Abstract
This study aimed to assess the toxic effect of chronic exposure to cadmium through a metabolomic approach based on ultra-performance liquid chromatography/mass spectrometry (UPLC–MS). Forty male Sprague–Dawley rats were randomly assigned to the following groups: control, low-dose cadmium chloride (CdCl2) (0.13 mg/kg body weight (bw)), middle-dose CdCl2 (0.8/kg bw), and high-dose CdCl2 (4.9 mg/kg bw). The rats continuously received CdCl2 via drinking water for 24 weeks. Rat urine samples were then collected at different time points to establish the metabolomic profiles. Multiple statistical analyses with principal component analysis and partial least squares–discriminant analysis were used to investigate the metabolomic profile changes in the urine samples and screen for potential biomarkers. Thirteen metabolites were identified from the metabolomic profiles of rat urine after treatment. Compared with the control group, the treated groups showed significantly increased intensities of phenylacetylglycine, guanidinosuccinic acid, 4-pyridoxic acid, 4-aminohippuric acid, 4-guanidinobutanoic acid, allantoic acid, dopamine, LysoPC(18:2(9Z,12Z)), and L-urobilinogen. By contrast, the intensities of creatinine, L-carnitine, taurine, and pantothenic acid in the treated groups were significantly decreased. These results indicated that Cd disrupts energy and lipid metabolism. Meanwhile, Cd causes liver and kidney damage via induction of oxidative stress; serum biochemical indices (e.g., creatinine and urea nitrogen) also support the aforementioned results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a highly toxic environmental and industrial pollutant, primarily produced by battery, pigment, alloy manufacturing, electroplating, and fertilizer industries (Naiya et al. 2009; Ognjanović et al. 2008). Cd is a toxic, non-biodegradable metal with a long biological half-life and is non-beneficial to plants, animals, and humans (Järup et al. 2000). The extensive application of Cd inevitably exposes populations to this heavy metal (Lu et al. 2007; Morrison et al. 2008). The major exposure sources are residues from food and water, tobacco smoking, and occupational means. For the non-professional population, food and water residues are the primary exposure route to Cd, which enters the human body through chronic, low-dose oral intake (Adefegha et al. 2015; Jarup 2003). Therefore, the adverse effects of Cd exposure on humans have received increasing concern.
Toxicological studies on Cd have focused on its acute (Wang et al. 2016), short-term (Boets et al. 2012), developmental (Chemek et al. 2016), and reproductive toxicities (Jahan et al. 2014) and carcinogenicity (Hartwig 2010). Many specific in vivo studies on Cd have been recently published. Some studies reported that Cd can alter renal tubular and glomerular functions (Kobayashi et al. 2008), whereas some papers stated that Cd can affect the antioxidant defense system (Kaushik and Kaur 2003). Although Cd toxicity has been thoroughly studied, its toxicity mechanism is not yet completely elucidated. Moreover, only few studies have explored Cd-induced toxicity caused by long-term, low-dose, especially amounting to chronic population exposure dose. Therefore, modern instrumental analysis technique has attracted considerable attention for studying the toxic effects of long-term, low-dose exposure to Cd.
Metabolomics is defined as “the quantitative measurement of dynamic multiparametric metabolic response of living system to pathophysiological stimuli or genetic modification” (Nicholson et al. 1999). All metabolites in biological fluids (typically plasma or urine) can be rapidly detected and interpreted with this technique. This method can also be applied to determine metabolic pathways and toxic effects. Metabolomics has been recently used in toxicology due to its special advantage (Coen et al. 2008). For example, PC-12 cells incubated with CdCl2 for 48 h, the metabolites were profiled by ultra-performance liquid chromatography (UPLC)–mass spectrometry (MS). The results revealed the neurotoxicity mechanism of cadmium in PC-12 cells (Zong et al. 2017). Liquid chromatography (LC), holding the advantages of rapid separation and high resolution and high sensitivity, especially when coupled with MS, has been used as a highly promising method for global metabolite profiling (Theodoridis et al. 2008). Consequently, studying Cd-induced toxicity through metabolomics has attracted incremental attention.
In the current study, urinary metabolomic analysis was conducted to determine whether or not the chronic exposure to Cd at equivalent to the population dietary exposure dose could cause toxicity in rats at body metabolic level. Furthermore, the potential exposure biomarkers and the mechanisms of Cd toxicity were investigated.
Materials and methods
Chemicals and reagents
Cadmium chloride (99.99% purity) was obtained from Sigma-Aldrich (India). Methanol and acetonitrile (HPLC grade) were supplied by Dikma Science and Technology Co. Ltd. (Canada). HPLC grade formic acid was purchased from Beijing Reagent Company (Beijing, China). Leucine enkephalin was purchased from Sigma-Aldrich (St. Louis, MO). Deionized water was filtered using a Milli-Q system (Millipore, Billerica). Assay kits for creatinine (CRE), blood urea nitrogen (BUN), and uric acid (UA) were obtained from Wako Pure Chemical Industries Ltd. (Nagoya, Japan). Kits for superoxide dismutase (SOD), catalase (CAT), L-Glutathione (GSH), and malondialdehyde (MDA) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). 4-Pyridoxic acid (98% purity), creatinine (98% purity), and taurine (99% purity) were purchased from Sigma-Aldrich (St. Louis, MO, US). Pantothenic acid (98% purity), 4-aminohippuric acid (98% purity), and L-carnitine (98% purity) were obtained from MedChemexpress LLC (USA). Allantoic acid (98% purity) was purchased from Toronto Research Chemicals (CA). 4-Guanidinobutanoic acid (98% purity) was obtained from Guangzhou Isun Pharmaceutical Co. Ltd. (Guangzhou, China). Guanidinosuccinic acid (98% purity) was purchased from AlabiotechInc (US). Phenylacetylglycine (98% purity) and dopamine (99% purity) were obtained from XiYa Chemical Technology Co. Ltd. (Chengdu or Shanghai, China).
