Abstract
Untreated wastewater is a risk factor for the spread of antibiotic resistance in the environment. However, little is known about the contribution of untreated wastewater to the burden of antibiotic resistance in the Nigerian environment. In this study, a total of 143 ceftazidime-/cefpodoxime-resistant bacteria isolated from untreated wastewater and untreated wastewater-contaminated surface and groundwater in Nigeria were screened for extended-spectrum β-lactamase (ESBL) genes, integrons and integron gene cassettes by PCR. The genetic environment of bla CTX-M-15 was mapped by PCR and potentially conjugative plasmids were detected among the isolates by degenerate primer MOB typing (DPMT). ESBL production was confirmed in 114 (79.7%) isolates and ESBL genes (bla SHV, bla CTX-M-15 and bla TEM) were detected in 85 (74.6%) ESBL-producing isolates. bla CTX-M-15 was associated with ISEcp1 and with orf477 in 12 isolates and with ISEcp1, IS26 and orf477 in six others. To the best of our knowledge, this is the first report of bla CTX-M-15 in hand-dug wells and borehole serving as sources of drinking water and a first report of the genetic environment of bla CTX-M-15 in environmental bacteria from Nigeria. The results of this study confirm untreated wastewater as an important medium for the spread of ESBL-producing bacteria within the Nigerian environment. Hence, the widespread practice of discharging untreated wastewater into the aquatic ecosystem in Nigeria is a serious risk to public health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The widespread occurrence of antibiotic resistant bacteria (ARB) and antibiotic resistance genes (ARGs) in the environment is currently a major global public health issue. Previously, the spread of resistance has been associated with selection pressure derived from the clinical use of antibiotics, but recent studies have shown that natural environmental reservoirs contribute significantly to the global proliferation of antibiotic resistance (Canton 2009; Finley et al. 2013). In particular, municipal wastewater treatment systems which concentrate wastewaters containing residual antibiotics, ARB and ARGs of different origins are hotspots of antibiotic resistance and a direct source for the dissemination of ARB and ARGs into the environment (Berendonk et al. 2015; Di Cesare et al. 2016). Hence, several recent studies have investigated the important role played by wastewater and wastewater treatment systems in environmental contamination with ARB and ARGs (LaPara et al. 2011; Munir et al. 2011; Czekalski et al. 2014; Du et al. 2014; Rodriguez-Mozaz et al. 2015). Most of these studies are however carried out in countries with well-established wastewater treatment systems. Studies investigating environmental contamination with ARB and ARGs from wastewater and/or wastewater treatment systems in developing countries including Nigeria are still not very common.
In many developing countries like Nigeria, wastewaters from hospitals and industrial, domestic and agricultural sources are discharged into open sewers, rivers and lakes without prior treatment. This practice will likely create high selection-pressure zones within recipient aquatic ecosystem where residual antibiotics, ARB and ARGs in the untreated wastewater and the resident microflora of the receiving ecosystem are continually mixed. Such ecosystems are likely to act as hotspots for the emergence, proliferation and eventual dissemination of ARB and ARGs into the human population through the water-human route. There is thus an acute need for studies evaluating the contribution of untreated wastewater to environmental contamination with ARB and ARGs in developing countries. Such studies are particularly important because in the absence of wastewater treatment systems as it is presently the case in many developing countries, untreated wastewater remains an important risk factor in environmental contamination with ARB and ARGs.
Presently, one of the mechanisms of resistance reducing the effect of modern antibiotics is the extended-spectrum β-lactamases (ESBLs). ESBL genes are often carried on mobile genetic elements and control the production of enzymes that inactivate cephalosporins via hydrolysis of the β-lactam ring. Beta-lactams are a class of antibiotics accounting for up to 50–70% of the total antibiotic use in several countries of the world (Korzeniewska and Harnisz 2013) including Nigeria (Ogbolu et al. 2013). Worldwide, resistance to β-lactams has been reported in bacterial strains isolated from human clinical sources, animals, food products and the environment (Lupo et al. 2012).
However, very little information exists on the occurrence and spread of ESBLs in the Nigerian aquatic ecosystems. More importantly, few studies, if any, have investigated the role of untreated wastewater as a source of contamination of the Nigerian aquatic ecosystem with ESBL genes. Available studies on ESBLs in Nigeria focused on clinical bacteria isolates (Soge et al. 2006; Ogbolu et al. 2011, 2013). This study therefore investigated the occurrence of ESBL genes in gram-negative bacteria isolated from untreated wastewater and untreated wastewater-impacted aquatic ecosystems in Nigeria. Our primary aim is to assess the possible contribution of untreated wastewater to the contamination of Nigeria’s aquatic ecosystem with clinically relevant ARB and ARGs such as ESBLs.
