Abstract
Biochar is a potential amendment for improving soil fertility due to its richness of nutrients, P, K, Ca, and Mg. However, soil amended with metal-rich biochars may pose a risk of heavy metal release to the environment. Biochars derived from pig manure and sewage sludge (PM-biochar and SS-biochar) were investigated for their nutrient and heavy metal release in two soils (acidic and alkaline soil) under simulated landfill and acid rain conditions. Results showed that under both environmental conditions, adding PM-biochar into the soil increased K, P, and Mg release significantly by about 40–50 times, while only 2–4 times increase of the nutrients was observed in the SS-biochar-amended soil. The Ca release was higher in the SS-biochar-amended soil than in the PM-biochar-amended soil. Higher P, Ca, and Mg nutrient release was observed in alkaline soil than in acidic soil under the two environmental conditions though K release was not significant in both soils. A kinetic study in solution illustrated that the release of nutrients from biochar was initially via desorption and diffusion under environmental conditions and then through slow dissolution of insoluble species. More release of nutrients and heavy metals was observed in the biochar-amended soil under the landfill condition than under the acid rain condition. Although this release was limited under the acid rain condition, leaching of Fe and Mn exceeded the limitations of the groundwater standard value of China. Overall, biochar could be utilized as a prospective soil fertilizer by supplying nutrients such as P, K, Ca, and Mg, while the release of Fe and Mn should be paid more attention due to the risk of these metals impacting groundwater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many studies have demonstrated that biochar could be used for soil amendment with multiple benefits including carbon sequestration, fertility improvement, and pollution control (Cao et al. 2009; Lehmann and Joseph 2009). Plenty of mineral constituents in biochar, especially those derived from manure or sewage sludge, could be a supplement of nutrients’ slow release (Ca, Mg, K, P, etc.) (Cantrell et al. 2012; Liang et al. 2014; Lone et al. 2015). However, soil receiving long-term biochar application may undergo a risk of heavy metal (Zn, Mn, Fe, Cu, Pb, etc.) accumulation, depending on the properties of biochar and soil (Jin et al. 2014; Liesch et al. 2010; Shen et al. 2016). Buss et al. (2016) investigated the suitability of 19 waste biomass-derived biochar for land application. The results showed that biochar with high mineral content could be a beneficial nutrient source, but biochar from biomass grown on potentially toxic element-rich soils tended to exceed guideline values. Before practical application of biochar, assessing its fertility and toxicity simultaneously is necessary. However, the assessment by taking into account the variety of environmental conditions is still lacking.
Bakshi et al. (2014) showed that amendment of hardwood biochar into a contaminated sandy soil increased K and Na leaching, while Ca and Mg were reduced in leachate. The increase of leachate K and Na could be attributed to the high ash content (~ 18.8%) of hardwood biochar, while the decrease in leachate Ca and Mg concentrations might be due to the increased CEC of biochar-amended soils. In another study, Major et al. (2012) showed that leaching of K, Ca, and Mg were all increased by biochar application to a Typic Haplustox clay soil. The increased nutrient leaching resulted from the higher soil solution concentrations and nearly unchanged water flow, since infiltration in the clayey soils may improve not at all with biochar addition. Conclusions about P release affected by biochar were also inconsistent. Leaching of total dissolved P was reduced by 69% when hardwood biochar was added to a manure-amended mesic Typic Hapludolls soil, while considerable increase of available P was observed in Hardsetting soil amended with poultry litter biochar (Chan et al. 2008; Laird et al. 2010). Yuan et al. (2016) reported that sewage sludge biochar produced at 300 °C increased the leaching concentration of PO4 3− by 12.8% in Typic Plinthudult soil. Biochar produced from poultry litter and sewage sludge had higher nutrient values than those produced from plant materials. The higher content of P in poultry litter and sewage sludge biochar may account for the increased P release in amended soil.
Heavy metals are often concentrated in biochars, depending on the source biomass. We previously investigated 20 biochars covering animal manure, wood waste, crop waste, food waste, aquatic plants, and municipal waste and concluded that the maximum input of total concentration-based heavy metals (Pb, Cr, Cu, Zn) was not high, well below the class I limits of China Environmental Quality Standard for Soils (Zhao et al. 2013). However, Wu et al. (2016) pointed out the high concentrations of Pb, Cr, Cu, and Mn from sludge biochars (86.0, 16.3, 575, and 219 mg kg−1, respectively) may impose potential contamination risks on the soils. Wesenbeeck et al. (2014) reported that biochar produced from sewage sludge could not be used for soil amendment because the content of Cr, Zn, and Mo exceeded state soil regulations.