Animal treatment
A total of 40 male Sprague–Dawley rats (180 ± 20 g) were supplied from Vital Laboratory Animal Technology Co. Ltd. (Beijing, China). They were acclimated for 1 week prior to the experiment. The rats were housed individually in metabolic cages at room temperature (22 ± 2 °C) with 50–60% humidity and under a 12-h light-dark cycle. They were fed with AIN-93 M diets and drinking water was available ad libitum. Water consumption of each animal was recorded every day during the experiment. Rats’ body weights were measured weekly. All experimental procedures were in accordance with the Institute of Zoology Animal and Medical Ethics Committee of Harbin Medical University.
After acclimatization, rats were randomly allocated to four groups (control, low-, middle-, and high-doses, respectively,) according to body weights (10 for each group). The low-dose CdCl2 is 0.13 mg/kg bw, equivalent to the conversion of provisional tolerable monthly intake (PTMI) of Cd noted by Joint FAO/WHO Expert Committee on Food Additives (JECFA) at its 73rd meeting (Sharma 2012), based on 1/720 half-lethal dose (LD50) of CdCl2 (((JECFA) JFWECoFA, 2004); the middle-dose CdCl2 is 0.80 mg/kg bw (based on 1/120 LD50 of CdCl2, according to the no observed adverse effect level (NOAEL) of kidney (Fingerle et al. 1982); the high-dose CdCl2 is 4.9 mg/kg bw (based on 1/20 LD50 of CdCl2). CdCl2 was dissolved in water according to the dose of each treatment group and daily administered to the rats continually for 24 weeks ad libitum. The control group was given normal deionized water. The volume of water given to each rat every day was its average amount of water consumption last week plus 5 ml. Water consumption of each rat was daily recorded during the experiment, and water consumption at each time point showed no significant changes between the treatment group and the control group (p > 0.05) (Table S1).
Sample collection and preparation
Urine samples were collected in the metabolic cages with ice packs for 24 h at each time point (24 h pre-dose, 4, 8, 12, 16, 20, 24 weeks post-dose). Urine samples were centrifuged at 3000 rpm (936 g) for 15 min, and the supernatants were stored at − 80 °C for analysis. Before the sample analysis, urine samples were thawed at 4 °C and then centrifuged at 12,000 rpm (13,201 g) for 10 min. The supernatants were diluted with distilled water at the ratio of 1:3 (vol/vol) and vortexed for metabolomic analysis. Meanwhile, quality control (QC) sample was prepared by blending equal volumes from each of 40 urine samples and was analyzed every 10 urine samples in order to evaluate the system’s stability and performance.
After the experiment, the rats were anesthetized with pentobarbital sodium by intraperitoneal injection; the blood samples were collected from the aorta abdominalis. Serum was obtained by centrifugation at 3000 rpm (936 g) for 15 min. The serum was used for biochemical analysis with a Hitachi 7100 automated biochemical analyzer (Hitachi Co., Japan) to test the level of BUN, UA, and CRE. Subsequently, the kidney was quickly removed and part of the rat kidneys were used to analyze the levels of malondialdehyde (MDA) and the enzymatic antioxidant activities.
Chromatography
Chromatographic separation was conducted by Waters’ ACQUITY UPLC System (Waters Corporation) with an HSS T3 column (100 × 2.1 mm, 1.8 μmi day; Waters Corporation, Milford, MA). The temperatures of the autosampler and column were kept at 4 and 35 °C, respectively. A 2-μl aliquot of each sample solution was injected onto the column and the flow rate was 0.45 ml/min. The mobile phase contained (A) water + 0.1% formic acid and (B) acetonitrile. A gradient elution was applied as follows: 0–2% B for 0–0.5 min, 2–5% B for 0.5–1 min, 5–12% B for 1–2 min, 12–20% B for 2–5 min, 20–32% B for 5–6.5 min, 32–45% B for 6.5–8.5 min, 45–65% B for 8.5–10 min, 65–98% B for 10–11 min, 98% B for 11–12 min, 98–30% B for 12–13 min, 30–2% B for 13–14 min, and 2% B for 14–16 min in the positive and negative ion modes.
Mass spectrometry
Mass spectrometry was conducted using a Xevo G2 Q-TOF mass spectrometer (Waters Crop, Milford, MA) with electrospray ionization (ESI) in positive and negative modes, and in full scan mode from m/z 50–1000 for 0–16 min. The MS parameters were as follows: cone gas (nitrogen, 50 l/h) and desolvation gas (nitrogen, 900 l/h). The source and desolvation temperatures were set at 120 and 450 °C, respectively. The capillary voltage was 0.5 KV both in positive and negative modes, and the cone voltage was 30 KV. To ensure the accurate mass acquisition, a lock mass of leucine enkephalin was utilized via a lock spray interface at a flow rate of 10 μl/min to monitor in the positive ion mode ([M + H]+ = 556.2771) and the negative ion mode ([M − H]− = 554.2615). The lock spray frequency was set at 10 s in the positive ion mode and 15 s in the negative ion mode.
