to accumulate more Cr in roots is a natural toxicity response in which Cr becomes immobilized in the vacuoles of root cells (Ramana et al. 2013, Ullah et al. 2015). Plants with high BCF values in the roots and low TF values are suitable for phytostabilization of toxic HMs in the soil (Korzeniowska and Stanislawska-Glubiak 2015). Moreover, bacteria produce phytohormones which increase plant biomass and metal uptake (Glick 2003, Sessitsch et al. 2013).
In addition to various growth-promoting characteristics, inoculant bacteria must have capacity of persistence and re-colonization in polluted environment, which is an important factor for bacterial-assisted phytoremediation. In the current study, the inoculated bacterial strains exhibited survival and persistence in the rhizosphere soil, roots, and shoots of B. mutica (Table 5). Several earlier studies have also reported that endophytic bacteria can colonize rhizosphere and internal tissues of host plants (Ma et al. 2011b, He et al. 2013, Yuan et al. 2014). The highest bacterial population was detected in the roots of L. fusca and B. mutica vegetated in soil with 50 mg Cr kg−1 as compared to the roots of plants vegetated in soil with 200 mg Cr kg−1. This shows that HMs in soil adversely affect survival and persistence of bacteria (Giller et al. 1998). Furthermore, maximum competence, survival, and persistence of inoculated bacteria in endosphere may be attributed to the additional protection provided by plants against the deleterious outer environment. A similar trend of bacterial colonization pattern in the plants’ endosphere was observed by different researchers (Andria et al. 2009, Afzal et al. 2012, Khan et al. 2015). In this study, the effect of endophytic augmentation in phytostabilization of HMs may be credited to the high population of potential endophytes in the rhizosphere and endosphere of plants. In a recent study, augmentation with endophytic bacteria enhanced the growth of L. fusca while aiding in the removal of both organic and inorganic pollutants from the tannery effluent. Moreover, bacterial augmentation decreased toxicity in the effluent as well. Higher number of Cr-resistant bacteria was isolated from the rhizosphere and endosphere of L. fusca inoculated with the endophytes than from uninoculated plants.
The efficacy of plants for phytostabilization of Cr is evaluated not only on the basis of metal accumulation but also the opportunity of gaining appropriate quantity of root biomass (Gołda and Korzeniowska 2016). Although, L. fusca roots accumulated greater amount of Cr (493 ± 23 mg kg−1) than that of B. mutica roots (235 ± 8.9 mg kg−1), the percent increase in total metal accumulation in roots per pot was found higher for B. mutica due to its denser root biomass than that of L. fusca (Figs. 3 and 4).
Conclusions
The study performed here emphasizes the applicability of endophyte-assisted phytoremediation for viable and economical restoration of Cr-contaminated soil and presents the prospects of assessing the efficacy of endophyte-assisted phytoremediation at field-scale studies. Most importantly, we have established that L. fusca and B. mutica can not only survive well in extremely contaminated soil but cleanup it as well. In this way, two entirely new additions have been made in the plant species that are well-known to be useable for remediation of Cr-contaminated lands. Bacterial consortium alleviated the negative effects of Cr contamination on plant growth and increased plant biomass, chlorophyll content, and also increased metal accumulation in L. fusca and B. mutica. Our experiment showed high values of root BCF (> 1) and low values for shoot BCF and TF (< 1) indicating the competency of both plants to tolerate and accumulate Cr in roots with less translocation to aerial parts, thus minimizing the chances of Cr uptake in the food chain.
