Abstract
The Lesina lagoon (southern Adriatic Sea, Mediterranean) is a coastal lagoon located in a highly intensified farming and tourist area. A monthly sampling was carried out in 1998 in five stations, representative of different hydrological features, in order to analyse phytoplankton composition and its relation to environmental parameters. Our results showed high spatial variability of abiotic variables. Phytoplankton abundances and biomass trends showed marked seasonality, with annual peaks occurring in late winter–early spring and summer periods. Phytoplankton blooms were due to the diatom Thalassiosira pseudonana and the dinoflagellate Prorocentrum cordatum. Statistical analyses showed that salinity and nutrients were the main factors affecting phytoplankton abundance and biomass. Phytoplankton dynamics was associated both to seasonality and the hydrodynamic regime of the system. Moreover, chemical–physical data were analysed together with those collected in the same stations in 2007, to compare their dynamics under different hydrological regimes. The two periods corresponded to the closure and opening, respectively, of canals connecting the lagoon to the sea. In general, abiotic variables (salinity, dissolved oxygen, pH, nitrate, phosphate and silicate concentrations) were significantly affected by the hydrodynamic regime. These data could provide a useful basis to complement the knowledge gained through current monitoring within the framework of the European Directives, as well as to implement conservation and management strategies of these transitional waters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal lagoons are characterised by peculiar functional and structural features, due to their position between land and sea. They generally show large time-related and space-related variations in hydrochemical characteristics and high biological diversity (Castel et al. 1996; Tett et al. 2003; Roselli et al. 2009). A short nutrient turnover is promoted by these shallow ecosystems, resulting in considerable primary productivity and fishing production (Yãnez-Arancibia et al. 1994; Paerl et al. 2010; Roselli et al. 2013).
Lagoons provide a wide range of ecosystem services, such as biodiversity conservation, food production, water quality improvement and recreational and cultural activities. These environments are also subject to high anthropogenic pressures, which may influence marine organisms by acting directly on their physiology and phenology, as well as by modifying environmental variables that govern their dynamics (Brugnano et al. 2011; Caroppo 2015).
Phytoplankton, which can rapidly respond to a wide range of environmental perturbations, are sensitive indicators of water quality and hydrological evaluations and of any ecological changes in coastal systems (Paerl et al. 2010; Lugoli et al. 2012; Caroppo et al. 2013; Garmendia et al. 2013). For this reason, within the European framework directives (WFD, EC 2000; MSFD, EC 2008), phytoplankton are used as a biological quality element for ecological status assessment of different types of water bodies (from lakes to open sea).
The Lesina lagoon (southern Adriatic, Mediterranean Sea) is a non-tidal lentic system, quite interesting for its relevant biological and economic value. Internationally known as a breeding area for many migratory birds, in 1995, it was designated ‘Site of Community Importance’. The main economic activities of this brackish environment are fishing, tourism, extensive fish farming and, in the past, macroalgal harvesting. More specifically, from 1970 to 1990, Rhodophyta Gracilaria gracilis (Stackhouse) Steentoft, Irvine et Farnham (as Gracilaria confervoides Greville) used to flourish in the lagoon (Sacca Occidentale) in large quantities (e.g. 100 metric tons dry weight in 1978). It was used for commercial purposes (agar-agar extraction) (Trotta 1994). After 1990, a drastic reduction in G. gracilis biomass production was observed due to over-exploitation. Other seaweeds, i.e. Valonia aegagropila C. Agardh and Cladophora sp. (not economically profitable) grew in the lagoon and sometimes reached bloom conditions. An extraordinary organic matter degradation process and anoxia occurred at every bloom of these macroalgae, usually in the summertime (Trotta 1994; Nonnis Marzano et al. 2003). Reduced water exchange with the sea, due to the closure of two canals (Acquarotta and Schiapparo), affected the lagoon trophic state. Therefore, these canals were opened again in April 2000 to try and improve water quality (Manini et al. 2005).
As to phytoplankton biodiversity and dynamics, recent information is scarce, except for the studies carried out in the past (Solazzi et al. 1972). Moreover, no data are available on phytoplankton biomass in terms of carbon content. The latest phytoplankton data in the Lesina lagoon were collected to evaluate the ecosystem health, in compliance with Italian regulation No. 152/2006 and for Water Framework Directive (WFD) implementation (Roselli et al. 2009). Other studies reported the effects of a dystrophic event on phytoplankton physiognomy in 2008 (Vadrucci et al. 2009; Basset et al. 2013).
The goal of this study was to collect information on phytoplankton assemblages and dynamics in the Lesina lagoon in relation to specific hydrological conditions, with a view to assessing this transitional water ecosystem in accordance with the WFD (Ferreira et al. 2011). In particular, besides the environmental variables already monitored in 1998, the following phytoplankton parameters were described: (i) cell abundances, (ii) biomass in terms of carbon content, (iii) taxonomic composition and (iv) abundance and biomass correlations with environmental variables. Moreover, two different monitoring periods (January–December 1998 and January–December 2007), representing two different hydrological regimes, were compared to evaluate the effects of the opening of the canals Acquarotta and Schiapparo. This study could provide useful information for conservation and management strategies of this lagoon in view of a better understanding of non-tidal lentic lagoonal ecosystems.
Methods
Study area
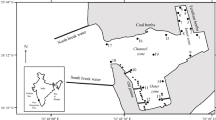
The Lesina lagoon (Fig. 1) is located on the southern Adriatic coast (Apulia, Italy) on the northern side of the Gargano promontor between the Fortore River and Rodi Garganico. It extends parallel to the coast for about 20 km, covering an area of about 51.5 km2, with a perimeter of 50 km, an average depth of about 0.9 m and of maximum 1.6 m (Solazzi et al. 1972). The lagoon is long and narrow, stretching along an east–west direction. It is divided into three different main areas: Sacca Occidentale, Area Mediale and Sacca Orientale (Solazzi et al. 1972). In the Sacca Occidentale and Area Mediale, the sediments are mostly silty, while mainly reed beds and sand and silty sediments characterise the Sacca Orientale (Ferrarin et al. 2014). The lagoon is connected with the Adriatic Sea through two canals: Acquarotta (on the western side) and Schiapparo (on the eastern side). The southern side of the lagoon receives numerous inputs. The Sannicandro Sewage canal, the Idrovora drainage canal, the rivers Lauro and Zannella and the Elce stream are the most important tributaries, bringing in groundwater and effluents, both raw and treated, from fish farming, agriculture and urban activities. They all end up into the lagoon, thus bringing a surplus of nutrients from intensive agriculture in the catchment basin, which, at 500 km2, is ten times larger than the lagoon area itself (Trotta 1994).
Map of the Lesina lagoon showing the location of sampling sites (modified from Roselli et al. 2009)
The lagoon shallow depth and the remarkable nutrient load are responsible for seasonal, and sometimes daily, fluctuations in physical and chemical parameters. Almost everywhere, the sediment redox potential in the lagoon is below 100 mV or even negative (Trotta 1994).
The lagoon current environmental conditions, with increased anthropogenic inputs, are described in Roselli et al. (2009).