Based on the above inconsistent results, it could be concluded that soil environment and soil properties such as pH, CEC, native minerals in soil, etc., and soil interaction with biochar, all will influence nutrient and heavy metal release. The objective of this study was to investigate the release properties of both nutrients and heavy metals from biochar-amended soils under landfill and acid rain conditions. Pig manure and sewage sludge were chosen for biochar production due to their being rich of minerals according to our previous findings (Zhao et al. 2013). We employed two soils with different properties, Argi-Udic Ferrosols and Ochri-Aquic Cambosols soil which were widespread in southern China and central China, respectively. The landfill condition was simulated by using toxicity characteristic leaching procedure (TCLP), while the acid rain environment was created by applying synthetic precipitation leaching procedure (SPLP). The two procedures were regulated representing the two conditions, respectively, by The United States Environmental Protection Agency (USEPA 1992, 1994). The mechanisms of their release were elucidated by means of solution experiment and kinetics analysis. The risk of heavy metals to groundwater was analyzed by comparing their leaching concentration with Chinese quality standard for groundwater (GB/T 14848-93) (China Ministry of Environmental Protection (CMEP) 1993).
Materials and methods
Biochar production and characterization
The pig manure and sewage sludge were collected from a farm and wastewater treatment plant, respectively, located in Baoshan district, Shanghai City, China. These organic waste samples were air-dried and ground to less than 2 mm. The ground waste was then put into a lab-scale pyrolysis system and heated to 500 °C under N2 atmosphere for 2 h for biochar production (Zhao et al. 2016). The pyrolysis heating rate was employed at 15 °C min−1 and nitrogen gas flows under standard conditions were 200 mL min−1. The produced biochars were ground and passed through a 0.5-mm sieve. For convenience, biochars produced from pig manure and sewage sludge were referred to as PM-biochar and SS-biochar, respectively.
The pH of biochar was determined in a mixture of 1 g biochar and 20 mL deionized water following 1 h equilibrium. Total carbon was measured on an element analyzer (Vario EL III, Elementar). Ash content was determined by weight loss after heating to 500 °C in an O2 atmosphere for 4 h. Cation exchange capacity (CEC) was measured using a modified barium chloride compulsive exchange method (Lee et al. 2010). Metal content was determined in the digested solution of biochar with a strong acid (HNO3 + H2O2) by using the inductively coupled plasma method (ICP-AES, ICAP6000 Radial, Thermo) (USEPA 1986). The pore structure characteristics were measured using a BET-N2 specific surface area (SSA) analyzer (BK122T-B, JWGB, China) according to the standard of the International Union of Pure and Applied Chemistry (IUPAC). The mineral phases of the biochars were detected by X-ray diffraction (XRD) patterns, which were obtained on a Rigaku D/Max-2550 PC (Rigaku Corporation) by scanning at a range from 20° to 35° at 2° per minute.
Soil sampling and characterization
Two soils, Argi-Udic Ferrosols and Ochri-Aquic Cambosols, used in this study were collected from Yingtan City, Jiangxi Province, and Fengqiu City, Henan Province in China, respectively. For simplicity, they were referred to as AUF and OAC soil, respectively. After being air-dried and passed through a 2-mm sieve, the soils were characterized. Soil pH was measured with a soil:water ratio of 1:20 after 1 h of equilibrium. Soil texture was analyzed following the method provided by the American Society for Testing and Materials (American Society for Testing and Materials (ASTM) 2000). Concentrations of nutrients and metals were measured using the same method with biochar.
Release of nutrients and heavy metals from biochar-amended soil
The biochars were well mixed with AUF or OAC soil at a rate of 10% dry weight (Hass et al. 2012; Parvage et al. 2013; Xu et al. 2014). Soil without biochar addition was set as control. Each treatment was incubated for 1 week at a moisture content of 60–70% of water-holding capacity. At the end of the incubation, about 10 g soil samples were collected from each treatment. Then, the soil samples were air-dried and subjected to nutrient and heavy metal release evaluation using the TCLP and SPLP, which simulate landfill and acid rain environmental conditions (USEPA 1992, 1994), respectively.