References
Abou-Shanab RA, Ghanem K, Ghanem N, Al-Kolaibe A (2008) The role of bacteria on heavy-metal extraction and uptake by plants growing on multi-metal-contaminated soils. World J Microbiol Biotechnol 24(2):253–262. https://doi.org/10.1007/s11274-007-9464-x
Adediran GA, Ngwenya BT, Mosselmans JFW, Heal KV, Harvie BA (2015) Mechanisms behind bacteria induced plant growth promotion and Zn accumulation in Brassica juncea. J Hazard Mater 283:490–499. https://doi.org/10.1016/j.jhazmat.2014.09.064
Afzal M, Khan QM, Sessitsch A (2014a) Endophytic bacteria: prospects and applications for the phytoremediation of organic pollutants. Chemosphere 117:232–242. https://doi.org/10.1016/j.chemosphere.2014.06.078
Afzal M, Shabir G, Iqbal S, Mustafa T, Khan QM, Khalid ZM (2014b) Assessment of heavy metal contamination in soil and groundwater at leather industrial area of Kasur, Pakistan. Clean Soil Air Water 42(8):1133–1139. https://doi.org/10.1002/clen.201100715
Afzal M, Yousaf S, Reichenauer TG, Sessitsch A (2012) The inoculation method affects colonization and performance of bacterial inoculant strains in the phytoremediation of soil contaminated with diesel oil. Int J Phytoremediat 14(1):35–47. https://doi.org/10.1080/15226514.2011.552928
Ahemad M (2015) Enhancing phytoremediation of chromium-stressed soils through plant-growth-promoting bacteria. J Genet Eng Biotechnol 13(1):51–58. https://doi.org/10.1016/j.jgeb.2015.02.001
Akhter J, Murray R, Mahmood K, Malik K, Ahmed S (2004) Improvement of degraded physical properties of a saline-sodic soil by reclamation with kallar grass (Leptochloa fusca). Plant Soil 258(1):207–216. https://doi.org/10.1023/B:PLSO.0000016551.08880.6b
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals—concepts and applications. Chemosphere 91(7):869–881. https://doi.org/10.1016/j.chemosphere.2013.01.075
Andria V, Reichenauer TG, Sessitsch A (2009) Expression of alkane monooxygenase (alkB) genes by plant-associated bacteria in the rhizosphere and endosphere of Italian ryegrass (Lolium multiflorum L.) grown in diesel contaminated soil. Environ Pollut 157(12):3347–3350. https://doi.org/10.1016/j.envpol.2009.08.023
Arnon DI (1949) Copper enzyme in isolated chloroplast, polyphenoloxidase in Beta Vulgaris. Plant Physiol 24(1):1–15. https://doi.org/10.1104/pp.24.1.1
Arslan A, Imran A, Khan QM, Afzal M (2017) Plant-bacteria partnerships for the remediation of persistent organic pollutants. Environ Sci Pollut Res 24(5):4322–4336. https://doi.org/10.1007/s11356-015-4935-3
Ashraf S, Afzal M, Naveed M, Shahid M, Zahir ZA (2017): Endophytic bacteria enhance remediation of tannery effluent in constructed wetlands vegetated with Leptochloa fusca. Int J Phytoremediat (in press), 00. https://doi.org/10.1080/15226514.2017.1337072
Braud A, Jézéquel K, Bazot S, Lebeau T (2009) Enhanced phytoextraction of an agricultural Cr- and Pb-contaminated soil by bioaugmentation with siderophore-producing bacteria. Chemosphere 74(2):280–286. https://doi.org/10.1016/j.chemosphere.2008.09.013
Brunetti G, Farrag K, Soler-Rovira P, Ferrara M, Nigro F, Senesi N (2012) The effect of compost and Bacillus licheniformis on the phytoextraction of Cr, Cu, Pb and Zn by three brassicaceae species from contaminated soils in the Apulia region, Southern Italy. Geoderma 170:322–330. https://doi.org/10.1016/j.geoderma.2011.11.029
Chen F, Wang F, Wu F, Mao W, Zhang G, Zhou M (2010) Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance. Plant Physiol Biochem 48(8):663–672. https://doi.org/10.1016/j.plaphy.2010.05.001
Chen YX, He YF, Luo YM, Yu YL, Lin Q, Wong MH (2003) Physiological mechanism of plant roots exposed to cadmium. Chemosphere 50(6):789–793. https://doi.org/10.1016/S0045-6535(02)00220-5
Cheng S-F, Huang C-Y, Lin Y-C, Lin S-C, Chen K-L (2015) Phytoremediation of lead using corn in contaminated agricultural land—an in situ study and benefit assessment. Ecotoxicol Environ Saf 111:72–77. https://doi.org/10.1016/j.ecoenv.2014.09.024