Sample collection and abiotic factors
From January to December 1998, monthly samplings were conducted in five stations (Fig. 1) located at the Sacca Occidentale (stations 1 and 13), Area Mediale (stations 15 and 20) and Sacca Orientale (station 28) of the lagoon. Water samples were taken at the surface of each station (n = 5) using a 5-L Niskin bottle. Transparency was measured by Secchi disk. Temperature, salinity, dissolved oxygen and pH were recorded by an Idronaut Ocean Seven 501 multiprobe and compared with in situ (electronic thermometers) and laboratory (Guildline Autosal 8400 B salinometer, Winkler and pH meter) measurements. Nutrients (NH4 +–N, NO2 −–N, NO3 −–N, PO4 3−–P, SiO3 2−–Si) were measured according to Strickland and Parsons (1972). Environmental data collected from the same stations from January to December 2007 were consistent with the two different hydrological regimes of closure (1998) and opening (2007) of the sea-connecting canals.
Phytoplankton samples, freshly collected in 1998, were fixed with Lugol’s iodine solution and examined by an inverted microscope (Labovert FS Leitz) by following the Utermöhl method (Utermöhl 1958). Phytoplankton were counted along transects (1–4) or in random fields (30–60) with 400× magnification. Moreover, to better evaluate less abundant microphytoplankton taxa, half of the Utermöhl chambers were also examined (200× magnification) (Zingone et al. 2010). Nanophytoplankton (2–20 μm) were counted in 15 randomly selected fields with 630× magnifications.
Biovolume was calculated by approximating species shapes to geometrical models (Hillebrand et al. 1999). Carbon content was evaluated from mean cell biovolumes using Menden-Deuer and Lessard formula (2000).
Statistical analysis
The experimental data were processed using the STATISTICA software package (StatSoft Inc., Tulsa, OK, USA).
Mean values and standard deviations of the variables were regarded as space-related variations of each variable. One-way variance analysis (ANOVA) was applied to environmental data to evaluate the differences between the two hydrological conditions in 1998 and 2007, respectively. Furthermore, ANOVA was carried out on the variables collected in 1998 and 2007 to describe the system space–time variation in each sampling period (Underwood 1997).
A standard Pearson correlation was used to quantify direct correlations between phytoplankton abundance and biomass and environmental parameters. In order to obtain a visual representation of the correlations between environmental variables and phytoplankton, a principal component analysis (PCA) was performed on the whole dataset collected in 1998. Logarithmic transformation [log10(x + 1)] was used on phytoplankton data prior to statistical analyses, in order to obtain a normal distribution. Moreover, PCA was also performed by using both 1998 and 2007 environmental data, in order to compare the two different hydrological regimes.
Results
Environmental data
The average values of the parameters considered during the two sampling periods are shown in Figs. 2 and 3, together with the relative standard deviations as spatial variation for each variable.
Water temperature followed a seasonal trend for both 1998 and 2007 sampling years, with minimum temperatures in winter (January 1998 with 6.90 ± 0.14 °C and December 2007 with 10.30 ± 1.02 °C) and maxima in July (29.65 ± 0.53 and 27.30 ± 0.73 °C, respectively, 1998 and 2007) (Fig. 2). Temperature showed a significant time-related variation (p < 0.001), while no significant differences were found among the stations, in both sampling periods.
In 1998 and 2007, salinity showed similar seasonal trends, characterised by an increase of values from winter (18.38 ± 1.65 in February 1998 and 10.37 ± 2.21 in January 2007) to summer (35.78 ± 4.98 in September 1998 and 28.01 ± 4.51 in August 2007) (Fig. 2). In the late summer–fall period values decreased. Salinity was particularly affected by the change of the hydrological conditions; in fact it was significantly higher in 1998 than in 2007 (p < 10−4), especially in summer, when evaporation was higher and freshwater input scarce. ANOVA showed also significant time-related variations in salinity (1998: p < 0.001; 2007: p < 0.05). Furthermore, significant differences among the stations (p < 0.05) were recorded with the western side stations characterised by higher values than the eastern side stations in both periods.
Dissolved oxygen (saturation percentage) showed significantly different values in the two hydrological conditions (p < 10−4), and higher values were usually detected in 1998. Dissolved oxygen displayed opposite trends from May to August comparing the two sampling periods (Fig. 2). In 1998, saturation percentage values ranged between 100.73 ± 12.84% (November) and 155.79 ± 24.65% (July), while in 2007 they were comprised between 83.78 ± 19.13% (July) and 131.48 ± 31.22% (April). In both periods, only significant time-related variations were observed (1998: p < 0.001; 2007: p < 0.05).
ANOVA evidenced statistically significant differences (p < 10−4) of pH values in two sampling years. Seasonal trends were comparable and higher values were always detected in 2007 (Fig. 2). In 1998, values ranged between 8.08 ± 0.04 (December) and 8.47 ± 0.18 (July). In 2007, pH values were lowest in August (8.39 ± 0.34) and highest in March (8.82 ± 0.22). In both sampling periods, no significant time-related and space-related changes were recorded.
As far as the concentration of N, P and Si on the Lesina lagoon was concerned, ANOVA showed statistically significant differences in N–NO3 − (p < 0.001), P–PO4 3− (p < 0.05), Si–SiO2 − (p < 10−4) concentrations and N/P (p < 0.001) and Si/N (p < 0.01) ratios before and after the canals opened.
As regards N–NH4 + seasonal patterns, they did not show differences between the two considered periods. The lowest concentrations of N-NH4 + were recorded in June (1998: 0.88 ± 0.46 μM; 2007: 0.82 ± 0.60 μM) while the highest in December 1998 (4.31 ± 0.75 μM) and in May 2007 (4.39 ± 6.47 μM) (Fig. 3). N–NO2 − and particularly N–NO3 − showed a changing trend over time (Fig. 3). N–NO2 − levels showed opposite trends throughout the years. In particular, in 1998 higher values were monitored in winter. On the contrary, in 2007, greater concentrations were usually detected in the other periods of the year and sporadic peaks were detected in May, September and December. In 1998, values ranged between 0.02 ± 0.03 μM (October) and 1.97 ± 0.31 μM (January). In 2007, N–NO2 − values were lowest in July (0.16 ± 0.07 μM) and highest in May (1.62 ± 3.08 μM). As regards N–NO3–, before the canals opened, temporal distribution of concentrations was characterised by greater values in late winter (up to 6.77 ± 3.47 μM, March 1998) (Fig. 3). In the other periods of the year, concentrations decreased, by reaching the minimum average value of 0.20 ± 0.04 μM (July). In 2007, probably because of heavy rains and nutrient load in freshwater inputs, N–NO3 − concentrations were higher than in 1998 and values showed peaks throughout all the seasons, with maxima values in December 2007 (30.21 ± 44.89 μM). Finally, N–NH4 +, N–NO2 − and N–NO3 − concentrations showed no significant time-related and space-related variations in both sampling periods.