In detail, 1 g biochar was mixed with 20 mL TCLP extraction fluid#1 (pH = 2.88) or SPLP extraction fluid (pH = 3.20), and then agitated at 60 rpm on a reciprocating shaker. The mixtures were agitated for 18 h and then centrifuged at 3000 rpm for 10 min using a centrifuge (GENIUS 6K-C). The supernatants were filtered through 0.45-mm Millipore filters, and the filtrate solutions were analyzed for nutrient (P, K, Ca, Mg, etc.) and heavy metal (Fe, Mn, Cu, Zn, etc.) concentration.
Mechanism study by solution experiment
To elucidate the mechanisms of element release from the soil-biochar mixture and the biochar contribution, the maximum release and release kinetics of nutrients and heavy metals from biochar solely (without soil) were determined using the same leaching procedures, TCLP and SPLP. The biochar solution was agitated and sampled at 0, 1, 6, 12, 18, 36, and 72 h, respectively. The release curves were simulated using two kinetic models, i.e., pseudo-second-order and pseudo-first-order models.
Results and discussion
Basic properties of biochar and soil
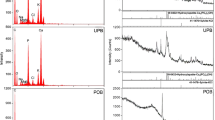
The selected physicochemical properties of PM- and SS-biochars are shown in Table 1. The C content of SS-biochar was about 26.5%, being much lower than that of the PM-biochar, 42.7%. A previous work also showed that biochars produced from municipal waste tended to have lower C than that from animal manure (Zhao et al. 2013). Both PM- and SS-biochars had a high content of Ca (3.5% for PM-biochar, 6.6% for SS-biochar), which was present as calcite CaCO3 (Fig. 1). The relatively higher content of Ca in SS-biochar was probably originated from hydrated lime in wastewater treatment (Netzer et al. 1974). The high content of Mg (2.80%) was detected in PM-biochar, which allowed its association with Ca and P, present as the mineral dolomite (Ca,Mg)(CO3)2 and whitlockite (Ca,Mg)3(PO4)2 (Fig. 1). Fe is abundant in SS-biochar (2.2%) and generally exists as FeOOH (Fig. 1). Overall, PM-biochar contained more nutrients (K, P, and Mg) than SS-biochar, but less heavy metals (Zn and Fe) (Table 1).
SSA of the biochars is not high, being only 47.4 m2 g−1 for PM-biochar and 71.6 m2 g−1 for SS-biochar. But the pore volume and average pore size of the former were a little higher than those of the latter (Table 1). The higher pH of PM-biochar (9.90) than SS-biochar (8.10) might result from the higher content of alkali and alkali earth metals (K, Ca, Mg, etc.) in PM biochar (Table 1). These alkali and alkali earth metals were the main alkaline substances in biochar (Yuan et al. 2011). Besides, the higher content of Fe (2.2%) in SS-biochar than in PM-biochar (0.696%) could hydrolyze to generate H+ in the solution, resulting in the lower pH in SS-biochar than that in PM-biochar.
Two soils with different properties were employed in this study to explore the interaction of soil with biochar components concerning the element release. AUF soil is acidic (pH = 5.05) and rich in Fe (47.0 g kg−1) and Mn (0.67 g kg−1) oxides, while OAC soil is alkaline (pH = 7.77) and contained more K (19.7 g kg−1), P (1.59 g kg−1), Ca (22.3 g kg−1), and Mg (9.62 g kg−1), but less Fe and Mn than the AUF soil (Table 2). OAC soil contained more organic carbon (10.4 g kg−1) and had a higher CEC (12.7 cmol kg−1) than the AUF soil. Both soils are silt loam with silt content of over 55%, especially for OAC soil with the silt content of 71.3%. It was assumed that these soil properties could affect the capacity of metal and nutrient release from biochar amendments.
Nutrient release from biochar-amended soil under landfill and acid rain conditions
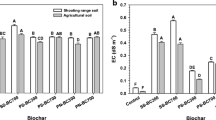
The addition of biochars into soil dramatically increased the release of nutrients, compared to the soil without biochar. Especially, PM-biochar-amended soil showed a much higher K, P, and Mg release (0.89–1.03, 1.57–2.39, and 1.67–2.55 mg g−1, respectively) than SS-biochar-amended soil, which were 0.03–0.10 mg g−1 K, 0.24–0.69 mg g−1 P, and 0.35–1.23 mg g−1 Mg, while Ca release was contrary for the two biochars, i.e., Ca release was higher in the SS-biochar-amended soil than in the PM-biochar-amended soil (Fig. 2). The release trends are consistent with the content of these elements in the two biochars (Table 1), thus biochar, especially PM-biochar, could be a potential supply pool of nutrients for soil. Previous studies also reported similar results (Hass et al. 2012; Laird et al. 2010; Liang et al. 2014).
