Abstract
Climate change is predicted to influence the heat budget of aquatic ecosystems and, in turn, affect the stability of the water column leading to increased turbulence coupled with enhanced turbidity. However, the synergetic effects of turbulence and turbidity on zooplankton community structure remain to be understood in large, shallow lakes. To determine the possible synergetic effects of these factors on zooplankton communities, a 15-day mesocosm experiment was carried out and tested under four turbulence and turbidity regimes namely control (ɛ = 0, 7.6 ± 4.2 NTU), low (ɛ = 6.01 × 10−8 m2 s−3, 19.4 ± 8.6 NTU), medium (ɛ = 2.95 × 10−5 m2 s−3, 55.2 ± 14.4 NTU), and high (ɛ = 2.39 × 10−4 m2 s−3, 741.6 ± 105.2 NTU) conditions, which were comparable to the natural conditions in Lake Taihu. Results clearly showed the negative effects of turbulence and turbidity on zooplankton survival, which also differed among taxa. Specifically, increased turbulence and turbidity levels influenced the competition among zooplankton species, which resulted to the shift from being large body crustacean-dominated (copepods and cladocerans) to rotifer-dominated community after 3 days. The shift could be associated with the decrease in vulnerability of crustaceans in such environments. Our findings suggested that changes in the level of both turbidity and turbulence in natural aquatic systems would have significant repercussions on the zooplankton communities, which could contribute to the better understanding of community and food web dynamics in lake ecosystems exposed to natural mixing/disturbances.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zooplankton are usually regarded as excellent indicators of the trophic status of aquatic ecosystems due to their pivotal position in the food webs as mediators between the phytoplankton and the higher trophic levels, and through their top-down feedback mechanisms (Sotton et al. 2014). These, in turn, are reflective of the conditions or quality of a water body (Jones et al. 2015). The rich body of knowledge provides insights into the parameters that affect zooplankton populations and community structure (Shurin 2000). Generally, zooplankton are influenced by a number of abiotic (e.g., UV radiation, wind, precipitation, turbidity, and hydrology) and biotic (e.g., competition, parasitism, disease, and predation) factors (see review by Dejen et al. 2004). The relative importance of these factors to zooplankton assemblages however varies among species and seasons (Thorp and Mantovani 2005; Jones et al. 2015).
Recent simulation models predicted increases in wind speed and wave height in the global scale due to climate change (Young et al. 2011), which would also likely affect the stability and turbidity of the water column particularly of shallow and wind-exposed aquatic systems such as lakes. However, the effects of these hydrological changes to the plankton communities have received little attention (Harkonen et al. 2014). Many of the world’s lakes are shallow and thus would be susceptible to substantial sediment resuspension during windy conditions (Levine et al. 2005). The amount of suspended materials in water mass is related to changes in turbulence, and the resulting turbidity affects the productivity of the zooplankton community (Miquelis et al. 2001). Hydrodynamic turbulence together with sediment laden inputs (turbidity) could regulate the zooplankton communities by influencing predation of larger invertebrates and fishes in the system (Horppila et al. 2009; Schulze 2011). In addition, turbidity has been shown to eliminate the most sensitive organisms and modify competition among zooplankton taxa (Jack et al. 1993). Field observations further revealed that zooplankton abundance correlated most strongly with turbidity and/or turbulence over seasons and space in a large lake (Dejen et al. 2004; G- Tóth et al. 2011). For example, horizontal spatial gradients in turbidity affect the occurrence and distribution of zooplankton populations (Hart 1990). However, most of these studies that were performed in laboratory or field focused on either turbidity or turbulence, and not many studies have considered the synergetic effects of the two factors in natural settings (Miquelis et al. 2001).