Materials and methods
Bacterial isolates
A total of 143 bacteria with resistance to ceftazidime (6 μg/mL) or cefpodoxime (6 μg/mL) were included in this study. The bacterial isolates were collected between 2009 and 2013 from six states located in three geo-political regions of Nigeria. The bacteria were collected within the framework of a larger project examining the role of untreated wastewater commonly released into the Nigerian environment from several point and non-point sources as a potential medium for the spread of ARB and ARGs into the Nigerian environment. The project focused on wastewater from three different sources considered of interest to Nigeria, namely (a) hospital sources, (b) domestic sources and (c) aquaculture.
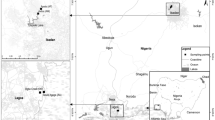
Samples from hospital sources consisted of untreated wastewater and groundwater (from hand-dug wells and boreholes) within the vicinity of the wastewater discharge points of seven hospitals located in Ogun State (n = 5), southwestern Nigeria, and Benue State (n = 2), North Central Nigeria. The Ogun State samples were made up of untreated wastewater and groundwater collected from boreholes and wells between September 2009 and August 2011 while untreated wastewater samples were collected from the two hospitals in Benue State in March and April 2013. Aquaculture samples consisting of effluents from three fish farms located in Ibadan (IBD 1 and IBD 2; with seven and two ponds, respectively) and Ilesha (ILE1 with six ponds) in the southwest of Nigeria were collected in the second and fourth weeks of March 2013. Up till the time of sample collection, oxytetracycline and neocloxin (a mixture of oxytetracycline, chloramphenicol and neomycin) were commonly used in IBD 1 and IBD 2 ponds to treat fish infections while no previous history of antibiotic use was known at ILE 1. Partially treated wastewater from domestic sources was collected from the wastewater oxidation pond at the University of Ibadan, Oyo State, Nigeria. Additionally, water from three polluted rivers receiving untreated wastewater from domestic and industrial sources in the south of Nigeria was also collected. The three rivers were as follows: the Zik River flowing through the Campus of the University of Ibadan in Oyo State, southwest of Nigeria, which receives untreated wastewater from the university’s hostel facilities, and the Ikpoba River and the River Cross located in Edo and Cross River States in the south-south region of Nigeria which receives untreated wastewater from domestic and industrial sources. Samples were collected monthly from the Zik River between August 2012 and February 2013 and from the wastewater oxidation pond in March and April 2013. Samples from Ikpoba and Cross Rivers were collected during an environmental impact assessment trip in December 2012.
Bacteria isolation, antimicrobial susceptibility testing and screening for ESBL production
Bacteria were isolated on Muller Hinton agar (MHA) or Eosin Methylene Blue Agar (EMB) amended with ceftazidime (6 μg/mL) or cefpodoxime (6 μg/mL). Samples were serially diluted in sterile normal saline and aliquots (200 μl) of diluted samples plated on ceftazidime- or cefpodoxime-supplemented EMB and MHA. Non-duplicate colonies of each morphological types observed on the plates after overnight incubation at 35 °C were picked and streaked on fresh plates to obtain pure cultures which were stored frozen in glycerol broth (15%) pending transfer to the Institute of Hydrobiology, Technical University of Dresden, Dresden, Germany, for genotyping. Isolates selected on MHA plates were subjected to gram staining and 3% KOH test (Buck 1982) to confirm them as gram negatives before inclusion in the study. Where two isolates from the same sample selected from EMB and MHA plates showed exactly the same pattern of resistance and identity, only one was included in the study. Bacterial isolates were tested for susceptibility to four antibiotics (Oxoid™): sulphamethoxazole/trimethoprim (SXT, 25 μg), tetracycline (TET, 30 μg), ciprofloxacin (CIP, 5 μg) and ceftazidime (CAZ, 30 μg) by the agar disc diffusion method. Zones of growth inhibition around each disc were measured and interpreted as described by the Clinical and Laboratory Standards Institute (CLSI 2011). Isolates confirmed as showing resistance or intermediate resistance to ceftazidime were selected for double disc synergy test (DDST) (CLSI 2011) to confirm ESBL production.
Bacteria identification
Suspected Escherichia coli (colonies showing greenish metallic sheen on EMB agar) were identified by streaking on chromogenic selective Brilliance™ E. coli/coliform agar while all other isolates, including isolates showing ambiguous colony appearance on Brilliance E. coli/coliform agar, were identified by PCR amplification and sequencing (GATC Biotech, Köln, Germany) of the 16S rDNA (Lane 1991). Sequences obtained were blasted against reference sequences in the GenBank (http://blast.ncbi.nlm.nih.gov/blast.cgi) to identify the bacteria strains. Genomic DNA for 16S rDNA amplification was extracted with the microwave method (Orsini and Romano-Spica 2001).