Release of K from soil under landfill and acid rain conditions did not show any difference in both soils (Fig. 2a). The alkali metal K in biochar is mainly present as soluble KCl salt (Fig. 1) and often occurs in free ion form (K+), and its dissolution is not strongly reliable on the soil acidity or alkalinity. For P, Ca, and Mg, the difference between landfill and acid rain conditions was also not significant in AUF soil (pH = 5.05), but the significant difference was observed in OAC soil (pH = 7.77) (Fig. 2). In the OAC soil, P, Mg, and Ca presented a much higher release under landfill condition than under acid rain condition. This is because the acetic acid in the landfill extraction fluid was readily dissociated into acetate (CH3COO−) in the alkaline OAC soil, which can complex with P, Mg, and Ca from less soluble CaCO3, (Ca,Mg)(CO3)2, or (Ca,Mg)3(PO4)2, thereby enhancing mobility of P, Mg, and Ca. Moreover, OAC soil itself contains high Ca and Mg (Table 2), which could be activated by CH3COO− in landfill condition rather than in acid rain condition (Fig. 2c, d). The release of K and P in control soil without biochar was very low regardless of the leaching conditions (Fig. 2a, b), indicating the large biochar contribution for their release from the soil.
Furthermore, more obvious enhancement was observed in biochar-amended AUF soil than biochar-amended OAC soil under the same conditions. For example, the release of K increased almost 50 times in PM-biochar-amended acid AUF soil compared with soil alone under acid rainfall condition, while in alkaline OAC soil, the release of K only increased about 25 times. It can be seen from Table 2 that the TOC (4.84 g kg−1) and CEC (9.88 cmol kg−1) of AUF soil were lower than those of OAC soil, accounting for the higher K release.
Kinetics and mechanisms of nutrient release from biochar itself
Release kinetic curves showed that biochar nutrients were released rapidly in the initial 1 h, and then slowed down suddenly until the equilibrium was reached after about 36 h (Fig. 3). For example, almost 60% of the total K in PM-biochar was released in the initial 1 h under the landfill condition, accounting for about 80% of the potential maximum released K, while in the acid rain environment, only 10% of the total K was released in the initial 1 h, accounting for about 42% of the potential maximum released K. A previous study also showed the cumulative K in switchgrass biochar released rapidly in water within the first 1 h followed by a more gradual release rate afterwards (Kim et al. 2014).
The release process over time could be divided into “stage I (0–18 h)” and “stage II (18–72 h)”. For all the biochars, nutrient release in stage I fitted the pseudo-second-order kinetic model well with a R 2 being 0.816–1.000 (Table 3), indicating that the release process in stage I was dominated by chemical desorption and diffusion of labile nutrients (Hamdi et al. 2013). This rapid element-detaching reactions also could be attributed to ion exchange or desorption (Mcdowell et al. 2001). Dünisch et al. (2007) ascribed the fast release of nutrients to those being more available on the outer surface of smaller particles where nutrients released faster than those in the pores of larger particles. At stage II, the slow and steady nutrient release was probably due to the dissolution of less soluble minerals such as (Ca,Mg)3(PO4)2 or (Ca,Mg)(CO3)2 (Cao and Harris 2010; Liang et al. 2014), which were presented in biochars (Fig. 1).
Except Ca, the PM-biochar released much more nutrients, accounting for 1.5–54.9% of the total nutrients in biochar, compared with the SS-biochar (0.3–40.4%). This was mainly due to the higher total nutrients in PM-biochar than in SS-biochar (Table 1). The difference in pore size of biochar may be another factor. Our previous study reported that SS-biochar had micropores (< 2 nm) that accounted for about 50% of the pore diameter, while the pore size of PM-biochar was almost distributed in 2–50 nm ranges (Zhao et al. 2014). Nutrients presented in the micropores of SS-biochar were less accessible and therefore released less than those presented in the mesopores of PM-biochar. Higher Ca release in SS-biochar than in PM-biochar resulted from the higher total Ca in biochar (Fig. 3 and Table 1). The release trends of all four nutrients in biochar alone agreed with those in the amended soils, further confirming the dominant contribution of biochar to the nutrient release.