Data processing and metabolite identification
UPLC-MS data was performed using the Progenesis QI software (version 2.1; Waters Corporation, Milford MA). The metabolites were filtered according to one-way analysis of variance (ANOVA), p value < 0.5, and max fold change > 2. Subsequently, the selected data was performed by the EZ info statistical analysis software (version 2.0; UmetricsAB, Umea, Sweden). Prior to multivariate statistical analysis, the data were mean-centered and Pareto-scaled. Principal component analysis (PCA), containing QC samples, to visualize the data, values the reproducibility and quality. Then, the partial least squares–discriminant analysis (PLS-DA) model was employed. To avoid the over-fitting of PLS-DA models, the test with 200 random permutations was performed by the SIMCA-P software (version 12.0; Umetrics AB, Umea, Sweden). The metabolites with the variable importance in the project (VIP) value above 1.0 would be selected.
The selected compounds were imported into the Progenesis QI software for metabolite identification. The identification for metabolites was conducted using the Human Metabolome Database (HMDB) (the tolerance mass was set at 10 ppm or 5 mDa), to multiple ambiguous identifications for each compound. To reduce the false-positive matches, the chromatographic retention behavior and fragmentation information should be also taken into consideration. Finally, metabolites were confirmed by comparing retention time and fragmentation pattern with standard substances. The potential pathways of biomarkers were interpreted by using databases, including HMDB (http://www.hmdb.ca/) and KEEG (http://www.kegg.com/).
Statistical analysis
Statistical analysis was performed using SPSS (version 19.0; Beijing Stats Data Mining Co. Ltd., China). Differences among groups were analyzed by ANOVA or the non-parametric Kruskal–Wallis test; p < 0.05 was considered statistically significant. Receiver operator characteristic (ROC) curve analysis was conducted by SPSS to determine the area under the curve (AUC), used to compare the predictive ability of the metabolites.
Results
Body weight
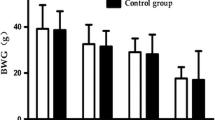
The rat body weights at each time point for each group are shown in Fig. 1. These weights evidently increased in the first 16 weeks and then slowly stabilized in the last 8 weeks. The rat body weights in all experimental groups were not significantly different from those of the time-matched control groups for all time points (p > 0.05).
Biochemical indices
Several serum biochemical indices were measured at the end of the experiment (Table 1). The CRE, UA, and BUN levels significantly increased in the high-dose group (D3; p < 0.01) as compared with those in the control group. No significant changes were observed in the low- and middle-dose groups (D1, D2; p > 0.05) compared with those in the control group.
Antioxidant enzyme activities and MDA levels
The antioxidant enzyme activities and the MDA levels in the rat kidneys are listed in Table 2. The superoxide dismutase (SOD) and catalase (CAT) activities and L-glutathione (GSH) levels were significantly decreased in group D3 compared with those in the control group (p < 0.01). By contrast, the MDA levels significantly increased in this group relative to those of the control group (p < 0.05). No significant difference was observed in groups D1and D2 as compared with group C.
Metabolic profiling
Urine samples from the control and treated animals were analyzed by UPLC–MS in the positive and negative modes to ensure the widest possible detection coverage. The typical base peak intensity chromatograms obtained from the urine analysis in the positive mode are presented in Fig. 2. As shown, the control and high-dose groups only slightly differed with retention time and peak intensity.
The score plots of PCA in the positive and negative modes are displayed in Fig. 3. The QC samples were tightly clustered. Six ions (m/z 124.1338, 213.2280, 314.3894, 379.4930, 424.1794, and 583.8849 in the positive mode) from the chromatographic peaks were selected to validate the reliability of the method. The relative standard deviations (RSDs) of the retention times and m/z and peak intensities were 0–0.91%, 0.04–0.28%, and 0.25–1.31%, respectively. PLS-DA scores in the positive and negative modes are shown in Figs. S1 and 4. The PLS-DA scores of both modes at four different time points (0, 8, 12, and 24 weeks) were created to observe the metabolic changes and characterize the metabolite profiles of the experimental and control samples. The data points of the different groups did not separate from each other at week 0 before administration (Fig. 4a). After 8 weeks of treatment, the data points of the middle- and high-dose groups were separated from those of the control group. However, the data points of the low-dose and control groups were relatively closely contacted (Fig. 4b). After 12 weeks of treatment, the data points of the treated groups were separated from those of the control group, but overlaps existed between the low-dose and control groups (Fig. 4c). After 24 weeks of treatment, the separation of data points became clear among the different groups, and the cluster of each group was reasonably close (Fig. 4d). Permutation tests of the PLS-DA models after 24 weeks of treatment were subsequently conducted in the positive and negative modes. The PLS-DA model parameters, including the R2Y and Q2 values (0.988 and 0.887, respectively, in the positive mode and 0.960 and 0.831, in the negative mode, respectively.) exceeded 0.5, which indicates that the models were appropriate for recognition analysis. The R2 values denoted in green and the Q2 values in blue on the left were lower than the original points on the right (Fig. S2).