PCR detection of ESBL genes, integrons and conjugative relaxases
Presumptive ESBL-producing bacteria were screened by standard PCR for the presence of bla TEM , bla SHV , bla CTX-M, ampC gene bla FOX and integrons classes 1, 2 and 3 as described (Pérez-Pérez and Hanson 2002; Mulvey et al. 2003; Machado et al. 2005; Calbo et al. 2011). Gene cassettes were amplified with primers targeting the variable regions of class 1 integrons (Machado et al. 2005). The genetic contexts of detected bla CTX-M were mapped as described by Dihanji et al. (2011). Plasmids of ESBL-producing isolates were extracted with a Nucleospin plasmid purification kit (Qiagen) according to the manufacturer’s instructions. Degenerate primers targeting ten relaxase MOB families MOB F11, F12, P11, P12, P13, P14, P3, P4, H121, and Q11 were used to amplify conjugative relaxase genes as described by Alvarado et al. (2012) using the extracted plasmid DNA as templates. The selected primer pairs targeted relaxases of the plasmid incompatibility groups IncN, IncW, IncP9, IncF complex, Inc9, IncP1 complex, Incl1 complex, IncK, IncB/O, IncL/M, IncQ1, IncQ2, IncP6, IncX, IncA/C and IncU (Alvarado et al. 2012). Representatives of correct size amplicons of all detected genes were sequenced to confirm their identity.
Results
Identification of ESBL-producing bacteria and their antibiotic susceptibility
A total of one hundred and forty-three (143) gram-negative bacteria with reduced susceptibility to ceftazidime or cefpodoxime were isolated from the different samples analysed in this study. DDST screening revealed the production of ESBLs in 114 isolates (79.7%) where 3.5% (n = 4) originated from the oxidation pond, 7.9% (n = 9) from rivers, 40.4% (n = 46) from hospitals and 48.2% (n = 55) from fish farm effluents.
ESBL-producing isolates of hospital/river/domestic wastewater origin were identified as mostly members of the family Enterobacteriaceae, while non-lactose fermenting gram-negative bacteria predominated in the fish farm effluents (Table 1). In the wastewater from the hospitals, the majority of isolates were identified as E. coli and Proteus mirabilis, the water samples from the river/domestic wastewater oxidation pond instead displayed a higher bacterial diversity but E. coli was unrepresented in these samples. In the fish farm effluents, Stenotrophomonas maltophilia dominated among the ESBL-producing bacteria.
All the 114 isolates showed resistance to CAZ while 75, 90, 93.5 and 94.5% of isolates from oxidation pond, rivers, hospital and fish farms were resistant to TET. From the same samples, 75, 66.7, 89 and 52.7% showed resistance to CIP, and 75, 66.7, 93.5 and 11% are resistant to SXT (Fig. 1). Most of the isolates showed resistance to more than one of the tested antibiotics with 95.5, 92.3 and 72.7% of isolates from hospital, river/domestic wastewaters and fish farms displaying a multi-resistance phenotype (Table 1). Interestingly, isolates of Stenotrophomonas from the fish farms showed low levels of resistance to sulphamethoxazole/trimethoprim (SXT) (Fig. 1). This is in contrast to other reports, describing the recent global emergence of resistance to sulphamethoxazole/trimethoprim in species of Stenotrophomonas (Toleman et al. 2007; Huang et al. 2014).
Characterisation of ESBL determinants and their relationship to aquatic habitats
Eighty-five of 114 (74.6%) isolates with reduced susceptibility to ceftazidime and positive in DDST carried at least one of the ESBL genes bla SHV, bla TEM, bla CTX-M included in this study. None of the isolates carried a bla FOX gene (Table 1). bla genes were detected in 40 of 46 (86.9%) isolates from hospital wastewater, 36 of 55 (65.5%) isolates from fish farm ponds and 9 of 13 (69.2%) river/domestic wastewater isolates. Overall, bla SHV was detected in 47 (55.3%) isolates, bla TEM in 39 (45.9%) and bla CTX-M in 18 (21.2%). The majority of the bla CTX-M -positive isolates were detected in the wastewater from hospitals, specifically in Shigella flexnerii from the north central region hospitals and in E. coli, E. hermannii and Shigella sonnei from the hospitals of the southwest region (Table 1).
None of the isolates from fish farms carried bla CTX-M but the gene was present in two isolates (Escherichia fergusonii and Klebsiella pneumoniae subsp. pneumoniae) originating from the Zik River. Interestingly, two ESBL-producing bacteria carrying bla CTX-M (Shigella sonnei and the E. coli) were also isolated from borehole and hand-dug wells near the hospital wastewater collection points in the southwest region, highlighting the potential public health risk associated with the use of these waters for domestic purposes. bla SHV occurred most frequently among isolates from rivers/domestic wastewaters (61.5%) and fish farm ponds (73.8%) but less frequently in the wastewater from hospitals (20%). Sequencing of representative amplicons confirmed the identity of the detected ß-lactam genes. All bla CTX-M and the selected bla TEM amplicons sequenced shared sequence identities (99–100%) with bla CTX-M-15 (accession no: KJ451410.1) and bla TEM-1b (accession no: KF 976462.2) respectively in the GenBank. In contrast, sequencing of seven representative bla SHV amplicons showed they shared 99–100% sequence identities with bla SHV-1, bla SHV-11, bla SHV-12 and bla SHV-121 (GenBank accession no. KC699840.1, GU083599.1, DQ219473.1, KF585135.1, KJ083256.1, NG_050005.1).