The release of P, K, Ca, and Mg from the biochars under landfill condition was 9.9–54.9%, being much higher than that under acid rain condition (0.3–32.4%). It was because the landfill extraction fluid was mainly composed of CH3COO−, which had high complexation ability with cations, thus enhancing their mobility (Cao and Dermatas 2008; Halim et al. 2004). In addition, acidity of landfill extraction fluid was stronger (pH = 2.80) than that of acid rain extraction fluid (pH = 3.20). Silber et al. (2010) concluded that the release amount of P, Ca, and Mg from biochar was pH-dependent, exhibiting a dramatic increase in released quantities when pH decreased from 8.9 to 4.5.
When biochar was amended into soil, nutrient release behavior must be changed by soil components, and from this change, we could deduce the interaction of soil with biochar. The nutrient release from biochar in soil was obtained by subtracting the release amount of soil control from that of biochar-amended soil. Compared to the biochar in solution, biochar in soil released less K and P under the landfill condition. For example, only 3.9–30.2% of total K and 6.4–56.6% of total P were released in the biochar-amended soil (Fig. 2), being lower than the potential maximum release amount in solution, 9.9–54.9% (K) and 26.6–59.2% (P) in biochar alone, respectively (Fig. 3). Biochar amendment provided more negative charges by introducing the organic groups such as –COOH, –OH, etc., which retained the released K via electrostatic attraction forces (Beesley et al. 2011; Lehmann et al. 2011; Yao et al. 2012; Zheng et al. 2013). With regard to P, the competition between pore sequestration and electrostatic repulsion determined the P retention and release (Yuan et al. 2011, 2016). Electrostatic repulsion generally inhibits the adsorption of PO4 3− to the biochar surface. However, in acidic environment, the electrostatic repulsion was absent; thus, PO4 3− could be adsorbed and sequestrated in the pores, resulting in the lower release ratio of P in acidic AUF soil (6.4–38.3%) than in alkaline OAC soil (19.4–56.6%). However, situation is the opposite in the acid rain environment. The potential maximum release amount of K and P in solution was 2.0–13.7% and 0.3–6.2%, respectively (Fig. 3), which were much lower than those released in soil, 12.6–30.1% of total K and 19.4–46.1% of total P (Fig. 2). The release of Ca and Mg was also enhanced when biochar was added to the two soils. The enhanced nutrient release in soil might result from further release of less soluble minerals such as Ca(CO3) and Ca,Mg(CO3)2 during the relative long-term incubation (1 week in soil vs 72 h in solution), in addition to the initial readily release of Ca and Mg (Fig. 1). Besides, the soil itself contains a certain amount of organic components, which could form coordination complexes with Ca and Mg and enhanced their higher release.
Heavy metal release from biochar-amended soil
The detected heavy metals from biochar-amended soil mainly included Cu, Zn, Fe, and Mn (Fig. 4). Under the landfill condition, high concentrations of Cu (7.0 mg kg−1), Zn (5.5 mg kg−1), and Fe (9.4 mg kg−1) were released in the control AUF soil without biochar addition, while the concentration of Mn was very low, less than 3.0 mg kg−1 (Fig. 4). On the contrary, the control OAC soil released a relatively high concentration of Mn (42.3 mg kg−1), while the release of Cu, Zn, and Fe could be neglected. Here, it could be inferred that the higher content of Fe/Al oxide in acid soil and the low pH were responsible for the release of Cu, Zn, and Fe due to the repulsive force, while the negative charges on the alkaline soil had electrostatic attraction to the cations.
The addition of the PM- and SS-biochar increased the release of all four heavy metals in alkaline OAC soil, compared to the control soil, while the release of Cu and Fe in the biochar-amended acidic AUF soil was greatly decreased (Fig. 4). This further demonstrated that when biochar was added into soil, sorption or desorption of heavy metals depends on the soil properties (Beesley et al. 2010; Shen et al. 2016). It was speculated that for the acid soil (AUF soil), the negative charge on the surface of biochar could form electrostatic attraction with the positive charge of the soil components, which could strengthen the soil adsorption to some cations, while for the alkaline soil (OAC soil), the addition of biochar induced a repulsive force and resulted to the release of more metals.