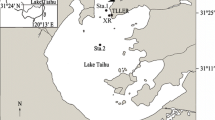
Lake Taihu (Taihu), the third largest freshwater lake in China, is located in the subtropical zone of the Yangtze River Delta region. It covers a total area of 2338 km2 with a mean depth of 2 m, and the maximum depth is not more than 3 m with relatively uniform flat-bottomed bathymetry (Qin et al. 2007). Taihu is highly susceptible to substantial sediment resuspension, making it difficult to control eutrophication and proliferation of cyanobacteria blooms in the lake (Zhu 2005; Zhu et al. 2014). Under wind-induced turbulent conditions, the resuspension of sediments into the water column, which increases turbidity coupled with decolorization, interferes with light penetration. Depending on the extent to which light is attenuated, turbidity can decrease or increase primary production in lakes. Since competitors are unequally affected by turbidity and/or turbulence, zooplankton community structure is also likely to be affected in the process (Zehrer et al. 2015).
In the present study, we investigated the synergetic effects of wind-driven turbulence and turbidity on the structure of zooplankton communities in Taihu. We hypothesize that physical conditions linked to turbidity and turbulence will negatively influence zooplankton dynamics and that this coupling would significantly shift the competitive balance of zooplankton species. To carry out the study, we used a mesocosm experiment to test four levels of turbidity with varied levels of turbulence where natural zooplankton communities were incubated. Results of this study would help in the further understanding of zooplankton responses to fluctuations in environmental conditions and would allow for the implementation of informed management strategies.
Materials and methods
Experimental setup and design
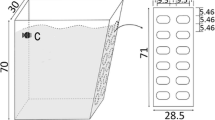
Mesocosm experiments were carried out in a total of 12 customized tanks made of 8-mm-thick Plexiglass. Each tank was right trapezoid with an external length, width, and height measuring 67 (up), 53 (down) × 30 × 70 cm (see Fig. 1a), and has maximal capacities of 126 L. Artificial waves were generated by frequency conversion wavemaker pumps (WP, Jebao, China; Fig. 1c) with slopes (5:1) and dissipation plates on the sides (Fig. 1a, b) to reduce and lessen the effects of rebounds. Potential chemical contaminants in the materials used for the tanks were removed by immersing them in water for 15 days before being used for any experiment.
The sediments (median diameter was 13 μm) used in the experiments were collected in Meiliang Bay (N 31.43544°, E 120.18846°) using a 0.025-m3 modified Peterson grap. To focus on turbidity, organisms and organic matter were removed from the sediments. Sediments were first air-dried in a circulating oven (60 °C) for a day and then placed in the muffle oven for 4 h to burn off any remaining organic matter (Jones et al. 2015). The sediment was then mixed with sterilized ultrapure water and added in each tank to a height of 5 cm. The pre-cleaned tanks were filled with lake water pumped from 0.2-m subsurface (ca. 96 L) without disturbing the sediments.
The four levels of turbulence (energy dissipation rates, ɛ) used in this study were 0 (control (C)), 1.12 × 10−6 (low (L)), 2.95 × 10−5 (medium (M)), and 1.48 × 10−4 m2 s−3 (high (H)), which were based on actual conditions previously observed in Lake Taihu (ɛ, 6.01 × 10−8–2.39 × 10−4 m2 s−3) during the summer of 2013. Turbulences generated and measured in the experimental setups were detailed in Zhou et al. (2015, 2016). The desired turbidity levels were achieved by adding silt under the four turbulent intensities. All treatments were conducted in triplicates.
Natural conditions (e.g., light, water temperature) were simulated by floating and fixing the tanks in an outside artificial pond (10 × 10 × 2 m) filled with lake water and dug 1 m into the ground. The duration of the experiments was limited to 15 days to lessen potential variances associated with bottle effects. These experiments were performed from 28 August to 13 September 2014 at Taihu Laboratory for Lake Ecosystem Research on the shores of Lake Taihu, in Wuxi.