Occurrence of multiple ß-lactam determinants in ESBL-producing bacteria
The occurrence of multiple ß-lactam resistance genes was detected in 18.8% of the ESBL-positive isolates (Table 1). bla TEM and bla SHV co-occurred in six isolates from hospital wastewater (Proteus mirabilis n = 5) and Citrobacter freundii isolated from the Zik River. Co-occurrence of bla TEM and bla CTX-M-15 was mainly found in hospital wastewater (E. coli n = 5) and water from neighbouring hand-dug wells (E. coli n = 1) in the southwest region of Nigeria. bla CTX-M-15 and bla SHV occurred together in one E. coli isolated from borehole water also in the southwest of Nigeria. Co-occurrence of the three bla genes was found in three isolates from hospital wastewater (E. coli and E. hermannii) and river water (K. pneumoniae subsp. pneumoniae) from the southwest of Nigeria. No isolate from fish farms carried multiple ß-lactam resistance determinants (Table 1).
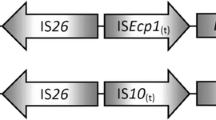
Genetic environment of bla CTX-M-15
The bla CTX-M-15 was flanked upstream and downstream by ISEcp1 and orf477 in 12 isolates similar to the international bla CTX-M-15 genetic environment commonly reported for clinical bacterial strains. In the remaining six isolates, the bla CTX-M-15 was flanked upstream by a combination of ISEcp1 and IS26 and downstream by orf477. The six isolates where bla CTX-M-15 is associated with ISEcp1 and IS26 are from wastewater (E. coli: n = 4), well water (E. coli: n = 1) and borehole water (S. sonnei: n = 1) from Ogun State, southwest Nigeria (Table 2). ISEcp1 has been previously reported upstream of bla CTX-M-15 in bacteria from clinical (Soge et al. 2006; Aibinu et al. 2012; Iroha et al. 2012; Inwezerua et al. 2014; Fortini et al. 2015 and Raji et al. 2015) and food animal sources (Ojo et al. 2016) in Nigeria. However, to the best of our knowledge, this is the first time the genetic environment of bla CTX-M-15 is being reported in bacteria from environmental sources in Nigeria.
PCR-mapping of class 1 integron
Overall, integrons were detected in 54 (63.5%) of the isolates. Int1 was the most prevalent class in hospital and domestic/river water and was associated with the presence of at least one ESBL gene in 46 isolates (Table 1). Integrons were rarely detected in fish farm isolates, being found in 8 of 55 isolates including 5 isolates where none of the tested bla genes was detected. Int2 was detected only in two isolates from hospital wastewater which were also positive for Int1. Int3 was not detected in any isolate in this study. Different sized amplicons were obtained with primers targeting the variable region of class 1 integrons in 23 (42.6%) of class 1 integron-positive isolates, indicating that the remaining isolates are either carrying empty integrons without gene cassettes or are harbouring Tn5090-like class 1 integron lacking the 3′conserved end. The amplicons were randomly assigned to four classes (classes 1–4) based on size of the products and the products sequenced (GATC Biotech, Konstanz, Germany).
Group 1 gene cassettes (ca. 1.5 kb) occurred in 12 isolates with 11 (3 P. mirabilis from Makurdi, 6 E. coli and 1 S. sonnei from Ogun state, and 1 E. fergusonni from the Zik River) sharing 99% sequence identity with dfrA17 and aadA5 on class 1 integron of reference strains in the GenBank (accession no: KX573886.1 and JQ823009.1). The last member of this group found in K. pneumoniae subsp. rhinoscleromatis OZ49 isolated from oxidation pond in Ibadan shared 99% sequence identity with drfA16 and aadA2 present on class 1 integron In36 of E. coli plasmid pHSH1 (AY259085.1). All group 2 gene cassettes (ca. 850 bp) shared 99% sequence identity with dfrA7 on class 1 integron of E. coli G8 (KR952342.1) and Salmonella enterica subsp. enterica serovar Enteritidis D7795 (LN879484.1). Sequences of group 3 cassettes (> 850 bp) obtained from four isolates shared 98–99% identity with aadA1 on class I integrons of S. enterica subsp. enterica serovar Typhimurium (LN794248.1) and E. coli EC4951 (JN814922.1). Only two isolates, P. rettgeri CR4 and CR230, from Cross River carried group 4 (> 1.5 kb) cassette which shared 99% sequence identity with class 1 integron of Aeromonas hydrophila M-X4A containing drfA32, ereA and aadA2 (KJ543558.1) (Table 1).