P–PO4 3− accounted for the least abundant element among the nutrients studied with extremely low values on average below 1 μM for the 2 years under examination (Fig. 3). The seasonal variation of P–PO4 3− was different during the two periods. Higher concentrations of this nutrient were monitored in 1998, particularly in spring-summer (from April to September). Instead, in 2007 the higher levels were observed in February–March period (Fig. 3). The lowest P–PO4 3− values were recorded in January (0.08 ± 0.02 μM) and the highest in September 1998 (0.94 ± 0.28 μM). In the second period, the lowest P–PO4 3− values were recorded in January 2007 (0.04 ± 0.02 μM) and the highest ones in March 2007 (0.77 ± 1.05 μM). In 1998, P–PO4 3− showed significant variations from month to month (p < 0.001), but not among stations. In 2007, no space-time-related variations were detected.
Si–SiO2 − seasonal pattern was similar in the two investigated periods (Fig. 3). Si–SiO2 − concentrations were the lowest in August 1998 (23.69 ± 3.84 μM) and the greatest in December 1998 (73.31 ± 7.96 μM). In 2007, the lowest concentrations were recorded in August (15.62 ± 8.26 μM) and the highest one in May (73.35 ± 38.59 μM) (Fig. 3). Si–SiO2 − showed no significant time- and space-related differences in the 2 years.
N/P ratio seasonal distribution appeared, on average, significantly higher in 2007 than in 1998 (p < 0.001). It was usually greater than the theoretical value of 16 for all the sampling periods, except for the summer–fall period in 1998 when the minimum value of 0.88 ± 0.43 (July) was detected (Fig. 3). Statistically significant time-related variations (p < 10−4) and space-related variations (p < 0.001) were observed in 1998 and 2007, respectively. Si/N ratio was always greater than the theoretical value (~ 1) and showed a changing trend over the years (Fig. 3). The highest values were monitored in summer 1998 (up to 58.05 ± 26.53, June). In 2007, peaks were detected throughout the year. Moreover, statistically significant temporal (p < 0.001) and spatial (p < 10−4) differences were recorded in 1998 and 2007, respectively.
Phytoplankton communities and harmful species
Phytoplankton communities were reported with a wide range of abundance (119 × 103–20.0 × 106 cells L−1) and biomass (6.7–805.6 μg C L−1) and with significant time-related variations (ANOVA, p < 10−4). On average, seasonal abundance and biomass trends were similar and higher from January to April (up to 20.0 × 106 cells L−1 and 805.6 μg C L−1, station 15) when a peak was detected in all stations, except for station 1 (Fig. 4a, b). Abundance and biomass decreased from May on and peaked again in August only in stations 20 and 28 (up to 10.4 × 106 cells L−1 and 415.8 μg C L−1, station 28).
As to spatial distribution, ANOVA revealed no significant differences between the stations (p > 0.05). The highest annual mean cell abundance was observed in station 15 (6.3 ± 7.6 × 106 cells L−1), while station 28 reported the highest biomass (213.0 ± 171.5 μg C L−1). The lowest annual mean abundance and biomass were recorded in station 1, where 1.5 ± 1.9 × 106 cells L−1 and 87.3 ± 73.6 μg C L−1 were detected, respectively.
The phytoplankton community was generally dominated by ‘others’, including several phytoplankton groups. This was the case in all stations and mainly in summer and autumn (Fig. 5). These algae on average accounted for about 60.7 ± 34.1% of total abundance and 49.7 ± 26.6% of total biomass. Diatoms accounted on average for 29.3 ± 31.5% of total abundance and 18.1 ± 17.9% of total biomass. They most contributed to the phytoplankton community in winter (abundance) and spring (biomass) (Fig. 5). Dinoflagellates accounted for 10.0 ± 9.4% of total abundance and 32.2 ± 24.7% of total carbon. They reached their highest abundance and biomass percentage in winter and spring, respectively (Fig. 5). All phytoplankton groups only showed significant time-related (p < 10−4) variability.
A total of 59 taxa corresponding to 34 diatoms, 20 dinoflagellates and 5 others were identified (Table 1). Time-related variations of the six most abundant and frequent taxa in the Lesina lagoon are reported in Fig. 6.
The group of ‘others’ was an important component in the phytoplankton community, in terms of abundance (1.3 ± 1.2 × 106 cells L−1) and contribution to total biomass (66.4 ± 65.4 μg C L−1). The highest abundances were recorded in April 1998 (up to 5.4 × 106 cells L−1–290.9 μg C L−1, station 15). They were mainly represented by small (< 10 μm) phytoflagellates of uncertain taxonomic identification, accounting for 65.5 ± 32.6% of total abundances. Undetermined, > 10 μm phytoflagellates, prymnesiophyceans, cryptophyceans, dictyochophyceans, prasinophyceans and euglenophyceans (Table 1) with low cell concentrations were generally detected less frequently. Among them, the most abundant were prasinophycean Tetraselmis wettsteinii and euglenophycean Euglena acusformis. T. wettsteinii was most abundant in the late fall–winter period (up to 1.2 × 106 cells L−1 station 15, December) (Fig. 6) and E. acusformis developed in summer. Its greatest 38.0 × 103 cells L−1 abundance was monitored in station 20 (August).
Diatoms were detected with average 2.2 ± 3.4 × 106 cells L−1 abundances, with 25.8 ± 26.6 μg C L−1 contribution to total biomass. The highest abundance and biomass were reported in winter and spring in all stations (Fig. 5). In summer (as well as in autumn), diatoms showed the lowest abundance and biomass in all stations except for stations 20 and 28. The nano-sized species Thalassiosira pseudonana was responsible for these blooms. In winter–spring, this species reached its highest 11.9 × 106 cell L−1 concentration (station 15, February), accounting for 74.9% of total phytoplankton abundance and 33.6% biomass. Other representative diatom species were Navicula sp.p. (Fig. 6), Cylindrotheca closterium, Chaetoceros sp.p., Amphora coffaeformis, Amphora sp.p. and Cocconeis placentula.
Dinoflagellates (on average, 0.5 ± 0.7 × 106 cells L−1 and 71.4 ± 101.2 μg C L−1) increased in late winter and spring, while in summer the only biomass increase was observed in station 28 (Fig. 5). In spring, and specifically in April in station 15, different species reached their highest abundance: Prorocentrum cordatum (2.5 × 106 cell L−1) (Fig. 6), Gymnodinium sp. (198.6 × 103 cells L−1) and the heterotrophic Diplopsalis group (116.8 × 103 cells L−1). In summer, the nano-sized Heterocapsa niei developed in the lagoon, reaching its highest growth in August (up to 140.2 × 103 cells L−1, station 28). In late summer and fall, Prorocentrum scutellum was the most abundant species (up to 87.6 × 103 cells L−1, station 13, October) (Fig. 6). Scrippsiella acuminata with its wide time-related distribution was observed throughout the year (Fig. 6), even if its abundance was never particularly high (up to 58.4 × 103 cells L−1, August, station 28).