Measurements
Physico-chemical parameters were measured every day from day 0 until day 9, and at days 12 and 15, between 7:00 and 8:00 in the morning. Water temperature (WT), dissolved oxygen (DO), pH, and nephelometric turbidity units (NTUs) were determined using a 6600 multi-sensor sonde (Yellow Springs Instruments, San Diego, CA, USA). Then, samples were collected by sampling 0.75 L of vertically integrated water using a tube sampler. Nutrients analyzed included total nitrogen (TN), total dissolved nitrogen (TDN), ammonium (NH4 +), nitrate (NO3 −), nitrite (NO2 −), total phosphorus (TP), total dissolved phosphorus (TDP), soluble reactive phosphorus (SRP), and suspended solids (SSs), following the methods described in Zhu et al. (2014). The chlorophyll a (Chl a) concentrations were measured by spectrophotometric method (Papista et al. 2002). Samples were first filtered through GF/F filters and frozen at − 20 °C, and pigments were extracted with 90% hot acetone.
Observations for biological variables started at day 0 until day 15, with sampling conducted every 3 days. The same tube sampler was used to obtain a vertically integrated 2 L sample of the water column. The water samples were then concentrated with a 64-μm mesh net and preserved in 4% formaldehyde (final concentration). The filtrates were placed back into their corresponding tanks. The preserved zooplankton samples were analyzed at ×40 magnification using an Olympus microscope (Olympus Corporation, Tokyo, Japan) and identified to species or genus level (Zhang and Huang 1991). From each crustacean taxon, 30 individuals were measured to convert abundances to biomass (wet weight) using length-weight regressions (Zhang and Huang 1991).
Statistical analysis
All statistical analyses were performed in R version 3.4.0 using the “Vegan” package (R Core Team 2017). Analysis of variance (ANOVA) was performed to test whether the environment parameters varied significantly among different treatments with Bonferroni correction. Pairwise analysis of similarity (ANOSIM) was used to compare the similarity of the zooplankton community between each treatment. Diversity indices were determined using the Vegan package (Oksanen et al. 2015). Zooplankton abundances were presented as heat map generated using “heatmap3” package in the R environment (Zhao et al. 2014).
Results
Turbulence and turbidity conditions
The levels of turbulence and turbidity during the study are summarized in Table 1. The ɛ in the treatments C, L, M, and H were 0, 1.12 × 10−6, 2.95 × 10−5, and 1.48 × 10−4 m2 s−3, with corresponding turbidities of 7.6 ± 4.2, 19.4 ± 8.6, 55.2 ± 14.4, and 741.6 ± 105.2 NTU, respectively. The SSs differed significantly among the setups ranging from 0.7 to 1027.5 mg L−1 (p < 0.05). In Lake Taihu, naturally observed turbulence dramatically varied, which ranged from 6.01 × 10−8 to 2.39 × 10−4 m2 s−3, as well as turbidity which was from 0.6 to as high as 1160.8 (mean 48.5) during the summer of 2013. The conditions used in the experiments then were similar to those existing in Taihu.
Environmental characteristics
Water temperature generally ranged from 24.2 to 27.5 °C (p > 0.05). DO and pH were generally higher in the control (C) than the treatments (L, M, and H) during the experiment (Table 2). The average concentrations of the total nutrients (TN and TP) also varied dramatically (Table 2). For example, the average TN and TP were higher in the treatments (L, M, and H) than in the C (Table 2). The same was also true for the concentrations of different nitrogen (TDN, NH4 +, NO3 −, and NO2 −) and phosphorus (TDP and SRP) samples (Table 2). Also, the 15-day average of Chl a in the C and H were lower compared to L and M (p > 0.05). All of these variables however, except for TN, did not differ significantly among the various treatments (p > 0.05, Table 2).
Impacts of turbulence and turbidity on the zooplankton communities
A total of 34 zooplankton species were observed for the entire duration of the experiment, namely 7 copepods, 5 cladocerans, and 22 rotifers. The dominant copepod species were Mesocyclops leuckarti and nauplii, while Bosmina coregoni and Ceriodaphnia cornuta dominated the cladocerans. In addition, Brachionus calyciflorus, Brachionus angularis, and Keratella valga were the most dominant rotifers.