Dary M, Chamber-Perez MA, Palomares AJ, Pajuelo E (2010) “In situ” phytostabilisation of heavy metal polluted soils using Lupinus luteus inoculated with metal resistant plant-growth promoting rhizobacteria. J Hazard Mater 177(1-3):323–330. https://doi.org/10.1016/j.jhazmat.2009.12.035
Dell’Amico E, Cavalca L, Andreoni V (2005) Analysis of rhizobacterial communities in perennial Graminaceae from polluted water meadow soil, and screening of metal-resistant, potentially plant growth-promoting bacteria. FEMS Microbiol Ecol 52(2):153–162. https://doi.org/10.1016/j.femsec.2004.11.005
Dell’Amico E, Cavalca L, Andreoni V (2008) Improvement of Brassica napus growth under cadmium stress by cadmium-resistant rhizobacteria. Soil Biol Biochem 40(1):74–84. https://doi.org/10.1016/j.soilbio.2007.06.024
Fatima K, Imran A, Amin I, Khan QM, Afzal M (2016) Plant species affect colonization patterns and metabolic activity of associated endophytes during phytoremediation of crude oil-contaminated soil. Environ Sci Pollut Res 23(7):6188–6196. https://doi.org/10.1007/s11356-015-5845-0
Gill RA, Zang L, Ali B, Farooq MA, Cui P, Yang S, Ali S, Zhou W (2015) Chromium-induced physio-chemical and ultrastructural changes in four cultivars of Brassica napus L. Chemosphere 120:154–164. https://doi.org/10.1016/j.chemosphere.2014.06.029
Giller KE, Witter E, McGrath SP (1998) Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils. Soil Biol Biochem 30(10-11):1389–1414. https://doi.org/10.1016/S0038-0717(97)00270-8
Glick B, Cheng Z, Czarny J, Duan J (2007) Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur J Plant Pathol 119(3):329–339. https://doi.org/10.1007/s10658-007-9162-4
Glick BR (2003) Phytoremediation: synergistic use of plants and bacteria to clean up the environment. Biotechnol Adv 21(5):383–393. https://doi.org/10.1016/S0734-9750(03)00055-7
Gołda S, Korzeniowska J (2016) Comparison of phytoremediation potential of three grass species in soil contaminated with cadmium. Ochrona Srodowiska i Zasobów Naturalnych 27:8–14
He H, Ye Z, Yang D, Yan J, Xiao L, Zhong T, Yuan M, Cai X, Fang Z, Jing Y (2013) Characterization of endophytic Rahnella sp. JN6 from Polygonum pubescens and its potential in promoting growth and Cd, Pb, Zn uptake by Brassica napus. Chemosphere 90(6):1960–1965. https://doi.org/10.1016/j.chemosphere.2012.10.057
Ijaz A, Imran A, Anwar-ul-Haq M, Khan QM, Afzal M (2016b) Phytoremediation: recent advances in plant-endophytic synergistic interactions. Plant Soil 405(1-2):179–195. https://doi.org/10.1007/s11104-015-2606-2
Ijaz A, Iqbal Z, Afzal M (2016a) Remediation of sewage and industrial effluent using bacterially assisted floating treatment wetlands vegetated with Typha domingensis. Water Sci Technol 74(9):2192–2201. https://doi.org/10.2166/wst.2016.405
Ijaz A, Shabir G, Khan QM, Afzal M (2015) Enhanced remediation of sewage effluent by endophyte-assisted floating treatment wetlands. Ecol Eng 84:58–66. https://doi.org/10.1016/j.ecoleng.2015.07.025
Jiang C-Y, Sheng X-F, Qian M, Wang Q-Y (2008) Isolation and characterization of a heavy metal-resistant Burkholderia sp. from heavy metal-contaminated paddy field soil and its potential in promoting plant growth and heavy metal accumulation in metal-polluted soil. Chemosphere 72:157–164
Karczewska A, Lewińska K, Gałka B (2013) Arsenic extractability and uptake by velvetgrass Holcus lanatus and ryegrass Lolium perenne in variously treated soils polluted by tailing spills. J Hazard Mater 262:1014–1021. https://doi.org/10.1016/j.jhazmat.2012.09.008
Khan MU, Sessitsch A, Harris M, Fatima K, Imran A, Arslan M, Shabir G, Khan QM, Afzal M (2015) Cr-resistant rhizo-and endophytic bacteria associated with Prosopis juliflora and their potential as phytoremediation enhancing agents in metal-degraded soils. Front Plant Sci 5:10.3389
Khan S, Afzal M, Iqbal S, Khan QM (2013a) Plant-bacteria partnerships for the remediation of hydrocarbon contaminated soils. Chemosphere 90(0):1317–1332. https://doi.org/10.1016/j.chemosphere.2012.09.045