Among the dinoflagellates, besides Prorocentrum cordatum, various harmful, potentially biotoxin producing species were detected in the lagoon. Alexandrium minutum was observed mainly in summer (July–August) in all stations with concentrations ranging between 2.9–70.1 × 103 cells L−1. Amphidinium carterae was detected only in stations 20 and 28, with abundances ranging between 0.4 and 2.9 × 103 cells L−1. With its wide time-related distribution, Gonyaulax spinifera was reported throughout the year, although with a low frequency (14% of the total samples), with abundances ranging between 0.7 and 5.8 × 103 cells L−1.
Also, Akashiwo sanguinea, a high biomass producer, was identified, mainly in autumn, with abundances ranging between 0.4 and 13.1 × 103 cells L−1.
Besides Diplopsalis group, other heterotrophic dinoflagellates, made up of different species of Protoperidium (Table 1), were detected with abundances ranging between 0.7 and 75.9 × 103 cells L−1 and a sampling frequency of 28.1%.
Correlations between environmental variables and phytoplankton
Correlation analysis pointed out that seasonal phytoplankton variations in abundance and biomass were significantly correlated with environmental data, especially salinity and nutrients (Table 2). Total phytoplankton, the ‘others’, diatom and dinoflagellate abundance and biomass showed negative correlations with salinity. Moreover, phytoplankton, and especially dinoflagellate dynamics was significantly correlated with N–NH4 +, N–NO2 −, N–NO3 −, P–PO4 3− and Si–SiO2 −. The ‘others’ (abundance and biomass) and total phytoplankton (abundance) showed significant correlations with N/P ratio. Moreover, dinoflagellate biomass was significantly correlated with Si/N ratio.
PCA applied to the 1998 dataset and the combined plot of scores and loadings allowed us to recognise groups of samples with similar behaviours and any existing correlation with the original variables. Three principal components were extracted accounting for 91% variability. The loading of variables on the first two principal components (Fig. 7) showed that temperature, N–NO2 −, N–NO3 −, P–PO4 3− and N/P ratio were dominant variables on PC1, whereas N–NH4 + and dinoflagellates were dominant variables on PC2. The scatter plot of the scores on the first two principal PC1 and PC2 components (Fig. 8) showed that in summer, autumn and late spring, all stations had a positive score on PC1, while in winter and at the beginning of spring all stations had a negative score on the same component. Conversely, in spring (April), station 15 had a more negative score compared to the other stations on PC2. Therefore, when their physical–chemical parameters were compared, all stations had the same behaviour, except for station 15, which in April showed higher dinoflagellate abundances.
PCA was also carried out to compare physical–chemical parameters following the closing (A) and opening of the canals (B), respectively. Three principal components were extracted, accounting for 87% variability. The scatter plot of the scores on the first two principal components PC1 and PC2 (Fig. 9) showed that the stations were quite apart. Indeed, in January and February, station 28 had a more negative score compared to the other stations on PC1, while station 20 had a more positive score compared to the other stations on PC2.
Loading of the variables on the first two principal components detected in the Lesina lagoon in the two periods (Fig. 10) showed that the dominant variables on PC1 were temperature, N–NO2 −, N–NO3 − in period A and temperature and salinity in period B in station 28. Moreover, the dominant variables on PC2 were pH, N–NH4 +, N–NO2 − and N–NO3 − in period B in station 20.
The results obtained from PCA showed that during the two examined periods, two stations showed a different behaviour in terms of nitrogen compound concentrations. In period A, the highest nitrogen compound concentrations (N–NO2 − and N–NO3 −) were observed in station 28, while in period B they were detected in station 20. Stations 20 and 28 were located near the watercourses flowing into the lagoon, along its southern edge and near the Schiapparo canal, respectively, connecting the lagoon to the sea. Considering that roughly 80% of annual freshwater was discharged into the eastern part of the lagoon, the low or scarce circulation in 1998 could have caused a nutrient build-up in station 28. The opening of the canals favoured an increase in nitrogen content in station 20 compared to the other stations, probably due to the prevalence of industrial and urban waste inputs. The effects of station ‘behaviours’ were more easily visible in the stations close to the Schiapparo canal than in those close to the Acquarotta canal, presumably because the first one is shorter than the latter, hence with more effective exchange with seawater.
Discussions
Environmental data and their correlation with different hydrological conditions
Because of its shallow waters, the hydrological characteristics of the Lesina lagoon are easily impacted by weather conditions, natural freshwater inflows, industrial and urban wastewaters and tidal exchanges. Both in 1998 and 2007 sampling periods, temperature followed a time-related trend typical of the Mediterranean region, with the highest average temperature values recorded in summer. In winter, temperature and salinity were lowest, due to abundant rainfalls and continental inputs. Salinity was the highest in summer 1998, when higher evaporation rates were recorded, linked to higher temperature and concurrent lower freshwater inputs. In this period, lower dissolved oxygen values were also recorded, especially near the Acquarotta canal, where the accumulated macroalgal biomass began decomposing. The area close to the Schiapparo canal was characterised by lower salinity, because of more abundant natural freshwater inputs from the eastern side of the lagoon. However, from September to December 2007, salinity was lower than in winter 1998. During the period when the canal was closed (1998), dissolved oxygen reached its lowest values (82% saturation) near the Acquarotta canal in summer, and its highest peaks near the Schiapparo canal, probably due to currents and tidal exchanges. As concerning the significantly high pH values detected in 2007, they might have been caused by the discharge of wastewaters and the greater concentrations of phosphorus (Coelho et al. 2007).
In both considered periods, the Lesina lagoon was characterised by time-related fluctuations in chemical–physical parameters and phytoplankton, while only salinity and N/P and Si/N ratios (only in 2007) showed significant space-related differences.
In general, nutrient concentrations (N–NO2 −, N–NO3 −, P–PO4 3−) as well as N/P and Si/N ratios showed different trends in 1998 and 2007. High nitrogen compound concentrations detected in winter and spring could be due to agricultural runoff. Indeed, the highest concentrations were registered in both hydrological conditions in the south-eastern stations, close to the main freshwater inputs. N–NH4 + concentrations are also linked to microbial decomposition of organic matter in the bottom sediments. N–NO2 − concentrations showed opposite trends in the various sampling periods, with higher mean values in winter 1998 and spring 2007, respectively. N–NO3 − concentrations showed higher mean values in 2007 (12.44 ± 15.65 μM) than in 1998 (1.98 ± 1.31 μM), presumably due to increased agriculture activity throughout these years. P–PO4 3− concentrations, on the one hand, were related to biogeochemical processes and to organic matter resuspension from the bottom caused by turbulence conditions (Gikas et al. 2006). On the other hand, they could be due to inputs carrying urban wastewaters and runoff. However, since P–PO4 3− concentrations were higher in 1998 than in 2007, limited seawater exchange through the canals could be assumed to be the cause of increased P–PO4 3− levels. Nitrogen and phosphorus concentrations are normally associated with anthropogenic pressures in limited seawater exchange systems (Newton and Mudge 2005; Pérez-Ruzafa et al. 2005; Viaroli et al. 2005; García-Pintado et al. 2007). Therefore, the south-eastern part of the lagoon seemed to be the main source of exogenous nitrogen, because of freshwater inputs in both hydrological conditions. Actually, observed high N/P ratios are indication of phosphorus-limited conditions in that part of the system. Conversely, the western part of the lagoon showed low N/P ratios, probably due to scarce freshwater input and high phosphorus concentration. Silicate concentrations fluctuated during the sampling periods, with high concentrations detected in the eastern part of the lagoon where freshwater inputs were higher. Whenever the Si/N ratio was higher than 1, diatoms were able to compete with the other algal classes that require less quantities of silicon (Conley et al. 1993).