Initially, the zooplankton community (% relative biomass) was mainly consisting of adult copepods (90.0%), cladocerans (6.3%), rotifers (1.4%), and nauplii (1.3%). Throughout the experiment, the total biomass of zooplankton was significantly higher in the C than the treatments with turbidity and turbulence (p < 0.05, Fig. 2). In the C, zooplankton was mostly composed of copepods at the initial stages of experiment but was later replaced by cladocerans, which gradually became the dominant phyla after 6 days (Fig. 2a). However, in the treatments (L, M, and H), the total zooplankton biomass dramatically decreased to almost being sparse at the end of the experiment (Fig. 2b–d). Interestingly, in the L, while copepods (70.0 ± 29.0%) dominated the initial stages of the incubation, the rotifers took over from day 6 to day 9 (23.0 ± 29.0%, Fig. 2b). In contrast, in the M and H, rotifers (about 70.8 ± 45.8%) immediately dominated the community right after day 3 (Fig. 2c).
The heat map based on zooplankton abundance revealed variations in community structure among treatments with four distinct clusters (Fig. 3). All treatments grouped into a distinct cluster after 6 days (Fig. 3). Specifically, in the C, the initially dominant Asplanchna brightwellii, Conochilus dossuarius, Polyarthra trigla, Brachionus caudatus, and M. leuckarti were replaced by B. coregoni, C. cornuta, and nauplii after day 6 (Fig. 3). In the L, M, and H treatments on the other hand, Brachinonus budapestiensis, Lepadella patella, and B. calyciflorus were rare before day 9 but dominated the community after replacing Limnoithona sinensis, B. angularis, and Brachionus urceus (Fig. 3). Interestingly, zooplankton communities remained similar in the C during the experiment (Fig. 3). Compared to C, zooplankton communities drastically changed and mainly grouped into a distinct cluster in the turbulence and turbidity treatments (except the L3; Fig. 3). Pairwise ANOSIM comparisons further showed that the community structure was significantly different between C, and M and H (p < 0.05), suggesting that the competitive balance of zooplankton species shifted under strong turbulence and turbidity conditions.
Furthermore, species richness varied from 3 to 17 with no significant differences between each treatment (Fig. 4a, p > 0.05). Shannon’s diversity index (H) ranged from 2.7 to 8.7, which were higher in L (7.3) and M (5.3) than both C (4.9) and H (3.9, p > 0.05, Fig. 4b). In addition, the Pielou evenness index (J) also varied among the various treatments and highest in the L, but there is no significant difference between each treatment (p > 0.05, Fig. 4c).
Discussion
Zooplankters not only comprise a substantial amount of the biomass of the plankton community but also play as major predators of phytoplankton in lakes (Stromberg et al. 2009; Vargas et al. 2010). However, the role of turbidity and hydrodynamics in the productivity and structure of zooplankton communities has been rarely investigated. This study demonstrated the negative effects of turbidity in association with turbulence on zooplankton biomass by shifting competitive advantage among zooplankton species (Figs. 2 and 3). These observations however were expected since the detrimental effects of intense turbulence and high silt loads on the physiological well-being of most zooplankters have been widely documented in the literature (Dejen et al. 2004; David et al. 2006; Modéran et al. 2010; Pécseli et al. 2014; Zehrer et al. 2015). Specifically, variables such as feeding, growth, and reproduction of different zooplankton species could be adversely affected by turbulence and turbidity (see review by Carrasco et al. 2013). Since zooplankton quickly responds to environmental change (in 3 days, Fig. 3), they are useful indicators of the trophic status and water quality of freshwater ecosystems. This study provides baseline knowledge for future studies since Taihu is strongly influenced by wind waves and sediment resuspension.