PCA score plot of urine obtained from the UPLC/ESI-Q-TOF/MS in the positive and negative modes after 24 weeks treatment. a The positive mode. b The negative mode. Black dot, the control group; red diamond, low-dose group; green star, middle-dose group; blue diagonal cross, high-dose group; orange inverted triangle, quality control group
Thirteen metabolites (11 from the positive mode and 2 from the negative mode) were identified by the aforementioned method (Table 3 and S2). The retention times, measured masses, calculated masses, and elemental compositions of these metabolites are listed in Table 3. The intensities of these metabolites in the positive and negative modes are shown in Table S3. With respect to the control group, the treatment groups exhibited statistically significant changes for some metabolites. Increased levels of phenylacetylglycine (PAG), guanidinosuccinic acid (GSA), 4-pyridoxic acid, 4-aminohippuric acid (PAH), 4-guanidinobutanoic acid (GBA), allantoic acid, and dopamine in the positive mode and LysoPC(18:2(9Z,12Z)) and L-urobilinogen in the negative mode but reduced levels of CRE, L-carnitine, taurine, and pantothenic acid were found in the treated group.
ROC curves were constructed to evaluate the sensitivity and specificity of the potential metabolites. AUC values for the 13 metabolites all exceeded 0.7 at a 95% confidence interval (Fig. S3). This result implies the increased sensitivity and specificity of all identified potential biomarkers.
Discussion
The experiment lasted for 24 weeks. No significant difference in body weight was observed among the control and treated groups at each time point (p > 0.05). This result indicated that the body weight was not affected by exposure to Cd.
In this study, the urine samples were detected by UPLC–Q-TOF–MS. The PCA score plot conducted on the QC and other groups showed that the QC samples were clustered (Fig. 3), as well as the relative standard deviations of extracting six ions, which implied the excellent stability and reproducibility of the metabolomic platform. To facilitate better characterization of the urine metabolite profiles of the treated groups and inspect the systemic metabolic changes with time and dose, a PLS-DA model was established on the basis of three dose groups and a control group (Figs. 4 and S1). The data points of the treated groups gradually deviated from those of the control with the increasing Cd dosage and treatment time. This deviation revealed that the toxic effects of exposure to Cd were dependent on time and dose.
Thirteen metabolites were identified as potential biomarkers of Cd-induced toxicity. Moreover, several indices (serum CRE, UA, and BUN, renal SOD and CAT activities, and GSH and MDA levels) were determined to provide extra support to the metabolomic results. In the current study, the relationship between potential biomarkers and the toxic effects of Cd was discussed through four pathways.
The first pathway involves the metabolism of energy and lipids (Fig. 5). As an important sulfonic amino acid, taurine is essential to normal skeletal muscle function. Taurine can preferentially rescue genes that are related to fatty acid metabolism in the liver or involved in oxidative phosphorylation and tricarboxylic acid (TCA) cycle in skeletal muscles (Mortensen et al. 2010). In the present study, the taurine levels significantly decreased in the high-dose group relative to those in the control group. This result indicated that exposure to Cd can disturb the TCA cycle and fatty acid metabolism. Pantothenic acid is a precursor to coenzyme A and is primarily involved in energy production and lipid metabolism through the TCA cycle and the β-oxidation pathway, respectively (Ferreira et al. 2015). The decrease in pantothenic acid levels in the treated group suggested that exposure to Cd affects the energy metabolism and lipid metabolism of the rats in this study. L-Carnitine is a required nutrient in energy production, and its deficiency can reduce energy availability to vital organs (Thangasamy et al. 2008). L-Carnitine also transports fatty acids into the mitochondria involved in energy production by fat burning related with β-oxidation (Indiveri et al. 2011). In the current study, L-carnitine levels significantly decreased in the treated groups with respect to those in the control group, which indicated that Cd affects the energy and fatty acid metabolism of the rats.
PAG is an acyl glycine and is a normally minor metabolite of fatty acids. Increased amounts of this metabolite in urine have been reportedly associated with mitochondrial fatty acid β-oxidation (Tanaka et al. 1981). In the present work, the augmented PAG levels in the high-dose group relative to those of the control group implied that the exposure to Cd affects the lipid metabolism of the rats. Lysophosphatidylcholine (LPC) is produced through the hydrolysis of phosphatidylcholine (PC) by phospholipase A2 (PLA2). Patients with focal segmental glomerulosclerosis present with increased urinary LPC and decreased PC levels, which signify the enhanced PLA2 activity. Erkan et al. (2016) speculated that intensified PLA2 activity in damaged tubular epithelial cells could augment the urinary concentrations of LPC. LPC is also a major product of low-density lipoprotein (LDL) oxidation. Approximately half of the fatty acids in LDL are polyunsaturated fatty acids that can be oxidized by free radicals (Mertens and Holvoet 2001). In the present study, the LysoPC(18:2(9Z,12Z)) levels were significantly increased in the high-dose group relative to those of the control group. Hence, the exposure to Cd could affect the lipid metabolism of rats.