Previous studies carried out in 2007 and aimed to evaluate the trophic state and water quality of the Lesina lagoon evidenced a high productivity of this ecosystem, which went from a good state, in the areas near the canals, to a poor state in those closed to freshwater inputs, especially during the winter months (Roselli et al. 2009). Results of this research show that the Lesina lagoon was a highly productive ecosystem also in the past, like other coastal brackish Mediterranean environments (e.g. Tett et al. 2003, Basset et al. 2006; Newton et al. 2014). This is confirmed by the high biomass and cell concentration of phytoplankton communities. The lagoon has been experiencing severe urban and agricultural pollution, which has increased over the years (Roselli et al. 2009). There was also a moderate freshwater flow, bound to increase in spring and fall due to rainfalls. The average water renewal time ranged between 50 days (eastern lagoon) and 250 days (western lagoon) (Ferrarin et al. 2014). Data collected in 1998–1999 indicated a water turnover time of around 100 days (Manini et al. 2005). Moreover, in these years, rainfalls were low, particularly during summer (0.5 mm day−1). Also, the closure of the canals led to high evaporation rates (3.7 mm day−1) (Manini et al. 2005), with consequent hypersalinity (up to 41.9) of the western area. With high waste discharges, low renewal capacity and shallow waters, the Lesina lagoon had a strong eutrophication potential. During the year of our investigation (1998), a massive growth of the macroalga Valonia utricularis (Roth) C. Agardh was observed, which had also occasionally caused dystrophic crises (Cozzolino 1995). However, during the following years Valonia disappeared (D’Adamo et al. 2009) and in 2007 the macrophytobenthos in the Lesina lagoon was mostly accounted for by rhizophytes Ruppia cirrhosa and Nanozoostera noltii and macroalgae Chaetomorpha sp., Chara sp., while in the basin western area, an extensive meadow of Gracilaria sp. was present. In general, the species succession depends on salinity fluctuations. In particular, Valonia spp. is associated with high salinity conditions (D’Adamo et al. 2009), as was found in the 1998 sampling period.
Phytoplankton community
Recorded phytoplankton densities were lower than those in other estuarine environments (Pereira Coutinho et al. 2012; Pachés et al. 2014; Coelho et al. 2015). They were higher than those monitored by Roselli et al. (2009) in the lagoon (0.07–11.21 × 106 cells L−1), but lower than those reported by Vadrucci et al. (2009) during the dystrophic crisis in the summer of 2008 (max value 5.48 × 107 cells L−1). Compared with previous studies, our abundance data are higher than those recorded by Solazzi et al. (1972) (10–448.0 × 103 cells L−1), when only the Schiapparo canal was opened. Our phytoplankton biomass estimates in terms of carbon content (the first for Lesina lagoon) show that maximum measured values are in the upper range reported to date for other Mediterranean transitional systems (Fanuko and Valčić 2009; Bosak et al. 2012) and are comparable to values recorded in the Fusaro lagoon (Sarno et al. 1993).
In the Lesina lagoon, the autotrophic biomass could be controlled by freshwater inputs, availability/limitation of nutrients, mineralisation processes, water residence times, tidal exchanges and the macrophytobenthic component in the basin as a whole. Actually, our data show a high correlation of phytoplankton with salinity (negative) and with nutrients. Presumably, when the canals were closed leading to less water renewal and higher phosphate availability, phytoplankton benefited from high nutrient concentrations thus reaching higher density than during the period in which the canals were opened and circulation guaranteed (2007). In addition, at the beginning of the summer 2008, the whole of the western part of the Lesina lagoon suffered a severe anoxia for about a month. This dystrophic event was caused by high temperature and the absence of wind, in conjunction with microbial decomposition of the macroalgal biomass. The latter was mostly made up of Gracilaria spp., which over the previous 2 years had homogeneously covered the bottom. Moreover, this event occurred with the temporary partial blockage of the Acquarotta canal, through which seawater was allowed to enter the lagoon, especially in summer. This partial block, in turn, further increased phytoplankton biomass concentration. The eastern part of the lagoon was not affected by these dystrophic conditions, probably thanks to freshwater inputs and the efficiency of the Schiapparo canal allowing for a good lagoon-seawater exchange. The importance of properly functioning tidal canals is well-known and this study confirms their fundamental role in lagoon environmental management.
Regarding time-related variations, they showed some differences from to the past (Solazzi et al., 1972), when the seasonal cycle was characterised by increased cell concentrations in spring, and maximum summer concentrations. During our survey, the maximal standing stock was registered in spring (April 1998), with increased summer abundances and biomass detected only in the eastern stations. Moreover, in winter (February 1998), high cell numbers were recorded, despite lower total biomass due to the small size of the blooming species (Thalassiosira pseudonana and Prorocentrum cordatum).
The community was dominated by smaller organisms: phytoflagellates and nano-sized diatoms and dinoflagellates. The high nutrient input in the lagoon seemed to favour autotrophic small-sized organisms capable of exploiting quickly available resources and characterised by high surface/volume ratios and reproductive rates (r-strategist). No information is available about picophytoplankton in the Lesina lagoon. However, based on data collected in other temperate transitional waters (Caroppo 2015 and references herein), including the adjacent Varano lagoon (Caroppo 2000), we would assume that this component plays an important role in phytoplankton physiognomy.
Phytoflagellates, considered typical autotrophs of the Adriatic Sea (Fonda Umani et al. 1992; Caroppo et al. 1999) were present throughout the year. The nano-sized diatom Thalassiosira pseudonana bloomed in late winter-spring, promoted by high nutrient availability. Unlike other diatoms, which usually grow in turbulent environments, T. pseudonana developed even in stable water conditions, probably due to its low sinking rate, as already observed in other coastal and brackish environments (Zingone et al. 1990; Giacobbe et al. 1996). Dinoflagellates played a minor role in terms of abundance, but were very important in terms of biomass, and significantly contributed to community diversity. Also, worth noting is that coccolithophorids—important components of phytoplankton assemblages in the coastal Adriatic Sea (Fonda Umani et al. 1992; Caroppo et al. 1999)—were rarely observed.
Less than 10 species accounted for the main bulk of phytoplankton population. The scarcity of phytoplankton species could be associated to eutrophication, responsible for strong selection and consequent species number reduction in some coastal sites (Sarno et al. 1993; Caroppo 2000). Moreover, phytoplankton dynamics was affected by limited tidal range and poor sea exchange.