Many studies proved the ability of biochars in immobilizing heavy metals in contaminated acidic soil (Cao et al. 2009; Cao and Harris 2010; Xu et al. 2013). The decrease of Cu release in acidic AUF soil again proved the strong complexation of organic acid in biochar with Cu (Qin et al. 2004; Wang et al. 2009). According to the theory of “hard-soft-acid-base” by Pearson, Cu2+ is a soft acid with a relatively low electric density and a high ionic radius, which enables it to be the sole metal ion that could form inner layer complexes with organic matter. Under the acid rain condition, biochar addition increased release of all four heavy metals in both soils except Fe. Biochar addition increased Zn release obviously in both soils under two environmental conditions and the landfill environment could release Zn almost 2–4 times than the acid rain environment (Fig. 4b). Zn was a metal of high mobility, especially after the complexation with organic matters in soil.
Release kinetics of heavy metals from biochars was conducted in solution. Although the total concentrations of Cu, Zn, Fe, and Mn in the biochars were as high as 380–780, 1010–1520, 6960–22,100, and 450–1230 mg kg−1, respectively (Table 1), their leachable amount was quite low with only 0–1.3% of Cu, 0.1–3.4% of Zn, 0–0.3% of Fe, and 0.1–21.5% of Mn leached (Fig. 5). Like in the soil, the release of these metals in solution under landfill condition was much higher than that under acid rain condition by 0.1–1.3%, 3.2–3.3%, 0–0.3%, and 9.8–21.5% for Cu, Zn, Fe, and Mn, respectively (Fig. 5). The high content of Fe in SS-biochar was hardly leached out, which was probably due to the presence of stable FeOOH that was less influenced by lower pH and complexation of organic acid.
Potential environmental risks to groundwater
In addition to the supply of nutrients, amendment of biochar into soil may pose a leaching risk of heavy metals, especially in the acid environment, which may affect the groundwater. Acid rain may happen in many areas of China, especially in the southern China. Here, leaching concentration of heavy metals in the biochar-amended soil by simulated rain water was compared with China groundwater standard (GB/T 14848-93) to evaluate the risk of heavy metals to groundwater. Class II groundwater standard represents natural background levels of these heavy metals in groundwater; standard class III is mainly applied to restrict heavy metals in the water source for drinking and ensure human health; groundwater that reaches class IV could be used as industrial water and some as agricultural water. As shown in Table 4, the concentration of Zn in acid rain leaching condition from AUF soil was much higher than the values in class II, but it was lower than class III; concentrations of Fe and Mn leached from AUF soil were higher than the values in class III; the Mn concentration in AUF soil amended with PM-biochar was closed to and even higher than the class IV standard values. It means that when acid rain falls into AUF soil amended with biochar, metals such as Fe and Mn may be leached into groundwater and cause pollution to drinking water sources. The concentration of Cu, Zn, and Fe in OAC soil was well below the class II groundwater standard values of China. However, the concentration of Mn was much higher than the class IV standard values. When acid rain falls into biochar-amended OAC soil, the high risk of Mn to groundwater contamination is possible. It should be pointed out that the main limitations of this comparison include the following: (1) The simulative leaching solution (TCLP and SPLP) is from the standard method of toxicity characteristic leaching procedure, which deviates a little from real acid rain or small molecular acid environment; and (2) the metal concentrations in the leachate depend on the solid (soil)/liquid (leaching solution) ratio. Thus, this comparison of the leachate concentrations with standard regulations for groundwater only represents the results under a given condition. Although it is a simple and approximate method, it could provide us some valuable information.
Conclusions
This study investigated the release of nutrients (K, P, Ca, and Mg) and heavy metals (Cu, Zn, Fe, and Mn) from soil amended with biochars under simulated environmentally relevant conditions. In the simulated landfill environment, low molecular weight organic acid showed a significant activation effect on nutrients and heavy metals, accounting for their much higher release potential than in the acid rain environment. Pig manure biochar could strengthen the nutrient (K, P, and Mg) release from soil under both simulated landfill and acid rain environment. Sewage sludge biochar had less K, P, and Mg release, but more Ca leaching than pig manure biochar. The alkaline soil favors nutrient release than the acidic soil under the two environmental conditions. The release of heavy metals, especially Fe and Mn, was significant, which must be paid more attention since excessive leaching of Fe and Mn may affect the groundwater. Therefore, biochar could be utilized as a fertilizer for soil, while the potential risks caused by heavy metals of biochar must be considered together with local environmental conditions and soil properties.