In a previous study, we evaluated the response of the zooplankton communities to a series of turbulence levels (the same used in the present study), which were comparable to the hydrodynamic conditions in Taihu (Zhou et al. 2016). We found that turbulence alone decreased the zooplankton biomass and affected the competitive advantage of the different size classes of zooplankton species (Zhou et al. 2016). However, in large shallow lakes, effects of turbulence cannot be separated from turbidity. Therefore, we hypothesized that their synergetic effects would also alter zooplankton community. In fact, turbidity and turbulence are both highly variable environmental parameters that change in both time and space over many orders of magnitude (Visser et al. 2009) and suggested to be the most important factors regulating zooplankton community structure (Ohman and Romagnan 2015). As a typical shallow and eutrophic lake with strong wind and wave influences, which also experiences extreme events (e.g., typhoon and flood several times a year; Zhu et al. 2014), Taihu is a highly turbid and turbulent lake. In Taihu, the monitored turbidity varied from 0.6 to 1160.8 with an average of 48.5 during summer (e.g., 2013), which were similar to those reported in Lake Kentucky (0 to 1400 NTU; Dasari 2012) and Lake St. Lucia (10 to 1000 NTU; Tirok and Scharler 2013). This supports that the turbidity levels (1.2 to 723.5) we used in the experiment were well within the natural ranges of shallow lakes.
Zooplankton may be directly impacted by the increasing turbidity and turbulence (Bickel et al. 2011). In the present study, we found that zooplankton biomass was negatively correlated with suspended clay concentrations and turbulence (Fig. 2). This corroborates what is known about the effects of inorganic suspended solids and turbulence on zooplankton densities. Specifically, inorganic suspended solids could mechanically interfere with food collection or dilute gut contents, resulting to less food being assimilated (Dejen et al. 2004). Also, food limitation reduces the fecundity and ultimately the birth rate of the different species (Dejen et al. 2004; Jones et al. 2015). Moreover, intense turbulence could result to physical injury caused by abrasion and turbulent shear forces (Koenings et al. 1990). Prolonged or frequent sediment resuspension is, therefore, expected to result in less energy being transferred from lower to higher trophic levels (Zehrer et al. 2015). In the field, turbulence coupled with turbidity have also been mostly reported to negatively affect aquatic biota (Henley et al. 2000; Dejen et al. 2004; Carrasco et al. 2007, 2013; Pécseli et al. 2014; Jones et al. 2015). For example, Beaver et al. (2013) demonstrated that increased flow rates of moving water through a reservoir system could decrease zooplankton density during discharge events. A reduction in zooplankton abundance was also observed with increasing turbidity in Lake Chad (Ohman and Romagnan 2015). Our findings were also consistent with those observed in Lake St. Lucia, where a reduction in feeding rate of zooplankton was observed across the control-2500 NTU gradient (Carrasco et al. 2007, 2013).
Although the low zooplankton biomasses were consistently recorded in the turbulent and turbid treatments, an expected pattern of structure shift in the medium and high treatments was also observed. Generally, a hydrological regime of the system exerts a strong influence on the zooplankton structure (Baranyi et al. 2002; Lansactôha et al. 2009; Chaparro et al. 2011). Studies based on field observations also found that the structure of zooplankton communities was associated with the temporal and spatial turbulence and turbidity gradients in Itapeva Lake (Cardoso and Marques 2007). Previous studies also reported that changes in the plankton community of Lake Waihola were related to changes in turbidity (Zehrer et al. 2015). In this study, the population densities of copepods (e.g., Microcyclops varicans and M. leuckarti) and cladocerans (e.g., C. cornuta and B. coregoni) were negatively correlated with turbidity and turbulence, but rotifers (e.g., B. angularis, B. calyciflorus, and B. urceus) thrived in such conditions (Figs. 2 and 3). Body size in particular has been considered a key trait since metabolism, predation risk, and other characteristics covary with size (Litchman et al. 2013). Indeed, large-bodied copepods and cladocerans were more negatively affected by turbulence compared to small-bodied rotifers (Carrasco et al. 2013; Jones et al. 2015). Rotifers in particular have short development times and show fast population recovery from turbidity and turbulence (e.g., floods and typhoon), whereas microcrustaceans have slower growth rates and thus more negatively affected (Baranyi et al. 2002; Paidere 2009). Direct observations on the feeding behavior also showed that clay strongly reduces cladoceran and Daphnia feeding rates compared to rotifers (Levine et al. 2005). In this study, however, rotifers declined significantly after peaking at day 9 (Fig. 2), which was consistent with our previous study and could be associated with the accumulation of damages after long-term exposure to intense turbulence (Zhou et al. 2016).