Khan S, Afzal M, Iqbal S, Mirza MS, Khan QM (2013b) Inoculum pretreatment affects bacterial survival, activity and catabolic gene expression during phytoremediation of diesel contaminated soil. Chemosphere 91(5):663–668. https://doi.org/10.1016/j.chemosphere.2013.01.025
Korzeniowska J, Stanislawska-Glubiak E (2015) Phytoremediation potential of Miscanthus giganteus and Spartina pectinata in soil contaminated with heavy metals. Environ Sci Pollut Res 22(15):11648–11657. https://doi.org/10.1007/s11356-015-4439-1
Korzeniowska J, Stanislawska-Glubiak E, Igras J (2011) Applicability of energy crops for metal phytostabilization of soils moderately contaminated with copper, nickel and zinc. J Food Agric Environ 9:693–697
Kumar M, Mishra S, Dixit V, Kumar M, Agarwal L, Chauhan PS, Nautiyal CS (2016) Synergistic effect of Pseudomonas putida and Bacillus amyloliquefaciens ameliorates drought stress in chickpea (Cicer arietinum L.) Plant Signal Behav 11:e1071004
Lebeau T, Braud A, Jézéquel K (2008) Performance of bioaugmentation-assisted phytoextraction applied to metal contaminated soils. Environ Pollut 153(3):497–522. https://doi.org/10.1016/j.envpol.2007.09.015
Lee S, Flores-Encarnacion M, Contreras-Zentella M, Garcia-Flores L, Escamilla JE, Kennedy C (2004) Indole-3-acetic acid biosynthesis is deficient in Gluconacetobacter diazotrophicus strains with mutations in cytochrome c biogenesis genes. J Bacteriol 186(16):5384–5391. https://doi.org/10.1128/JB.186.16.5384-5391.2004
Lin Y-F, Severing EI, te Lintel Hekkert B, Schijlen E, Aarts MG (2014) A comprehensive set of transcript sequences of the heavy metal hyperaccumulator Noccaea caerulescens. Front Plant Sci 5:261
Lotfy SM, Mostafa AZ (2014) Phytoremediation of contaminated soil with cobalt and chromium. J Geochem Explor 144:367–373. https://doi.org/10.1016/j.gexplo.2013.07.003
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63(1):541–556. https://doi.org/10.1146/annurev.micro.62.081307.162918
Ma Y, Prasad M, Rajkumar M, Freitas H (2011b) Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol Adv 29(2):248–258. https://doi.org/10.1016/j.biotechadv.2010.12.001
Ma Y, Rajkumar M, Luo YM, Freitas H (2011a) Inoculation of endophytic bacteria on host and non-host plants-effects on plant growth and Ni uptake. J Hazard Mater 195:230–237. https://doi.org/10.1016/j.jhazmat.2011.08.034
Mohanty M, Patra HK (2012) Phytoremediation potential of paragrass—an in situ approach for chromium contaminated soil. Int J Phytoremediat 14(8):796–805. https://doi.org/10.1080/15226514.2011.619595
Nordberg GF, Fowler BA, Nordberg M (2014) Handbook on the toxicology of metals. Academic Press, Cambrige
Nunes da Silva M, Mucha AP, Rocha AC, Teixeira C, Gomes CR, Almeida CMR (2014) A strategy to potentiate Cd phytoremediation by saltmarsh plants—Autochthonous bioaugmentation. J Environ Manag 134:136–144. https://doi.org/10.1016/j.jenvman.2014.01.004
Prapagdee B, Chanprasert M, Mongkolsuk S (2013) Bioaugmentation with cadmium-resistant plant growth-promoting rhizobacteria to assist cadmium phytoextraction by Helianthus annuus. Chemosphere 92(6):659–666. https://doi.org/10.1016/j.chemosphere.2013.01.082
Qiu Z, Tan H, Zhou S, Cao L (2014) Enhanced phytoremediation of toxic metals by inoculating endophytic Enterobacter sp. CBSB1 expressing bifunctional glutathione synthase. J Hazard Mater 267:17–20. https://doi.org/10.1016/j.jhazmat.2013.12.043
Rajkumar M, Ae N, Freitas H (2009) Endophytic bacteria and their potential to enhance heavy metal phytoextraction. Chemosphere 77(2):153–160. https://doi.org/10.1016/j.chemosphere.2009.06.047
Ramana S, Biswas AK, Ajay K, Singh AB, Ahirwar NK, Subba Rao A (2013) Potential of rose for phytostabilization of chromium contaminated soils. Indian J Plant Physiol 18(4):381–383. https://doi.org/10.1007/s40502-013-0055-6
Sessitsch A, Kuffner M, Kidd P, Vangronsveld J, Wenzel WW, Fallmann K, Puschenreiter M (2013) The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol Biochem 60(100):182–194. https://doi.org/10.1016/j.soilbio.2013.01.012
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Int 31(5):739–753. https://doi.org/10.1016/j.envint.2005.02.003