The hydrodynamic regime role in the Lesina lagoon is more evident if we compare its phytoplankton dynamics and species composition to those in the adjacent Varano lagoon (Caroppo 2000). Here, the highest cell abundances were recorded in autumn and the succession of the various taxa appeared very similar to that monitored in the adjacent coastal waters of the southern Adriatic Sea (Caroppo et al. 1999). Presumably, in the Lesina lagoon, phytoplankton assemblages were not the result of passive transport. After their initial intrusion in the lagoon, species of marine origin might have been selected by the environment.
Some of these species such as Thalassiosira pseudonana and Prorocentrum cordatum (= P. minimum) are typical of enclosed and semi-enclosed basins, or estuarine waters (Sarno et al. 1993; Pereira Coutinho et al. 2012). Moreover, due to the lagoon shallow waters, ticopelagic species like Cocconeis scutellum, Licmophora gracilis and Synedra undulata were also detected.
In 2007, phytoplankton assemblage composition was similar to that observed in 1998 (data not shown), highlighting the adaptation of some species to lagoonal conditions. Cell concentrations seemed to have been controlled by basin water renewal depending on different hydrological conditions: closed canals (1998), opened canals (2007) and partially closed canals (2008). In 2008, the Acquarotta canal was closed for dredging operations and structural engineering works recording a concurrent increase in phytoplankton biomass dystrophic crises in the western part of the lagoon (Basset et al., 2013).
Taking into account the physiognomy of phytoplankton and their significant correlations with salinity and nutrients, this study furnishes evidence that phytoplankton provide useful information on the environmental quality of the lagoon and its canals over the years.
Harmful algal species
During our survey, the Lesina lagoon was affected by the presence of HABs. The blooming species Prorocentrum cordatum was one of them, the others were the toxin producers Alexandrium minutum, Gonyaulax spinifera and Amphidinium carterae and the high biomass producer Akashiwo sanguinea. Among the harmful dinoflagellates, Alexandrium minutum and Gonyaulax spinifera have been identified for the first time in the Lesina lagoon during our investigations.
Prorocentrum cordatum (= P. minimum) blooms are typical of brackish environments (e.g. Pereira Coutinho et al. 2012) and seem to be associated to eutrophication (Heil et al. 2005). Shellfish toxicity has been attributed to Prorocentrum cordatum from different coastal environments (Japan; France; Norway; Netherlands; New York, USA). However, a water-soluble neurotoxic component which killed mice was isolated only from Mediterranean clones collected along the coasts of France (Heil et al. 2005). Alexandrium minutum and Gonyaulax spinifera are common along the Adriatic Sea (Caroppo et al. 1999; Viličić et al. 2002; Arapov 2013) and the Ionian coasts (Rubino et al. 2009; Caroppo et al. 2016) and are known to produce paralytic shellfish poisoning (PSP) (Chang et al. 1997) and yessotoxins (YTXs) (Riccardi et al. 2009), respectively. Amphidinium carterae, besides being involved in ciguatera poisoning (Hallegraeff 2003), is known to cause red tides and has been implicated in mass fish mortality (Yasumoto 1990). This effect seemed to be related to hemolysins, low molecular weight compounds produced by this dinoflagellate. Laboratory studies evidenced also that this species exerts toxic effects on the early life stages of sea urchins (Pagliara and Caroppo 2012). Akashiwo sanguinea is a nontoxic bloom-forming organism, but it is suspected of causing seabird deaths through saponification. Through wind mixing and surfaction, this dinoflagellate forms a yellowish green foam. This foam acts as a surfactant, dissolving the natural oil cover from the feathers of seabirds which can result in hypothermia (Jessup et al. 2009). It is a very common species and can cause blooms in coastal waters throughout the world, such as the Black Sea (Gómez and Boicenco 2004), Oregon, USA (Du et al. 2011), Hong Kong (Lu and Hodgkiss 2004) and in brackish environments of the Mediterranean Sea (Caroppo 2002; Caroppo et al. 2016).
In more recent years, some of the harmful species detected in 1998 have been identified, including Prorocentrum cordatum (as P. minimum), but they have never reached high abundance rates (Vadrucci et al. 2009). However, the presence of these microalgae needs to be monitored in the future, in order to protect the high value services (tourism, fishing and aquaculture) offered by this lagoon.
Conclusions
This study confirms that phytoplankton is a good indicator of the ecosystem environmental quality, since phytoplankton dynamics and biodiversity were shown to be strictly associated to seasonality, hydrodynamic regime and environmental quality of the studied environment. On the one hand, a well-thought-out monitoring plan helps identify those elements that are likely to tilt an ecosystem balance; on the other hand, it is also fundamental for lagoon management, which must take account of nutrient load (wastewaters and/or fertilisers), use of surface waters for irrigation and the functioning of tidal canals. This study aimed to improve our knowledge on the dynamics of coastal lagoons, in order to promote better planning and the implementation of management strategies designed to guarantee the ecosystem conservation for both purposes: namely, a sustainable exploitation of its resources—such as fishing, aquaculture, biomass and biochemical production—and the implementation of cultural services, such as recreation and nature-based tourism, cultural heritage and identity.
References
Arapov J (2013) A review of shellfish phycotoxin profile and toxic phytoplankton species along Croatian coast of the Adriatic Sea. Acta Adriat 54(2):283–298
Basset A, Sabetta L, Carrada GC (2006) Conservation of transitional water ecosystems in the Mediterranean area: bridging basic ecological research and theories with requirements of application. Aquat Conserv 16:439–440
Basset A, Barbone E, Rosati I, Vignes F, Breber P, Specchiulli A, D’Adamo R, Renzi M, Focardi S, Ungaro N, Pinna M (2013) Resistance and resilience of ecosystem descriptors and properties to dystrophic events: a study case in a Mediterranean lagoon. Transit Waters Bull 7(1):1–22
Bosak S, Šilović T, Ljubešić Z, Kušpilić G, Pestorić B, Krivokapić S, Viličić D (2012) Phytoplankton size structure and species composition as an indicator of trophic status in transitional ecosystems: the case study of a Mediterranean fjord-like karstic bay. Oceanologia 54(2):255–286