The second pathway involves oxidative stress (Fig. 5). The antioxidant enzyme defense system (SOD, CAT, and GSH) protects cells against reactive oxygen species (ROS) generation and lipid peroxidation. Oxidative stress plays an important role in Cd-induced toxic effects (Liu et al. 2009). Cd-induced oxidative stress and lipid peroxidation are believed to help suppress the free radical-scavenging function and increase the ROS levels (Lopez et al. 2006; Szuster-Ciesielska, et al 2000). The primary route for Cd toxicity is the depletion of GSH and the binding of Cd to –SH groups of proteins (El-Boshy et al. 2015), which is the most probable cause of GSH level reduction in the Cd-treated group (Table 2). The reduced antioxidant activity (SOD and CAT) and GSH levels and the increased MDA levels in the kidney (Table 2) in the present study implied that the exposure to Cd can increase oxidative injury and lipid peroxidation. Many studies have shown that LPC can effectively promote asymmetric dimethylarginine production and oxidative stress by activating the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Inoue et al. 2001; Takeshita et al. 2000). The augmented LPC level in the high-dose group may be linked to the Cd-induced oxidative stress. Pyridoxal-5′-phosphate (PLP) is the active coenzyme form of vitamin B6, and 4-pyridoxic acid is derived from the degradation of PLP in urine. Some studies have suggested that vitamin B6 deficiency weakens the antioxidant defense system and elevates the oxidative stress in rat liver tissues (Taysi 2005). Increased 4-pyridoxic acid levels can reflect the decreased vitamin B6 levels. The elevated 4-pyridoxic acid level in the current study suggested that Cd can influence the antioxidant defense system. Allantoinase catalyzes the hydrolysis of allantoin to allantoic acid. Human allantoin is a reliable biomarker for oxidative stress, and its diminished levels in the urine are associated with oxidative stress (Feng et al. 2012; Yardim-Akaydin et al. 2006). In the present study, the increased allantoic acid levels were detected and indicated that Cd can induce oxidative stress.
The third pathway is related to kidney and liver function (Fig. 5). The kidney maintains the stability of the internal chemical environment in the body. PAH levels in the human saliva and urine are monitored in clinical laboratories to detect renal diseases. The PAH level in the urine samples collected from patients with renal disease is several times higher than that collected from healthy volunteers (Guan et al. 2005). It was reported that exogenous substances induced renal proximal tubule cell membrane functional injury, thus leading to PAH transport impairment and changes of PAH level, which mainly attributed to exogenous substance-induced oxidative stress (Cojocel and Thomson 2004). GBA and GSA are guanidino compounds (GCs); the latter is considered as a candidate of uremic toxin. The levels of GSA and other GCs are significantly increased in the serum, urine, and different brain regions of patients with renal failure (De Deyn et al. 2001). It was reported that GCs can increase the production of ROS from chronic kidney disease, leading to disturbances in renal metabolism and function (Levillain et al. 2001; Schepers et al. 2010). In the present study, the PAH, GSA, and GBA levels were significantly increased in the high-dose group compared with those in the control group. These results suggested that exposure to Cd could induce kidney injury. The changes in the parameters of routine kidney function (CRE, UA, and BUN in rat serum; Table 1) further supported the aforementioned results. GSH acts as a cofactor for conjugation reactions, which represent a major route for detoxification of heavy metals (Lee et al. 2014). In the present study, GSH level was significantly decreased in the kidney (Table 2). The result further indicated that redox metabolomic pathway may be associated with Cd-mediated chronic kidney injury. This result is consistent with those of Lee et al.
The liver is a vital organ that plays a key role in metabolism and detoxification. Urobilinogen is the end-product of bilirubin. Patients with liver disease will have an elevated urobilinogen level (Roberts, 2007). The protective effect of antioxidant bilirubin in liver disease had been described (Neimark and LeLeiko 2009). In the current study, the augmented urobilinogen level may reflect decreased bilirubin level, which is related to Cd-induced oxidative stress. The liver also plays a vital function in lipid metabolism. Patients with severe liver disease suffer from lipid metabolism disturbances (El-Kabbany et al. 2014). In the current work, PAG and LysoPC(18:2(9Z,12Z)) levels were significantly elevated in the high-dose group, which suggested that exposure to Cd can affect lipid metabolism and further induce liver damage. Cd-induced oxidative stress plays an important role in the liver damage. In our previous published article, GSH level and CAT and SOD activities were significantly decreased, while lipid preoxidation (MDA) was increased in the liver. Regarding liver markers, the increased activities of AST and ALT in serum (Hu et al. 2017) also further supported the metabolomic results.
The fourth pathway is related to the nervous system (Fig. 5). Dopamine (DA) is a catecholamine that serves as a neurotransmitter in the central and peripheral nervous systems. DA is synthesized by dopaminergic neurons, which are critically involved in different vital central nervous system functions, such as sleep, attention, memory, and learning. However, evidence on DA-induced cellular damage has accumulated mainly from studies on the central nervous system (Haque et al. 2003). Urinary DA levels are increased in patients with Parkinson’s disease. In the present study, the increased DA levels in the middle- and high-dose groups suggested that the exposure to Cd can affect the nervous system. Some studies showed that GSA acting as an N-methyl-D-aspartate receptor agonist and a gamma-aminobutyric acid receptor antagonist was suggested to be an important neurotoxin (Torremans et al. 2005). In addition, high GBA levels in the urine have been reportedly related to neurotoxicity (Wang et al. 2015). In the current study, the increased GBA and GSA levels in rat urine implied that the exposure to Cd can affect the nervous system of rats.