Shehzadi M, Fatima K, Imran A, Mirza M, Khan Q, Afzal M (2016) Ecology of bacterial endophytes associated with wetland plants growing in textile effluent for pollutant-degradation and plant growth-promotion potentials. Plant Biosyst 150(6):1261–1270. https://doi.org/10.1080/11263504.2015.1022238
Sheng XF, Xia JJ, Jiang CY, He LY, Qian M (2008) Characterization of heavy metal-resistant endophytic bacteria from rape (Brassica napus) roots and their potential in promoting the growth and lead accumulation of rape. Environ Pollut 156(3):1164–1170. https://doi.org/10.1016/j.envpol.2008.04.007
Singh N, Rai U, Tewari A, Singh M (2010) Metal accumulation and growth response in Vigna radiata L. inoculated with chromate tolerant rhizobacteria and grown on tannery sludge amended soil. Bull Environ Contam Toxicol 84(1):118–124. https://doi.org/10.1007/s00128-009-9875-5
Song B, Zeng G, Gong J, Liang J, Xu P, Liu Z, Zhang Y, Zhang C, Cheng M, Liu Y (2017) Evaluation methods for assessing effectiveness of in situ remediation of soil and sediment contaminated with organic pollutants and heavy metals. Environ Int 105:43–55. https://doi.org/10.1016/j.envint.2017.05.001
Souza R, Ambrosini A, Passaglia LM (2015) Plant growth-promoting bacteria as inoculants in agricultural soils. Genet Mol Biol 38(4):401–419. https://doi.org/10.1590/S1415-475738420150053
Srivastava S, Verma PC, Chaudhry V, Singh N, Abhilash PC, Kumar KV, Sharma N, Singh N (2013) Influence of inoculation of arsenic-resistant Staphylococcus arlettae on growth and arsenic uptake in Brassica juncea (L.) Czern. Var. R-46. J Hazard Mater 262:1039–1047. https://doi.org/10.1016/j.jhazmat.2012.08.019
Taghavi S, Garafola C, Monchy S, Newman L, Hoffman A, Weyens N, Barac T, Vangronsveld J, van der Lelie D (2009) Genome survey and characterization of endophytic bacteria exhibiting a beneficial effect on growth and development of poplar trees. Appl Environ Microbiol 75(3):748–757. https://doi.org/10.1128/AEM.02239-08
Ullah A, Heng S, Munis MFH, Fahad S, Yang X (2015) Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria. Environ Exp Bot 117:28–40. https://doi.org/10.1016/j.envexpbot.2015.05.001
Vejan P, Abdullah R, Khadiran T, Ismail S, Nasrulhaq Boyce A (2016) Role of plant growth promoting rhizobacteria in agricultural sustainability. Molecules 21(5):573. https://doi.org/10.3390/molecules21050573
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255(2):571–586. https://doi.org/10.1023/A:1026037216893
Wang W, Deng Z, Tan H, Cao L (2013) Effects of Cd, Pb, Zn, Cu-resistant endophytic Enterobacter sp. CBSB1 and Rhodotorula sp. CBSB79 on the growth and phytoextraction of Brassica plants in multimetal contaminated soils. Int J Phytoremediat 15(5):488–497. https://doi.org/10.1080/15226514.2012.716101
Wu SC, Cheung KC, Luo YM, Wong MH (2006) Effects of inoculation of plant growth-promoting rhizobacteria on metal uptake by Brassica juncea. Environ Pollut 140:124–135
Yousaf S, Afzal M, Anees M, Malik RN, Campisano A (2014) Ecology and functional potential of endophytes in bioremediation: a molecular perspective. In: Verma VC, Gange AC (eds) Advances in endophytic research. Springer, pp 301–320. https://doi.org/10.1007/978-81-322-1575-2_16
Yuan M, He H, Xiao L, Zhong T, Liu H, Li S, Deng P, Ye Z, Jing Y (2014) Enhancement of Cd phytoextraction by two Amaranthus species with endophytic Rahnella sp. JN27. Chemosphere 103:99–104. https://doi.org/10.1016/j.chemosphere.2013.11.040
Funding
This study was supported by the International Foundation of Science, Sweden, and the Organization for the Prohibition of Chemical Weapons (research grant number W/5104-2), and Higher Education Commission, Government of Pakistan (research grant number 20-3854/R&D/HEC/14).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Ahsan, M.T., Najam-ul-haq, M., Saeed, A. et al. Augmentation with potential endophytes enhances phytostabilization of Cr in contaminated soil. Environ Sci Pollut Res 25, 7021–7032 (2018). https://doi.org/10.1007/s11356-017-0987-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0987-x