Conjugative relaxase typing
Plasmid bands were detected in 56 (63.5%) of the ESBL-producing bacteria, more frequently in hospitals and rivers/domestic wastewaters than in fish farm isolates. Twenty-three (23) of the detected plasmids could not be typed with degenerate primer MOB typing (DPMT) described by Alvarado et al. (2012) while PCR using extracted plasmids DNA as template yielded amplicons for 33 isolates. Relaxases of the MOB F12 family were most prevalent and were found in 81.8% of the 33 typable plasmids, while MOB F11 and P11 were found in 24.2 and 21.2% of the isolates respectively (Table 3). All isolates of Stenotrophomonas positive for relaxase genes were from a single fish farm (IBD1) (Table 3). Multiple relaxases were detected in 7 (20.0%) of the isolates, all of which showed multiple plasmid detection bands in gel electrophoresis. Representative amplicons of the DPMT sequenced shared 100% identity with conjugative transfer relaxases TraI (Accession no KF732966.1).
Discussion
Direct discharge of untreated wastewater from domestic, industrial, hospital and agricultural sources into surface waters is a common practice in Nigeria. However, to the best of our knowledge, few studies have evaluated the risk of environmental contamination with ESBL-producing bacteria resulting from such untreated wastewater discharge in Nigeria. In this study, we demonstrated that discharge of untreated wastewater from hospitals and domestic and aquaculture sources is a risk factor for the release of ESBL-producing bacteria into the Nigerian aquatic ecosystem. Only recently, Obasi et al. (2017) reported the detection of ESBL-producing K. pneumoniae in wastewater collected from six pharmaceutical manufacturing industries in southwestern Nigeria. The authors however did not investigate surrounding aquatic ecosystems of the wastewater collection points and only detected ESBL genes in species of Klebsiella. Interestingly, even though sample types are different, we also detected the ESBL genes bla CTX-M-15 (detected in K. pneumoniae subsp. pneumoniae OZ63; E. coli EOd8, EOd11, EOd12, EOd19, EOd20, EOd21, EId3, EId10, EIw10,EAd9, EAd10, EWb3; E. fergusonii OZ24; Ent. hermannii EAd19; S. flexneri MK228 and S. sonnei EAw8) and bla SHV-12 (detected in E. coli EAd15), and the broad spectrum beta-lactamases bla SHV-1 (Ent. asburiae IK42, P. mirabilis MK221, S. maltophilia OZ85), bla SHV-11 (K. pneumoniae subsp. rhinoscleromatis OZ49, S. maltophilia IBD1–108) and bla TEM-1 among our isolates similar to the results of Obasi et al. (2017). A previous study has also detected bla SHV-12 in an Enterobaacter aerogenes isolated from the blood of a 2-year-old patient admitted to a tertiary hospital in southwestern Nigeria (Kasap et al. 2010), the same as the region of the present study and the study of Obasi et al. (2017). However, in contrast to a single species (K. pneumoniae) reported by Obasi et al. (2017), we detected the aforementioned genes in 18 different species of bacteria isolated from untreated wastewater and contaminated surface and groundwater in the proximity of our wastewater collection points indicating possible cross-contamination of the water sources. It has previously been hypothesised that human infections and faecal carriage of ESBL-producing Enterobacteriaceae are a major source of ESBL-producing gram-negative bacteria in wastewaters (Dolejska et al. 2011).
This is the first report of bla CTX-M-15 genes in hospital wastewaters, rivers, wells and boreholes used for domestic purposes in Nigeria with some bacteria isolates of public health importance such as E. coli, C. freundii and K. pneumoniae subsp. pneumoniae carrying additional bla-resistance genes. The presence of bla CTX-M-15 in bacteria from these sources is worrisome since this gene is the most common ESBL in ESBL-producing bacteria causing human infections. Moreover, the genetic environment of the detected bla CTX-M-15 is similar to that commonly found in clinical isolates. The isolation of these bacteria from the untreated wastewater-contaminated aquatic environment is an indication of the important role played by untreated wastewater in the spread of ESBL-producing bacteria into the Nigerian environment. Previous studies have ascribed a role for anthropogenic pollution (Tacão et al. 2012) and especially wastewater effluent (Amos et al. 2014) in the dissemination of bla CTX-M-15 in the aquatic environment. Additionally, the screening of wastewater from different sources showed that different beta-lactamase types dominated the different samples. Bacteria isolated from hospital wastewater and environments polluted with hospital wastewater displayed mostly bla CTX-M-15 and bla TEM genes while isolates from rivers and fish farms were mostly carrying bla SHV genes independent of the geographical location.