References
American Society for Testing and Materials (ASTM) (2000) Annual book of ASTM standards, soil and rock, vol. 4.08
Bakshi S, He ZL, Harris WG (2014) Biochar amendment affects leaching potential of copper and nutrient release behavior in contaminated sandy soils. J Environ Qual 43:1894–1902
Beesley L, Moreno-Jiménez E, Gomez-Eyles JL (2010) Effects of biochar and green waste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ Pollut 158:2282–2287
Beesley L, Moreno-Jiménez E, Gomez-Eyles JL, Harris E, Robinson B, Sizmur T (2011) A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ Pollut 159:3269–3282
Buss W, Graham MC, Shepherd JG, Mašek O (2016) Suitability of marginal biomass-derived biochars for soil amendment. Sci Total Environ 547:314–322
Cantrell KB, Hunt PG, Uchimiya M, Novak JM, Ro KS (2012) Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour Technol 107:419–428
Cao XD, Dermatas D (2008) Evaluating the applicability of regulatory leaching tests for assessing lead leachability in contaminated shooting range soils. Environ Monit Assess 139:1–13
Cao XD, Harris W (2010) Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour Technol 101:5222–5228
Cao XD, Ma L, Gao B, Harris W (2009) Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ Sci Technol 43:3285–3291
Chan KY, Van ZL, Meszaros I, Downie A, Joseph S (2008) Using poultry litter biochars as soil amendments. Soil Res 46:437–444
China Ministry of Environmental Protection (CMEP) (1993) Quality standard for ground water (GB/T14848–93). China Environmental Science Press, Beijing
Dünisch O, Lima VC, Seehann G, Donath J, Montóia VR, Schwarz T (2007) Retention properties of wood residues and their potential for soil amelioration. Wood Sci Technol 41:169–189
Halim CE, Scott JA, Natawardaya H, Amal R, Beydoun D, Low G (2004) Comparison between acetic acid and landfill leachates for the leaching of Pb(II), Cd(II), As(V), and Cr(VI) from cementitious wastes. Environ Sci Technol 38:3977–3983
Hamdi W, Pelster D, Seffen M (2013) Phosphorus sorption kinetics in different types of alkaline soils. Arch Agron Soil Sci 60:577–586
Hass A, Gonzalez JM, Lima IM, Godwin HW, Halvorson JJ, Boyer DG (2012) Chicken manure biochar as liming and nutrient source for acid Appalachian soil. J Environ Qual 41:1096–1106
Jin HM, Arazo RO, Gao J, Capareda S, Chang ZZ (2014) Leaching of heavy metals from fast pyrolysis residues produced from different particle sizes of sewage sludge. J Anal Appl Pyrol 109:168–175
Kim P, Hensley D, Labbé N (2014) Nutrient release from switchgrass-derived biochar pellets embedded with fertilizers. Geoderma 232-234:341–351
Laird D, Fleming P, Wang B, Horton R, Karlen D (2010) Biochar impact on nutrient leaching from a midwestern agricultural soil. Geoderma 158:436–442
Lee JW, Kidder M, Evans BR, Paik S, Buchanan AC, Garten CT, Brown RC (2010) Characterization of biochars produced from cornstovers for soil amendment. Environ Sci Technol 44:7970–7974
Lehmann J, Joseph S (2009) Biochar for environmental management. Science and Technology Earthscan, Ltd., London
Lehmann A, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota—a review. Soil Biol Biochem 43:1812–1836
Liang Y, Cao X, Zhao L, Xu X, Harris W (2014) Phosphorus release from dairy manure, the manure-derived biochar, and their amended soil: effects of phosphorus nature and soil property. J Environ Qual 43:1504–1509
Liesch AM, Weyers SL, Gaskin JW, Das KC (2010) Impact of two different biochars on earthworm growth and survival. Ann Environ Sci 4:1–9
Lone AH, Najar GR, Ganie MA, Sofi JA, Ali T (2015) Biochar for sustainable soil health: a review of prospects and concerns. Pedosphere 25:639–653
Major J, Rondon M, Molina D, Riha SJ, Lehmann J (2012) Nutrient leaching in a colombian savanna oxisol amended with biochar. J Environ Qual 41:1076–1086
Mcdowell R, Sharpley A, Brookes P, Poulton P (2001) Relationship between soil test phosphorus and phosphorus release to solution. Soil Sci 166:137–149