According to Connell’s intermediate disturbance hypothesis (IDH), zooplankton diversity within a community is maximal at intermediate frequencies and intensities of disturbances (Flöder and Sommer 1999). Similarly, the diversity was higher in the L and M than in the C and H, which was applicable to the IDH (Fig. 4). In addition, the critical time of the shift from large-bodied crustacean-dominated to small-bodied rotifer-dominated community was about 3 days (Fig. 2). Indeed, the response time of zooplankton in Taihu was very rapid and even evident within hours (Zhou et al. 2016). This could partially explain why rotifers probably did better in turbid lakes, not because they were favored in turbid conditions but rather because the harmful effects of competition and predation were partially alleviated by high suspended sediment loads under turbulent conditions (Thorp and Mantovani 2005). This could be the reason why small organisms such as rotifers and nauplii (microzooplankton) usually dominate zooplankton assemblages in shallow lakes (Chaparro et al. 2011). Our findings were also consistent with observations elsewhere, where rotifers fared better and microcrustaceans were negatively selected in turbid and turbulent rivers (Thorp and Mantovani 2005).
Conclusions
This study based on mesocosm experiments demonstrated the negative synergetic effects of turbulence and turbidity on zooplankton biomass, which also affected the competitive advantage of zooplankton species resulting to shift in zooplankton community structure. Copepods and cladocerans potentially preferred weakly turbulent and less turbid conditions, and only rotifers could survive and become dominant in strongly turbid and turbulent waters (ɛ ≥ 2.95 × 10−5, NTU ≥ 55.2 ± 14.4), but long-term exposure (> 9 days) could still become unfavorable. The shift from large-bodied crustacean-dominated to small-bodied rotifer-dominated community was observed after 3 days of constant turbulent and turbid exposures. The results were consistent with field-based studies and provided insights into the potentially important but less understood physical mechanisms affecting zooplankton communities. In the wake of the changing climate, which is predicted to affect world’s atmospheric system and weather patterns, increases in water surface-atmospheric interactions and turbidity in aquatic ecosystems would also be expected. These changes could have significant implications on the structure of zooplankton communities and, by implication, would have potential consequences on the entire food web.
References
Baranyi C, Hein T, Holarek C, Keckeis S, Schiemer F (2002) Zooplankton biomass and community structure in a Danube River floodplain system: effects of hydrology. Freshw Biol 47:473–482
Beaver J, Jensen D, Casamatta D, Tausz C, Scotese K, Buccier K et al (2013) Response of phytoplankton and zooplankton communities in six reservoirs of the middle Missouri River (USA) to drought conditions and a major flood event. Hydrobiologia 705:173–189
Bickel SL, Hammond JDM, Tang KW (2011) Boat-generated turbulence as a potential source of mortality among copepods. J Exp Mar Biol Ecol 401(1–2):105–109
Cardoso LS, Marques DM (2007) Hydrodynamics-driven plankton community in a shallow lake. Aquat Ecol 43:73–84
Carrasco NK, Perissinotto R, Miranda N (2007) Effects of silt loading on the feeding and mortality of the mysid Mesopodopsis africanain the St. Lucia Estuary, South Africa. J Exp Mar Biol Ecol 352:152–164
Carrasco NK, Perissinotto R, Jones S (2013) Turbidity effects on feeding and mortality of the copepod Acartiella natalensis (Connell and Grindley, 1974) in the St. Lucia Estuary, South Africa. J Exp Mar Biol Ecol 446:45–51