More importantly, all bla SHV carriers from the fish farms and one bla SHV carrier each from rivers and wastewater oxidation pond were identified as members of the genus Stenotrophomonas. This gene, which originated in and is mostly found among Enterobacteriaceae causing clinical infections, is now being reported in both Enterobacteriaceae and non-Enterobacteriaceae from different epidemiological settings covering human, animals, food and the environment (Pouget et al. 2013; Maravic et al. 2015; Rocha-Gracia et al. 2015; Zurfluh et al. 2013, 2015; Alcala et al. 2016), thus suggesting a changing epidemiology of this gene. The unusual widespread detection of bla SHV-producing Stenotrophomonas in this study is similar to the detection of bla SHV in S. maltophilia from a recreational lake in Serbia (Novovic et al. 2015). However, while plasmids were detected in 71.4% bla SHV-producing Enterobacteria isolates (n = 14) in this study consistent with the association of the gene with plasmids in Enterobacteriaceae, plasmids were detected in only 18% of bla SHV-producing Stenotrophomonas isolates (n = 33), suggesting that most of the allelic variants detected among the bla SHV-producing Stenotrophomonas may be chromosome associated. Remarkably, the detection and occurrence of bla SHV in Stenotrophomonas in the present study coincided with the first identification of bla SHV gene in S. maltophilia in Nigerian clinical settings (Ogbolu et al. 2013) raising concerns about the role of this bacterium as an opportunistic pathogen (Brooke 2012) and a reservoir of novel antibiotic resistance genes (Tada et al. 2014; Maravic et al. 2014). The presence of multi-resistant S. maltophilia in the fish farms is also worrisome because of the likelihood of its introduction into the food chain and subsequently humans (Ozaktas et al. 2012; Abgottspon et al. 2014).
To our knowledge, this study was the first attempt to use DPMT to investigate plasmid diversity in a polluted aquatic ecosystem. However, conjugative relaxases were detected mostly in isolated members of the Enterobacteriaceae family. Design of specific primers for relaxase characterisation among environmental microbial community is therefore needed before adopting this method in environmental and public health surveillance (Alvarado et al. 2012).
In conclusion, we demonstrated the important role of untreated wastewater as a source of environmental contamination with ESBL-producing bacteria in Nigeria. The widespread occurrence of ESBL producers across multiple bacterial hosts in Nigerian waters though worrisome was however not unexpected, but thus far had not been well documented.
References
Abgottspon H, Nuesch-Inderbinen MT, Zurfluh K et al (2014) Enterobacteriaceae with extended-spectrum- and pAmpC-type betalactamase-encoding genes isolated from freshwater fish from two lakes in Switzerland. Antimicrob Agents Chemother 58:2482–2484
Aibinu I, Pfeifer Y, Peters F, Ogunsola F, Adeonipekun E, Odungbemi T, Koenig W (2012) Emergence of bla CTX-M-15, qnrB1 and acc(6′)-Ib-cr resistance genes in Pantoea agglomerans and Enterobacter cloacae from Nigeria (sub-Saharan Africa). J Med Microbiol 61:165–167
Alcala L, Alonso CA, Simon C, Gonzalez-Esteban C, Oros J, Rezusta A et al (2016) Wild birds, frequent carriers of extended-Spectrum beta-lactamase (ESBL) producing Escherichia coli of CTX-M and SHV-12 types. Microb Ecol 72:861–869
Alvarado A, Garcillan-Barcia MP, de la Cruz F (2012) A degenerate primer MOB typing (DPMT) method to classify gamma-proteobacterial plasmids in clinical and environmental settings. PLoS One 7(7):e40438. https://doi.org/10.1371/journal.pone.0040438
Amos GCA, Hawkey PM, Gaze WH et al (2014) Waste water effluent contributes to the dissemination of CTX-M-15 in the natural environment. J Antimicrob Chemother 69:1785–1791
Berendonk TU, Manaia CM, Merlin C et al (2015) Tackling antibiotic resistance: the environmental framework. Nat Rev Microbiol 13:310–317
Brooke JS (2012) Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev 25:2–41
Buck JD (1982) Nonstaining (KOH) method for determination of gram reactions of marine bacteria. Appl Environ Microbiol 44:992–993
Calbo E, Freixas N, Xercavins M et al (2011) Foodborne nosocomial outbreak of SHV1 and CTX-M-15-producing Klebsiella pneumonia: epidemiology and control. Clin Infect Dis 52:743–749
Canton R (2009) Antibiotic resistance genes from the environment: a perspective through newly identified antibiotic resistance mechanisms in the clinical setting. Clin Microbiol Infect 15(Suppl.I):20–25
Clinical and Laboratory Standards Institute (CLSI) (2011) Performance standards for antimicrobial susceptibility testing: twenty-first informational supplement M100-S21. CLSI, Wayne