Netzer A, Wilkinson P, Beszedits S (1974) Removal of trace metals from wastewater by treatment with lime and discarded automotive tires. Water Res 10:813–817
Parvage MM, Ulén B, Kirchmann H (2013) A survey of soil phosphorus (P) and nitrogen (N) in Swedish horse paddocks. Agric Ecosyst Environ 178:1–9
Qin F, Shan XQ, Wei B (2004) Effects of low-molecular-weight organic acids and residence time on desorption of Cu, Cd, and Pb from soils. Chemosphere 57:253–263
Shen X, Huang DY, Ren XF, Zhu HH, Wang S, Xu C, He YB, Luo ZC, Zhu QH (2016) Phytoavailability of Cd and Pb in crop straw biochar-amended soil is related to the heavy metal content of both biochar and soil. J Environ Manag 168:245–251
Silber A, Levkovitch I, Graber ER (2010) pH-dependent mineral release and surface properties of cornstraw biochar: agronomic implications. Environ Sci Technol 44:9318–9323
USEPA (1986) Test methods for evaluating solid waste, laboratory manual physical/chemical methods, vol 1A, 3rd edn. SW-846, U.S. Government Printing Office, US Environmental Pollution Agency, Washington, DC
USEPA (1992) Method 1311: test methods for evaluating solid waste, physical/chemical methods, SW-846 3rd edn. US Environmental Pollution Agency, Washington, DC
USEPA (1994) Method 1312: test methods for evaluating solid waste, physical/chemical methods, SW-846 3rd edn. US Environmental Pollution Agency, Washington, DC
Wang YJ, Chen JH, Cui YX, Wang SQ, Zhou DM (2009) Effects of low-molecular-weight organic acids on Cu(II) adsorption onto hydroxyapatite nanoparticles. J Hazard Mater 162:1135–1140
Wesenbeeck SV, Prins W, Ronsse F, Antal MJ Jr (2014) Sewage sludge carbonization for biochar applications. Fate of heavy metals. Energy Fuel 28:5318–5326
Wu H, Che X, Ding Z, Hu X, Creamer AE, Chen H, Gao B (2016) Release of soluble elements from biochars derived from various biomass feedstocks. Environ Sci Pollut Res 23:1905–1915
Xu XY, Cao XD, Zhao L (2013) Comparison of rice husk- and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: role of mineral components in biochars. Chemosphere 92:955–961
Xu G, Sun JN, Shao HB, Chang SX (2014) Biochar had effects on phosphorus sorption and desorption in three soils with differing acidity. Ecol Eng 62:54–60
Yao Y, Gao B, Zhang M, Inyang M, Zimmerman AR (2012) Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 89:1467–1471
Yuan JH, RK X, Zhang H (2011) The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour Technol 102:3488–3497
Yuan H, Lu T, Wang Y, Chen Y, Lei T (2016) Sewage sludge biochar: nutrient composition and its effect on the leaching of soil nutrients. Geoderma 267:17–23
Zhao L, Cao X, Wang Q, Yang F, Xu S (2013) Mineral constituents profile of biochar derived from diversified waste biomasses: implications for agricultural applications. J Environ Qual 42:545–552
Zhao L, Zheng W, Cao XD (2014) Distribution and evolution of organic matter phases during biochar formation and their importance in carbon loss and pore structure. Chem Eng J 250:240–247
Zhao L, Cao X, Zheng W, Scott JW, Sharma BK, Chen X (2016) Copyrolysis of biomass with phosphate fertilizers to improve biochar carbon retention, slow nutrient release, and stabilize heavy metals in soil. ACS Sustain Chem Eng 21:409–414
Zheng H, Wang Z, Deng X, Zhao J, Luo Y, Novak J, Herbert S, Xing BS (2013) Characteristics and nutrient values of biochars produced from giant reed at different temperatures. Bioresour Technol 130:463–471
Funding
This study was supported by the National Natural Science Foundation of China (Nos. 21537002, 21577087, 21607002), Scientific and Technological Projects of Anhui Province (No. 1704e1002238), and Anhui Laimujia Biotechnology Co. Ltd.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhihong Xu
Rights and permissions
About this article
Cite this article
Zhao, Y., Zhao, L., Mei, Y. et al. Release of nutrients and heavy metals from biochar-amended soil under environmentally relevant conditions. Environ Sci Pollut Res 25, 2517–2527 (2018). https://doi.org/10.1007/s11356-017-0668-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0668-9