Brugnano C, D’Adamo R, Fabbrocini A, Granata A, Zagami G (2011) Zooplankton responses to hydrological and trophic variability in a Mediterranean coastal ecosystem (Lesina lagoon, south Adriatic Sea). Chem Ecol 27(5):461–480
Caroppo C (2000) The contribution of picophytoplankton to community structure in a Mediterranean brackish environment. J Plankton Res 22(2):381–397
Caroppo C (2002) Studio della variabilità spaziale del fitoplancton nelle lagune di Lesina e Varano (Mar Adriatico). Proc Ital Assoc Oceanol Limnol 15:111–122
Caroppo C (2015) Ecology and biodiversity of picoplanktonic cyanobacteria in coastal and brackish environments. Biodivers Conserv 24:949–971. https://doi.org/10.1007/s10531-015-0891-y
Caroppo C, Fiocca A, Sammarco P, Magazzù G (1999) Seasonal variations of nutrients and phytoplankton in the coastal SW Adriatic Sea (1995-1997). Bot Mar 42(4):389–400
Caroppo C, Buttino I, Camatti E, Caruso G, De Angelis R, Facca C, Giovanardi F, Lazzara L, Mangoni O, Magaletti E (2013) State of the art and perspectives on the use of planktonic communities as indicators of environmental status in relation to the EU Marine Strategy Framework Directive. Biol Mar Medit 20(1):65–73
Caroppo C, Cerino F, Auriemma R, Cibic T (2016) Phytoplankton dynamics with a special emphasis on harmful algal blooms in the Mar Piccolo of Taranto (Ionian Sea, Italy). Environ Sci Pollut Res 23:12691–12706
Castel J, Caumette P, Herbert R (1996) Eutrophication gradients in coastal lagoons as exemplified by the Bassin d’Ảrcachon and the Étang du Prévost. Hydrobiologia 329:9–28
Chang FH, Anderson DM, Kulis DM, Till DG (1997) Toxin production of Alexandrium minutum (Dinophyceae) from the Bay of Plenty, New Zealand. Toxicon 35(3):393–409
Coelho S, Gamito S, Pérez-Ruzafa A (2007) Trophic state of Foz de Almargem coastal lagoon (Algarve, South Portugal) based on the water quality and the phytoplankton community. Estuar Coast Shelf Sci 71:218–231
Coelho S, Pérez-Ruzafa A, Gamito S (2015) Phytoplankton community dynamics in an intermittently open hypereutrophic coastal lagoon in southern Portugal. Estuar Coast Shelf Sci 167:102–112
Conley DJ (2000) Biogeochemical nutrient cycles and nutrient management strategies. Hydrobiologia 410:87–96
Conley DJ, Schelske CL, Stoermer EF (1993) Modification of the biogeochemical cycle of silica with eutrophication. Mar Ecol Prog Ser 101:179–192
Cozzolino GC (1995) Macrofitobenthos della laguna di Lesina: cartografia estiva. Biol Mar Medit 2(2):361–363
D’Adamo R, Cecere E, Fabbrocini A, Petrocelli A, Sfriso A (2009) The lagoons of Lesina and Varano. In: Cecere E, Petrocelli A, Izzo G, Sfriso A (eds) Flora and vegetation of the Italian Transitional Water System. Corila Venezia, Italy, pp 159–171
Du X, Peterson W, McCulloch A, Liu G (2011) An unusual bloom of the dinoflagellate Akashiwo sanguinea off the central Oregon, USA, coast in autumn 2009. Harmful Algae 10(6):784–793
EC (2000) Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for community action in the field of water policy. J Eur Union 43:1
EC (2008) Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on environmental quality standards in the field of water policy, amending and subsequently repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and amending Directive 2000/60/EC of the European Parliament and of the Council Official. J Eur Union 105:1
Fanuko N, Valčić M (2009) Phytoplankton composition and biomass of the northern Adriatic lagoon of Stella Maris, Croatia. Acta Bot Croat 68(1):29–44
Ferrarin C, Zaggia L, Paschini E, Scirocco T, Lorenzetti G, Bajo M, Penna P, Francavilla M, D’Adamo R, Guerzoni S (2014) Hydrological regime and renewal capacity of the micro-tidal Lesina lagoon, Italy. Estuar Coast 37:79–93
Ferreira JG, Andersen JH, Borja A, Bricker SB, Camp J, Cardoso Da Silva M, Garcés E, Heiskanen AS, Humborg C, Ignatiades L, Lancelot C, Menesguen A, Tett P, Hoepffner N, Claussen U (2011) Overview of eutrophication indicators to assess environmental status within the European Marine Strategy Framework Directive. Estuar Coast Shelf Sci 93:117–131
Fonda Umani S, Franco P, Ghirardelli E, Malej A (1992) Outline of oceanography and the plankton of the Adriatic Sea. In: Colombo G, Ferrari I, Ceccherelli VU, Rossi R (eds) Marine eutrophication and population dynamics. Olsen and Olsen, Fredensborg, pp 347–365
García-Pintado J, Martínez-Mena M, Barber GG, Albaladejo J, Castillo VM (2007) Anthropogenic nutrient sources and loads from a Mediterranean catchment into a coastal lagoon: Mar Menor, Spain. Sci Total Environ 373:220–239
Garmendia M, Borja A, Franco J, Revilla M (2013) Phytoplankton composition indicators for the assessment of eutrophication in marine waters: present state and challenges within the European directives. Mar Pollut Bull 66:7–16
Giacobbe MG, Oliva FD, Maimone G (1996) Environmental factors and seasonal occurrence of the dinoflagellate Alexandrium minutum, a PSP potential producer, in a Mediterranean lagoon. Estuar Coast Shelf Sci 42:539–549
Gikas GD, Yiannakopoulou T, Tsihrintzis VA (2006) Water quality trends in a coastal lagoon impacted by non-point source pollution after implementation of protective measures. Hydrobiologia 563:385–406
Gómez F, Boicenco F (2004) An annotated checklist of dinoflagellates in the Black Sea. Hydrobiologia 517:289–311
Hallegraeff GM (2003) Harmful algal blooms: a global overview. In: Hallegraeff GM, Anderson DM, Cembella AD (eds) Manual on harmful marine microalgae. UNESCO, Paris, pp 25–49
Heil CA, Glibert PM, Fan C (2005) Prorocentrum minimum (Pavillard) Schiller a review of a harmful algal bloom species of growing worldwide importance. Harmful Algae 4:449–470
Hillebrand H, Durselen CD, Kirschtel D, Pollingher U, Zohary T (1999) Biovolume calculation for pelagic and benthic microalgae. J Phycol 35:403–424
Jessup D, Miller MA, Ryan JP, Nevins HM, Kerkeing HA, Mekebri A, Crane DB, Johnson TA, Kudela RM (2009) Mass stranding of marine birds caused by a surfactant-producing red tide. PLoS One 4(2). https://doi.org/10.1371/journal.pone.0004550
Lu SH, Hodgkiss IJ (2004) Harmful algal bloom causative collected from Hong Kong waters. Hydrobiologia 512:231–238
Lugoli F, Garmendia M, Lehtinen S, Kauppila P, Moncheva S, Revilla M, Roselli L, Slabakova N, Valencia V, Dromph KM, Basset A (2012) Application of a new multi-metric phytoplankton index to the assessment of ecological status in marine and transitional waters. Ecol Indic 23:338–355