Metal toxic effects are usually dependent on dose and time. Similarly, the data points of the treated groups in the PLS-DA model revealed that the toxic effects of exposure to Cd were dependent on time and dose. Notably, four new candidate biomarkers of Cd exposure were found in rat urine in this study. Among these biomarkers, GAS, GBA, and DA are associated with kidney function and the nervous system. PAH, as a typical biomarker of kidney function, was detected in the rat urine. These findings suggested that the toxic effect of chronic low-dose exposure to Cd differs from that of high-level, short-term exposure (Lee et al. 2014).
Conclusions
In conclusion, metabolomics was applied to study the metabolite profile of rats after chronic exposure to Cd. The chronic exposure to Cd at an equivalent to population exposure dose exerted no toxic effect on rats at the body metabolic level. Results indicated that Cd can disrupt rat metabolism (such as energy and lipid metabolism) and induce oxidative stress and liver and kidney damage. Further studies will be conducted to obtain tissue metabolic profiles and increase the comprehensive understanding of the toxic effects of Cd.
References
(JECFA) JFWECoFA (2004) Cadmium (addendum). INCHEM. http://www.inchem.org/documents/jecfa/jecmono/v52je22.htm. Accessed 15 Jan 2016
Adefegha SA, Omojokun OS, Oboh G (2015) Modulatory effect of protocatechuic acid on cadmium induced nephrotoxicity and hepatoxicity in rats in vivo. Spring 4(1):619. https://doi.org/10.1186/s40064-015-1408-6
Boets P, Lock K, Goethals PL, Janssen CR, De Schamphelaere KA (2012) A comparison of the short-term toxicity of cadmium to indigenous and alien gammarid species. Ecotoxicology 21(4):1135–1144. https://doi.org/10.1007/s10646-012-0868-5
Chemek M, Mimouna SB, Boughammoura S, Delbes G, Messaoudi I (2016) Protective role of zinc against the toxicity induced by exposure to cadmium during gestation and lactation on testis development. Reprod Toxicol 63:151–160. https://doi.org/10.1016/j.reprotox.2016.06.005
Coen M, Holmes E, Lindon JC, Nicholson JK (2008) NMR-based metabolic profiling and metabonomic approaches to problems in molecular toxicology. Chem Res Toxicol 21(1):9–27. https://doi.org/10.1021/tx700335d
Cojocel C, Thomson MS (2004) Protective effect of resveratrol against 6-hydroxydopamine-induced impairment of renal p-aminohippurate transport. Arch Toxicol 78(9):525–532. https://doi.org/10.1007/s00204-004-0566-z
De Deyn PP, D’Hooge R, Van Bogaert PP, Marescau B (2001) Endogenous guanidino compounds as uremic neurotoxins. Kidney Int Suppl 78:S77–S83. https://doi.org/10.1046/j.1523-1755.2001.59780077.x
El-Boshy ME, Risha EF, Abdelhamid FM, Mubarak MS, Hadda TB (2015) Protective effects of selenium against cadmium induced hematological disturbances, immunosuppressive, oxidative stress and hepatorenal damage in rats. J Trace Elem Med Biol 29:104–110. https://doi.org/10.1016/j.jtemb.2014.05.009
El-Kabbany ZA, Hamza RT, Ibrahim SA, Mahmoud NH (2014) Dyslipidemia and hyperinsulinemia in children and adolescents with chronic liver disease: relation to disease severity. Int J Adolesc Med Health 26(2):195–201. https://doi.org/10.1515/ijamh-2013-0302
Erkan E, Zhao X, Setchell K, Devarajan P (2016) Distinct urinary lipid profile in children with focal segmental glomerulosclerosis. Pediatr Nephrol 31(4):581–588. https://doi.org/10.1007/s00467-015-3239-7
Feng Z, Sun X, Yang J, Hao D, Du L, Wang H, Xu W, Zhao X, Sun C (2012) Metabonomics analysis of urine and plasma from rats given long-term and low-dose dimethoate by ultra-performance liquid chromatography-mass spectrometry. Chem Biol Interact 199(3):143–153. https://doi.org/10.1016/j.cbi.2012.07.004
Ferreira DW, Naquet P, Manautou JE (2015) Influence of vanin-1 and catalytic products in liver during normal and oxidative stress conditions. Curr Med Chem 22(20):2407–2416. https://doi.org/10.2174/092986732220150722124307
Fingerle H, Fischer G, Classen HG (1982) Failure to produce hypertension in rats by chronic exposure to cadmium. Food Chem Toxicol 20(3):301–306. https://doi.org/10.1016/S0278-6915(82)80296-2
Guan Y, Wu T, Ye J (2005) Determination of uric acid and p-aminohippuric acid in human saliva and urine using capillary electrophoresis with electrochemical detection: potential application in fast diagnosis of renal disease. J Chromatogr B Analyt Technol Biomed Life Sci 821(2):229–234. https://doi.org/10.1016/j.jchromb.2005.03.049