Czekalski N, Gascon-Díez E, Bürgmann H (2014) Wastewater as a point source of antibiotic-resistance genes in the sediment of a freshwater lake. ISME J 8:1381e1390
Di Cesare A, Eckert EM, D'Urso S, Bertoni R, Gillan DC, Wattiez R, Corno G (2016) Co-occurrence of integrase 1, antibiotic and metal resistance genes in municipal wastewater treatment plants. Water Res 94:208–214
Dihanji H, Patel R, Wall R, Doumith M, Patel B, Hope R, Livermore DM, Woodford N (2011) Variation in the genetic environments of blaCTX-M-15 in Escherichia coli from the faeces of travellers returning to the United Kingdom. J Antimicrob Chemother 66:1005–1012
Dolejska M, Frolkova P, Florek M, Jamborova I, Purgertova M, Kutilova I, Cizak A, Guenther S, Literak I (2011) CTX-M-15-producing Escherichia coli clone B2-025b-ST131 and Klebsiella spp. isolates in municipal wastewater treatment plant effluents. J Antimicrob Chemother 66:2784–2790
Du J, Ren H, Geng J, Zhang Y, Xu K, Ding L (2014) Occurrence and abundance of tetracycline, sulfonamide resistance genes, and class 1 integron in five wastewater treatment plants. Environ Sci Pollut R 21:7276–7284
Finley RL, Collignon P, Joakim-Larsson DG, McEwen SA, Li XZ, Gaze WH, Smith RR, Timinouni M, Graham DW, Topp E (2013) The scourge of antibiotic resistance and the important role of the environment. CID 57:704–710
Fortini D, Fashae K, Villa L, Feudi C, García-Fernández A, Carattoli A (2015) A novel plasmid carrying bla CTX-M-15 identified in commensal Escherichia coli from healthy pregnant women in Ibadan, Nigeria. J Global Antimicrob Resist 3:9–12
Huang YW, Hu RM, Lin YT et al (2014) Contribution of class 1 integron to antimicrobial resistance in Stenotrophomonas maltophilia. Microb Drug Resist. https://doi.org/10.1089/mdr.2014.0072
Iroha IR, Esimone CO, Neumann S, Marlinghaus L, Koerte M, Szabados F, Gatermann S, Kaase M (2012) First description of Escherichia coli producing CTX-M-15-extended spectrum beta lactamase (ESBL) in out-patients from south eastern Nigeria. Ann Clin Microbiol Antimicrob 11:19. https://doi.org/10.1186/1476-0711-11-19
Inwezerua C, Mendonça N, Calhau V, Domingues S, Adeleke OE, Da Silva GJ (2014) Occurrence of extended-spectrum beta-lactamases in human and bovine isolates of Escherichia coli from Oyo State, Nigeria. J Infect Dev Ctries 8(6):774–779
Kasap M, Fashae K, Torol S, Kolayli F, Budak F, Vahaboglu H (2010) Characterization of ESBL (SHV-12) producing clinical isolate of Enterobacter aerogenes from a tertiary care hospital in Nigeria. Ann Clin Microbiol Antimicrob 2010 9:1. http://www.ann-clinmicrob.com/content/9/1/1
Korzeniewska E, Harnisz M (2013) Extended spectrum beta-lactamase (ESBL)-positive Enterobacteriaceae in municipal sewage and their emission to the environment. J Environ Manag 128:904–911
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrant E, Goodfellov M (eds) Nucleic acids techniques in bacterial systematics. Wiley, Chichester, pp 115–148
LaPara TM, Burch TR, McNamara PJ, Tan DT, Yan M, Eichmiller JJ (2011) Tertiary-treated municipal wastewater is a significant point source of antibiotic resistance genes into Duluth-Superior Harbor. Environ Sci Technol 45(22):9543–9549
Lupo A, Coyne S, Berendonk TU (2012) Origin and evolution of antibiotic resistance: the common mechanisms of emergence and spread in water bodies. Front Microbio 3:18. https://doi.org/10.3389/fmicb.2012.00018
Machado E, Canton R, Baquero F et al (2005) Integron content of extended-spectrum-b-lactamase-producing Escherichia coli strains over 12 years in a single hospital in Madrid, Spain. Antimicrob Agents Chemother 49(5):1823–1829
Maravic A, Skocibusic M, Fredotovic Z et al (2014) Characterisation of environmental CTX-M-15 producing Stenotrophomonas maltophilia. Antimicrob Agents Chemother 58:6333–6334
Maravic A, Skocibusic M, Cvjetan S, Samanic I, Fredotovic Z, Puizina J (2015) Prevalence and diversity of extended-spectrum-beta-lactamase-producing Enterobacteriaceae from marine beach waters. Mar Pollut Bull 90:60–67
Munir M, Wong K, Xagoraraki I (2011) Release of antibiotic resistant bacteria and genes in the effluent and biosolids of five wastewater utilities in Michigan. Water Res 45:681–693
Mulvey MR, Soule G, Boyd D et al (2003) Characterisation of the first extended spectrum beta-lactamse producing Salmonella isolate in Canada. J Clin Microbiol 41:460–462
Novovic K, Filipic B, Veljovic K, Begovic J, Mirkovic N, Jovcic B (2015) Environmental waters and blaNDM-1 in Belgrade, Serbia: endemicity questioned. Sci Total Environ 511:393–398. https://doi.org/10.1016/j.scitotenv.2014.12.072