Chaparro G, Marinone MC, Lombardo R, Schiaffino MR, Guimarães A, O’Farrell I (2011) Zooplankton succession during extraordinary drought-flood cycles: a case study in a South American floodplain lake. Limnologica 4:371–381
Dasari S (2012) Inorganic elemental concentrations in water samples from Kentucky Lake, Clarks River and Ohio River: possible effects on establishment of zebra mussel population. Murray State University Murray, Kentucky, p 4
David V, Sautour B, Galois R, Chardy P (2006) The paradox high zooplankton biomass–low vegetal particulate organic matter in high turbidity zones: what way for energy transfer? J Exp Mar Biol Ecol 333:202–218
Dejen E, Vijverberg J, Nagelkerke LAJ, Sibbing FA (2004) Temporal and spatial distribution of microcrustacean zooplankton in relation to turbidity and other environmental factors in a large tropical lake (L. Tana, Ethiopia). Hydrobiologia 513:39–49
Flöder S, Sommer U (1999) Diversity in planktonic communities: an experimental test of the intermediate disturbance hypothesis. Limnol Oceanogr 44:1114–1119
G- Tóth L, Parpala L, Balogh C, Tátrai I, Baranyai E (2011) Zooplankton community response to enhanced turbulence generated by water-level decrease in Lake Balaton, the largest shallow lake in Central Europe. Limnol Oceanogr 56:2211–2222
Harkonen L, Pekcan-Hekim Z, Hellen N, Ojala A, Horppila J (2014) Combined effects of turbulence and different predation regimes on zooplankton in highly colored water—implications for environmental change in lakes. PLoS One 9:e111942
Hart R (1990) Zooplankton distribution in relation to turbidity and related environmental gradients in a large subtropical reservoir: patterns and implications. Freshw Biol 24:241–263
Henley WF, Patterson MA, Neves RJ, Lemly AD (2000) Effects of sedimentation and turbidity on lotic food webs: a concise review for natural resource managers. Rev Fish Sci 8:125–137
Horppila J, Eloranta P, Liljendahl-Nurminen A, Niemisto J, Pekcan-Hekim Z (2009) Refuge availability and sequence of predators determine the seasonal succession of crustacean zooplankton in a clay-turbid lake. Aquat Ecol 43:91–103
Jack JD, Wickham SA, Toalson SA, Gilbert JJ (1993) The effects of clays on freshwater plankton community: an enclosure experiment. Arch Hydrobiol 127(3):257–270
Jones S, Carrasco NK, Perissinotto R (2015) Turbidity effects on the feeding, respiration and mortality of the copepod Pseudodiaptomus stuhlmanni in the St. Lucia Estuary, South Africa. J Exp Mar Biol Ecol 469:63–68
Koenings JP, Burkett RD, Edmundson JM (1990) The exclusion of limnetic cladocera from turbid glacier-meltwater lakes. Ecology 71:57–65
Lansactôha FA, Bonecker CC, Velho LFM, Simões NR, Dias JD, Alves GM et al (2009) Biodiversity of zooplankton communities in the Upper Parana River floodplain: interannual variation from long-term studies. Braz J Biol 69:539–549
Levine SN, Zehrer RF, Burns CW (2005) Impact of resuspended sediment on zooplankton feeding in Lake Waihola, New Zealand. Freshw Biol 50:1515–1536
Litchman E, Ohman MD, Kiorboe T (2013) Traitbased approaches to zooplankton communities. J Plankton Res 35:473–484
Miquelis A, Rougier C, Pourriot R (2001) Impact of turbulence and turbidity on the grazing rate of the rotifer Brachionus calyciflorus (Pallas). Hydrobiologia 386:203–211
Modéran J, Bouvais P, David V, Noc SL, Simon-Bouhet B, Niquil N et al (2010) Zooplankton community structure in a highly turbid environment (Charente estuary, France): spatio-temporal patterns and environmental control. Estuar Coast Shelf Sci 88:219–232
Ohman MD, Romagnan JB (2015) Nonlinear effects of body size and optical attenuation on Diel Vertical Migration by zooplankton. Limnol Oceanogr 61:765–770
Oksanen J, Blanchet FG, Kindt R, et al. (2015) Vegan: Community Ecology Package, R Package Version 3.2–2, https://cran.r-project.org/web/packages/vegan/index.html