Haque ME, Asanuma M, Higashi Y, Miyazaki I, Tanaka KI, Ogawa N (2003) Apoptosis-inducing neurotoxicity of dopamine and its metabolites via reactive quinone generation in neuroblastoma cells. Biochim Biophys Acta 1619(1):39–52. https://doi.org/10.1016/S0304-4165(02)00440-3
Hartwig A (2010) Mechanisms in cadmium-induced carcinogenicity: recent insights. Biometals 23(5):951–960. https://doi.org/10.1007/s10534-010-9330-4
Hu L, Bo L, Zhang M, Li S, Zhao X, Sun C (2017) Metabonomics analysis of serum from rats given long-term and low-level cadmium by ultra-performance liquid chromatography-mass spectrometry. Xenobiotica 1–10. https://doi.org/10.1080/00498254.2017.1397811
Indiveri C, Iacobazzi V, Tonazzi A, Giangregorio N, Infantino V, Convertini P, Console L, Palmieri F (2011) The mitochondrial carnitine/acylcarnitine carrier: function, structure and physiopathology. Mol Asp Med 32(4-6):223–233. https://doi.org/10.1016/j.mam.2011.10.008
Inoue N, Takeshita S, Gao D, Ishida T, Kawashima S, Akita H, Tawa R, Sakurai H, Yokoyama M (2001) Lysophosphatidylcholine increases the secretion of matrix metalloproteinase 2 through the activation of NADH/NADPH oxidase in cultured aortic endothelial cells. Atherosclerosis 155(1):45–52. https://doi.org/10.1016/S0021-9150(00)00530-X
Järup L, Hellström L, Alfvén T, Carlsson MD, Grubb A, Persson B, Pettersson C, Spång G, Schütz A, Elinder CG (2000) Low level exposure to cadmium and early kidney damage: the OSCAR study. Occup Environ Med 57(10):668–672. https://doi.org/10.1136/oem.57.10.668
Jahan S, Khan M, Ahmed S, Ullah H (2014) Comparative analysis of antioxidants against cadmium induced reproductive toxicity in adult male rats. Syst Biol Reprod Med 60(1):28–34. https://doi.org/10.3109/19396368.2013.843039
Jarup L (2003) Hazards of heavy metal contamination. Br Med Bull 68(1):167–182. https://doi.org/10.1093/bmb/ldg032
Kaushik S, Kaur J (2003) Chronic cold exposure affects the antioxidant defense system in various rat tissues. Clin Chim Acta 333(1):69–77. https://doi.org/10.1016/S0009-8981(03)00171-2
Kobayashi E, Suwazono Y, Honda R, Dochi M, Nishijo M, Kido T, Nakagawa H (2008) Changes in renal tubular and glomerular functions and biological acid-base balance after soil replacement in Cd-polluted rice paddies calculated with a general linear mixed model. Biol Trace Elem Res 124(2):164–172. https://doi.org/10.1007/s12011-008-8125-8
Lee YK, Park EY, Kim S, Son JY, Kim TH, Kang WG, Jeong TC, Kim KB, Kwack SJ, Lee J, Kim S, Lee BM, Kim HS (2014) Evaluation of cadmium-induced nephrotoxicity using urinary metabolomic profiles in sprague-dawley male rats. J Toxicol Environ Health A 77(22-24):1384–1398. https://doi.org/10.1080/15287394.2014.951755
Levillain O, Marescau B, Possemiers I, Al Banchaabouchi M, De Deyn PP (2001) Influence of 72% injury in one kidney on several organs involved in guanidino compound metabolism: a time course study. Pflugers Arch 442(4):558–569. https://doi.org/10.1007/s004240100581
Liu J, Qu W, Kadiiska MB (2009) Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol Appl Pharmacol 238(3):209–214. https://doi.org/10.1016/j.taap.2009.01.029
Lopez E, Arce C, Oset-Gasque MJ, Canadas S, Gonzalez MP (2006) Cadmium induces reactive oxygen species generation and lipid peroxidation in cortical neurons in culture. Free Radic Biol Med 40(6):940–951. https://doi.org/10.1016/j.freeradbiomed.2005.10.062
Lu LT, Chang IC, Hsiao TY, Yu YH, Ma HW (2007) Identification of pollution source of cadmium in soil: application of material flow analysis and a case study in Taiwan. Environ Sci Pollut Res Int 14:49–59
Mertens A, Holvoet P (2001) Oxidized LDL and HDL: antagonists in atherothrombosis. FASEB J 15(12):2073–2084. https://doi.org/10.1096/fj.01-0273rev
Morrison L, Baumann HA, Stengel DB (2008) An assessment of metal contamination along the Irish coast using the seaweed Ascophyllum nodosum (Fucales, Phaeophyceae). Environ Pollut 152(2):293–303. https://doi.org/10.1016/j.envpol.2007.06.052
Mortensen OH, Olsen HL, Frandsen L, Nielsen PE, Nielsen FC, Grunnet N, Quistorff B (2010) A maternal low protein diet has pronounced effects on mitochondrial gene expression in offspring liver and skeletal muscle; protective effect of taurine. J Biomed Sci 17(Suppl 1):S38. https://doi.org/10.1186/1423-0127-17-s1-s38
Naiya TK, Bhattacharya AK, Das SK (2009) Adsorption of Cd(II) and Pb(II) from aqueous solutions on activated alumina. J Colloid Interface Sci 333(1):14–26. https://doi.org/10.1016/j.jcis.2009.01.003