Obasi A, Nwachukwu SC, Ugoji E, Kholer C, Ghöler A, Balau V, Pfeifer Y, Steinmetz I (2017) Extended-spectrum β-lactamase-producing Klebsiella pneumoniae from pharmaceutical wastewaters in south-western Nigeria. Microb Drug Resist. https://doi.org/10.1089/mdr.2016.0269
Ogbolu DO, Daini OA, Ogunledun A, Oyebode A, Terry-Alli OA, Webber MA (2013) Dissemination of IncF plasmids carrying beta-lactamase genes in gram negative bacteria from Nigerian hospitals. J Infect Dev Ctries 7(5):382–390. https://doi.org/10.3855/jidc.2613
Ogbolu DO, Daini OA, Ogunledun A, Alli AO, Webber MA (2011) High levels of multidrug resistance in clinical isolates of Gram-negative pathogens from Nigeria. Int J Antimicrob Agents 37:62–66
Ojo OE, Schwarz S, Michael GB (2016) Detection and characterization of extended-spectrum-β-lactamase-producing Escherichia coli from chicken production chains in Nigeria. Vet Microbiol 194:62–68
Orsini M, Romano-Spica V (2001) A microwave-based method for nucleic acid isolation from environmental samples. Lett Appl Microbiol 33:17–20
Ozaktas T, Taskin B, Gozen AG (2012) High level multiple antibiotic resistance among fish surface associated bacterial populations in non-aquaculture freshwater environment. Water Res 46:6382–6390. https://doi.org/10.1016/j.watres.2012.09.010
Pérez-Pérez FJ, Hanson ND (2002) Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol 40:2153–2162
Pouget JG, Coutinho FJ, Reid-Smith RJ, Boerlin P (2013) Characterization of blaSHV genes on plasmids from Escherichia coli and Salmonella enterica isolates from Canadian food animals (2006-2007). Appl Environ Microbiol 79:3864–3866
Raji MA, Jamal W, Ojemeh O, Rotimi VO (2015) Sequence analysis of genes mediating extended-spectrum beta-lactamase (ESBL) production in isolates of Enterobacteriaceae in a Lagos Teaching Hospital, Nigeria. BMC Infect Dis 7:215–259
Rodriguez-Mozaz S, Chamorro S, Marti E, Huerta B, Gros M, Sànchez-Melsió A, Borrego CM, Barceló D, Balcázar JL (2015) Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Res 69:234–242
Rocha-Gracia RC, Cortés-Cortés G, Lozano-Zarain P, Bello F, Martínez-Laguna Y, Torres C (2015) Faecal Escherichia coli isolates from healthy dogs harbour CTX-M-15 and CMY-2 β-lactamases. Vet J 203:315–319
Soge OO, Adeniyi BA, Roberts MC (2006) New antibiotic resistance genes associated with CTX-M plasmids from uropathogenic Nigerian Klebsiella pneumoniae. J Antimicrob Chemother 58:1048–1053
Tacão M, Correia A, Henriques I (2012) Resistance to broadspectrum antibiotics in aquatic systems: anthropogenic activities modulate the dissemination of bla CTX-M-like genes. Appl Environ Microbiol 78:4134–4140
Tada T, Miyoshi-Akiyama T, Dahal RK et al (2014) Identification of a novel 6′-N-aminoglycoside aminotransferase, ACC(6′)-lak, from a multidrug resistant clinical isolate of Stenotrophomonas maltophilia. Antimicrob Agents Chemother 58:6324–6327
Toleman MA, Bennett PM, Bennett DMC et al (2007) Global emergence of trimethoprim/sulfamethoxazole resistance in Stenotrophomonas maltophilia mediated by the acquisition of sul genes. Emerg Infect Dis 13:559–565
Zurfluh K, Hachler H, Nuesch-Inderbinen M, Stephan R (2013) Characteristics of extended-spectrum beta-lactamase- and carbapenemase-producing Enterobacteriaceae isolates from rivers and lakes in Switzerland. Appl Environ Microbiol 79:3021–3026
Zurfluh K, Nüesch-Inderbinen M, Morach M, Zihler Berner A, Hächler H, Stephan R (2015) Extended-spectrum-β-lactamase-producing Enterobacteriaceae isolated from vegetables imported from the Dominican Republic, India, Thailand, and Vietnam. Appl Environ Microbiol 81:3115–3120
Acknowledgements
We thank Dr. Dayo Sowunmi of the Department of Zoology, University of Ibadan, Nigeria, for his invaluable assistance in sample collection from Ikpoba and Cross Rivers and Dr. Jochen Müller of the Department of Environmental Biotechnology, Helmholtz Center for Environmental Research, Leipzig, Germany, for his assistance in the course of this project.
Funding
This work was supported by the ANTI-resist (02WRS1272A) Bundesministerium für Bildung und Forschung (BMBF), Germany risk management of new pollutants and pathogens in the water cycle (Riskwa) and the JPI water STARE project. O.O.A. was supported by the German Academic Exchange Service (DAAD) under the University Academics and Scientists Fellowship program during his stay at the Technical University of Dresden.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Diane Purchase
Rights and permissions
About this article
Cite this article
Adelowo, O.O., Caucci, S., Banjo, O.A. et al. Extended Spectrum Beta-Lactamase (ESBL)-producing bacteria isolated from hospital wastewaters, rivers and aquaculture sources in Nigeria. Environ Sci Pollut Res 25, 2744–2755 (2018). https://doi.org/10.1007/s11356-017-0686-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0686-7