Manini E, Breber P, D’Adamo R, Spagnoli F, Danovaro R (2005) South-eastern Italian coastal systems: lagoon of Lesina. In: Giordani G, Viaroli P, Swaney DP, Murray CN, Zaldívar JM (eds) LOICZ Reports & Studies No. 28, Marshall Crossland JI Texel, The Netherlands, pp 49–54
Menden-Deuer S, Lessard EJ (2000) Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol Oceanogr 45:569–579
Newton A, Mudge S (2005) Lagoon–sea exchanges, nutrient dynamics and water quality management of the Ria Formosa (Portugal). Estuar Coast Shelf Sci 62:405–414
Newton A, Icely J, Cristina S, Brito A, Cardoso AC, Colijn F, Dalla Riva S, Gertz F, Würgler Hansen J, Holmer M, Ivanova K, Leppäkoski E, Melaku Canu D, Mocennim C, Mudge S, Murray N, Pejrup M, Razinkovas A, Reizopoulou S, Pérez-Ruzafa A, Schernewski G, Schubert H, Carr L, Solidoro C, Viaroli P, Zaldívar JM (2014) An overview of ecological status, vulnerability and future perspectives of European large shallow, semi-enclosed coastal systems, lagoons and transitional waters. Estuar Coast Shelf Sci 140:95–122
Nonnis Marzano C, Scalera Liaci L, Franchini A, Gravina F, Mercurio M, Corriero G (2003) Distribution, persistence and change in the macrobenthos of the lagoon of Lesina (Apulia, southern Adriatic Sea). Oceanol Acta 26:57–66
Pachés M, Romero I, Martínez-Guijarro R, Martí CM, Ferrer J (2014) Changes in phytoplankton composition in a Mediterranean coastal lagoon in the Cullera Estany (Comunitat Valenciana, Spain). Water Environ J 28:135–144
Paerl HW, Rossignol K, Hall SN, Peierls BL, Wetz MS (2010) Phytoplankton community indicators of short- and long-term ecological change in the anthropogenically and climatically impacted Neuse River estuary, North Carolina, USA. Estuar Coast 33(2):485–497
Pagliara P, Caroppo C (2012) Toxicity assessment of Amphidinium carterae, Coolia cfr. monotis and Ostreopsis cfr. ovata (Dinophyta) isolated from the northern Ionian Sea (Mediterranean Sea). Toxicon 60(6):1203–1214
Pereira Coutinho MT, Brito AC, Pereira P, Gonçalves AS, Moita MT (2012) A phytoplankton tool for water quality assessment in semi-enclosed coastal lagoons: open vs closed regimes. Estuar Coast Shelf Sci 110:134–146
Pérez-Ruzafa A, Fernandez A, Marcos C, Gilabert J, Quispe J, Garcia-Charton A (2005) Spatial and temporal variations of hydrological conditions, nutrients and chlorophyll a in a Mediterranean coastal lagoon (Mar Menor, Spain). Hydrobiologia 550:11–27
Riccardi M, Guerrini F, Roncarati F, Milandri A, Cangini M, Pigozzi S, Riccardi E, Ceredi A, Ciminiello P, Dell’Aversano C, Fattorusso E, Forino M, Tartaglione L, Pistocchi R (2009) Gonyaulax spinifera from the Adriatic sea: toxin production and phylogenetic analysis. Harmful Algae 8(2):279–290
Roselli L, Fabbrocini A, Manzo C, D’Adamo R (2009) Hydrological heterogeneity, nutrient dynamics and water quality of a non-tidal lentic ecosystem (Lesina lagoon, Italy). Estuar Coast Shelf Sci 8:539–552
Roselli L, Cañedo-Argüelles M, Costa Goela P, Cristina S, Rieradevall M, D’Adamo R, Newton A (2013) Do physiography and hydrology determine the physico-chemical properties an trophic status of coastal lagoons? A comparative approach. Estuar Coast Shelf Sci 11:29–36
Rubino F, Saracino OD, Moscatello S, Belmonte G (2009) An integrated water/sediment approach to study plankton (a case study in the southern Adriatic Sea). J Mar Syst 78:536–546
Sarno D, Zingone A, Saggiomo V, Carrada GC (1993) Phytoplankton biomass and species composition in a Mediterranean coastal lagoon. Hydrobiologia 271:27–40
Solazzi A, Tolomio C, Andreoli C, Caniglia G, Colliva V (1972) La laguna di Lesina (Foggia): Carico microfitico in un ciclo annuale. Mem Biogeogr Adr 9:1–42
Strickland YDH, Parsons TR (1972) A practical handbook of seawater analysis. Bull Fish Res B Canada 16:1–311
Tett P, Gilpin L, Svendsen H, Erlandsson P, Larsson U, Kratzer S, Fouilland E, Janzen C, Lee JY, Grenz C, Newton A, Ferreira JG, Fernandes T, Scory S (2003) Eutrophication and some European waters of restricted exchange. Cont Shelf Res 23:1635–1671
Trotta P (1994) La pesca, la qualità delle acque, gli scarichi in laguna. In: C.N.R. Istituto Sfruttamento Biologico Lagune (ed) La laguna di Lesina: quali prospettive, quali soluzioni? Lesina (FG), pp 11–21
Underwood AJ (1997) Experiments in ecology. Cambridge University Press, Cambridge, p 522
Utermöhl H (1958) Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Mitt Int Ver Theor Angew Limnol 9:1–38
Vadrucci MR, Fiocca A, Vignes F, Fabbrocini A, Roselli L, D’Adamo R, Ungaro N, Basset A (2009) Dynamics of phytoplankton guilds under dystrophic pressures in Lesina lagoon. Transit Waters Bull 3(2):33–46
Viaroli P, Mistri M, Guerzoni S, Cardoso AC (2005) Structure, functions and ecosystems alteration in southern European coastal lagoon: preface. Hydrobiologia 550:7–9
Viličić D, Marasović I, Mioković D (2002) Checklist of phytoplankton in the eastern Adriatic Sea. Acta Bot Croat 61(1):57–91
Yãnez-Arancibia A, Lara Dominguez AL, Pauly D (1994) Coastal lagoons and fish habitats. In: Kjefve B (ed) Coastal lagoon processes. Elsevier Oceanographic Series 60, Amsterdam, pp 363–376
Yasumoto T (1990) Marine microorganism toxins—an overview. In: Graneli E, Sundstrom B, Edler L, Anderson DM (eds) Toxic marine phytoplankton. Elsevier Science Publications, New York, pp 3–8
Zingone A, Montresor M, Marino D (1990) Summer phytoplankton physiognomy in coastal waters of the Gulf of Naples. PSZNI Mar Ecol 11:157–172
Zingone A, Totti C, Sarno D, Cabrini M, Caroppo C, Giacobbe MG, Lugliè A, Nuccio C, Socal G (2010) Fitoplancton: metodiche di analisi quali-quantitativa. In: Socal G, Buttino I, Cabrini M, Mangoni O, Penna A, Totti C (eds) Metodologie di studio del plancton marino, Manuali e Linee Guida 56/2010, ISPRA SIBM, Roma, pp 213–237
Acknowledgements
The writing of this research was funded by the Project Bandiera RITMARE (Ricerca Italiana per il Mare), coordinated by the National Research Council and funded by the Ministry for Education, University and Research within the 2011–2013 National Research Programme.
The authors wish to thank the staff of the Institute of Marine Science of the National Council Research of Lesina (Foggia, Italy) where the research was carried out and the two anonymous referees for their valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Thomas Hein
Rights and permissions
About this article
Cite this article
Caroppo, C., Roselli, L. & Di Leo, A. Hydrological conditions and phytoplankton community in the Lesina lagoon (southern Adriatic Sea, Mediterranean). Environ Sci Pollut Res 25, 1784–1799 (2018). https://doi.org/10.1007/s11356-017-0599-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0599-5