Neimark E, LeLeiko NS (2009) Antioxidant effect of bilirubin and pediatric nonalcoholic fatty liver disease. Pediatrics 124(6):e1240–e1241. https://doi.org/10.1542/peds.2009-2487
Nicholson JK, Lindon JC, Holmes E (1999) ‘Metabonomics’: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 29(11):1181–1189. https://doi.org/10.1080/004982599238047
Ognjanović BI, Marković SD, Pavlović SZ, Zikić RV, Stajn AS, Saicić ZS (2008) Effect of chronic cadmium exposure on antioxidant defense system in some tissues of rats: protective effect of selenium. Physiol Res 57(3):403–411
Roberts JR (2007) Urine dipstick testing: everything you need to know. E M N 29:24–27
Schepers E, Glorieux G, Dou L, Cerini C, Gayrard N, Louvet L, Maugard C, Preus P, Rodriguez-Ortiz M, Argiles A, Brunet P, Cohen G, Jankowski J, Jankowski V, Massy Z, Rodriguez M, Vanholder R, for the European Uremic Toxin Work Group (EUTox) (2010) Guanidino compounds as cause of cardiovascular damage in chronic kidney disease: an in vitro evaluation. Blood Purif 30(4):277–287. https://doi.org/10.1159/000320765
Sharma AK (2012) Evaluation of certain food additives and contaminants, Seventy-third report of the Joint FAO/WHO Expert Committee on Food Additives. Indian J Med Res 136(6):1004–1010
Szuster-Ciesielska A, Stachura A, Słotwińska M, Kamińska T, Śnieżko R, Paduch R, Abramczyk D, Filar J, Kandefer-Szerszeń M (2000) The inhibitory effect of zinc on cadmium-induced cell apoptosis and reactive oxygen species (ROS) production in cell cultures. Toxicology 145(2-3):159–171. https://doi.org/10.1016/S0300-483X(00)00144-X
Takeshita S, Inoue N, Gao D, Rikitake Y, Kawashima S, Tawa R, Sakurai H, Yokoyama M (2000) Lysophosphatidylcholine enhances superoxide anions production via endothelial NADH/NADPH oxidase. J Atheroscler Thromb 7(4):238–246. https://doi.org/10.5551/jat1994.7.238
Tanaka K, Hine DG, West-Dull A, Lynn TB (1981) Gas-chromatographic method of analysis for urinary organic acids. I. Retention indices of 155 metabolically important compounds. Clin Chem 26:1839–1846
Taysi S (2005) Oxidant/antioxidant status in liver tissue of vitamin B6 deficient rats. Clin Nutr 24(3):385–389. https://doi.org/10.1016/j.clnu.2004.12.001
Thangasamy T, Subathra M, Sittadjody S, Jeyakumar P, Joyee AG, Mendoza E, Chinnakkanu P (2008) Role of L-carnitine in the modulation of immune response in aged rats. Clin Chim Acta 389(1-2):19–24. https://doi.org/10.1016/j.cca.2007.11.013
Theodoridis G, Gika HG, Wilson ID (2008) LC-MS-based methodology for global metabolite profiling in metabonomics/metabolomics. TrAC. Trends Anal Chem 27(3):251–260. https://doi.org/10.1016/j.trac.2008.01.008
Torremans A et al (2005) GSA: behavioral, histological, electrophysiological and neurochemical effects. Physiol Behav 84(2):251–264. https://doi.org/10.1016/j.physbeh.2004.12.001
Wang SY, Wang Y, Jin XW, Zhang Y, Chen JS, Ma WW, Wu YH, Wang DC (2015) A urinary metabolomics study of rats after the exposure to acrylamide by ultra performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry. Mol BioSyst 11(4):1146–1155. https://doi.org/10.1039/c4mb00682h
Wang X, Wu M, Ma J, Chen X, Hua L (2016) Modeling of acute cadmium toxicity in solution to barley root elongation using biotic ligand model theory. J Environ Sci (China) 42:112–118. https://doi.org/10.1016/j.jes.2015.06.019
Yardim-Akaydin S, Sepici A, Ozkan Y, Simsek B, Sepici V (2006) Evaluation of allantoin levels as a new marker of oxidative stress in Behcet’s disease. Scand J Rheumatol 35(1):61–64. https://doi.org/10.1080/03009740510026878
Zong L, Xing J, Liu S, Liu Z, Song F (2017) Cell metabolomics reveals the neurotoxicity mechanism of cadmium in PC12 cells. Ecotoxicol Environ Saf 147:26–33. https://doi.org/10.1016/j.ecoenv.2017.08.028
Acknowledgements
The laboratory of nutrition and food hygiene in Harbin Medical University is the key laboratory of Helongjiang Province and Helongjiang Higher Education Institutions.
Funding
Financial support from China National Center for Food Safety Risk Assessment is acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(PDF 667 kb).
Rights and permissions
About this article
Cite this article
Chen, S., Zhang, M., Bo, L. et al. Metabolomic analysis of the toxic effect of chronic exposure of cadmium on rat urine. Environ Sci Pollut Res 25, 3765–3774 (2018). https://doi.org/10.1007/s11356-017-0774-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0774-8