Paidere J (2009) Influence of flooding frequency on zooplankton in the floodplains of the Daugava River (Latvia). Acta Zool Lituanica 19:306–313
Papista E, Acs E, Boddi B (2002) Chlorophyll-alpha determination with ethanol—a critical test. Hydrobiologia 485:191–198
Pécseli HL, Trulsen JK, Fiksen Ø (2014) Predator-prey encounter and capture rates in turbulent environments. Limnol Oceanogr: Fluids Environ 4:85–105
Qin B, Xu P, Wu Q, Luo L, Zhang Y (2007) Environmental issues of Lake Taihu, China. Hydrobiologia 581:3–14
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna URL https://www.R-project.org/
Schulze PC (2011) Evidence that fish structure the zooplankton communities of turbid lakes and reservoirs. Freshw Biol 56:352–365
Shurin J (2000) Dispersal limitation, invasion resistance, and the structure of pond zooplankton communities. Ecology 81:3074–3086
Sotton B, Guillard J, Anneville O, Maréchal M, Savichtcheva O, Domaizon I (2014) Trophic transfer of microcystins through the lake pelagic food web: evidence for the role of zooplankton as a vector in fish contamination. Sci Total Environ 466:152–163
Stromberg K, Smyth T, Allen J, Pitois S, O’Brien T (2009) Estimation of global zooplankton biomass from satellite ocean colour. J Mar Syst 78:18–27
Thorp JH, Mantovani S (2005) Zooplankton of turbid and hydrologically dynamic prairie rivers. Freshw Biol 50:1474–1491
Tirok K, Scharler UM (2013) Dynamics of pelagic and benthic microalgae during drought conditions in a shallow estuarine lake (Lake St. Lucia). Estuar Coast Shelf Sci 118:86–96
Vargas C, Martinez R, Escribano R, Lagos N (2010) Seasonal relative influence of food quantity, quality, and feeding behaviour on zooplankton growth regulation in coastal food webs. J Mar Biol Assoc UK 90:1189–1201
Visser AW, Mariani P, Pigolotti S (2009) Swimming in turbulence: zooplankton fitness in terms of foraging efficiency and predation risk. J Plankton Res 31:121–133
Young IR, Zieger S, Babanin AV (2011) Global trends in wind speed and wave height. Science 332:451–455
Zehrer RF, Burns CW, Flöder S (2015) Sediment resuspension, salinity and temperature affect the plankton community of a shallow coastal lake. Mar Freshw Res 66:317
Zhang ZS, Huang XF (1991) Research methods on plankton in freshwater. Science Press, Beijing (in Chinese)
Zhao S, Guo Y, Sheng Q, Shyr Y (2014) Advanced heat map and clustering analysis using heatmap3. Biomed Res Int e986048:986048
Zhou J, Qin B, Casenave C, Han X, Yang G, Wu T, Wu P, Ma J (2015) Effects of wind wave turbulence on the phytoplankton community composition in large, shallow Lake Taihu. Environ Sci Pollut Res 22:12737–12746
Zhou J, Han X, Qin B, Casenave C, Yang G (2016) Response of zooplankton community to turbulence in large, shallow Lake Taihu: a mesocosm experiment. Fundam Appl Limnol 187(4):315–324
Zhu G (2005) Direct evidence of phosphorus outbreak release from sediment to overlying water in a large shallow lake caused by strong wind wave disturbance. Chin Sci Bull 50:577–582
Zhu M, Paerl HW, Zhu G (2014) The role of tropical cyclones in stimulating cyanobacterial (Microcystis spp.) blooms in hypertrophic Lake Taihu, China. Harmful Algae 39:310–321
Funding
This research was supported by the National Natural Science Foundation of China (41701098, 41230744, 41621002) and the International Scientific Cooperation Project (2014DFG91780).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Zhou, J., Qin, B. & Han, X. The synergetic effects of turbulence and turbidity on the zooplankton community structure in large, shallow Lake Taihu. Environ Sci Pollut Res 25, 1168–1175 (2018). https://doi.org/10.1007/s11356-017-0262-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0262-1